Abstract
Objective:
The aim of this study is to provide solid evidence to update the management of stage I colon cancer (CC) after surgery.
Background:
Given the low risk of recurrence of stage I CC, some international guidelines do not recommend intensive follow-up after surgery. However, data on the actual incidence, risk factors, and site of recurrences are scarce.
Methods:
This is a retrospective multicenter cohort study considering patients who underwent surgery at 25 Italian centers between 2010 and 2019, with a minimum follow-up of 24 months. A total of 1883 consecutive adult patients with stage I CC treated with curative surgery were considered, and 1611 fulfilled the inclusion criteria. The primary outcome was the rate of recurrence. Secondary outcomes included survival and risk factors for recurrence.
Results:
Eighty patients developed cancer recurrence (5.0%), of which 90% was systemic relapse. The event was more frequent in pT2 (6.0% vs 3.2%, P = 0.013), male patients (6.1% vs 3.6%, P = 0.021), in the presence of lymphovascular invasion (7.2% vs 3.6%, P = 0.01), and in cases of partial resection (11.1% vs 4.6%, P = 0.011). Also, preoperative carcinoembryonic antigen (P = 0.007) and tumor diameter (P < 0.001) were higher in the group who relapsed. Most patients had isolated cancer recurrence (90%). Recurrences peaked between 10 and 18 months after surgery and declined over time. Adjusted Cox regression analysis identified tumor diameter, carcinoembryonic antigen level, lymphovascular invasion, male gender, and less than 12 analyzed lymph nodes as significant risk factors for worse recurrence-free survival.
Conclusions:
This study showed that a not negligible rate of stage I CC recur after curative surgery. Most relapses occur at a single site within the first 3 years after surgery. This evidence could be used to optimize postoperative follow-up.
Keywords: colon cancer; follow-up; prognosis; recurrence, stage I
INTRODUCTION
Patients with stage I colon cancer (CC) are treated with surgical resection and adjuvant chemotherapy is not considered in this subset of patients.1–3 Some international guidelines, including those from the National Comprehensive Cancer Network, recommend less intensive follow-up or sole endoscopic follow-up due to the theoretically low risk of recurrence.1,4–6
However, about 5% of patients with stage I CC will develop a recurrence within 5 years from surgery.7 Despite the good prognosis of stage I CC, clinical and pathological factors beyond the standard TNM staging may be considered as prognostic factors and may be helpful in prognostic stratification.8–12 Though some authors investigated the role of pathological and clinical factors influencing the prognosis of stage II and III CC patients,7,10,12–14 few data are available for stage I CC patients.15–17 Identification of clinical and pathological characteristics associated with worse prognosis may explain heterogeneity within stage. Also, it could provide the most individualized and accurate estimate of patient outcomes, leading to personalized follow-up. The identification of high-risk patients is a fundamental strategy for optimizing healthcare resource utilization. In fact, it may promote efficient resource allocation, resulting in improved patient outcomes and a more effective healthcare system.
Therefore, this retrospective multicentric study aimed to analyze a large cohort of stage I CC undergoing surgical treatment at referral centers to assess the incidence and the sites of recurrence. Moreover, clinical and pathological characteristics of patients who developed a recurrence were evaluated to identify those affecting recurrence.
MATERIALS AND METHODS
Study Design and Inclusion Criteria
A national, multicenter, retrospective, cohort study under the patronage of the Colorectal Cancer Network of the Italian Society of Surgical Oncology was carried out. Patients data were collected from 25 centers following the guidelines set out in the Strengthening the Reporting of Observational Studies in Epidemiology statement.18 Data were retrieved from each center’s database and shared with the promoting center in an anonymized spreadsheet. Guidelines of common definitions on clinical, pathological, and surgical data were formulated by the promoting center and shared with the participating centers. Access to patients’ data was restricted to authorized members of the promoting center. All methods used in this study were performed following the relevant ethical guidelines and regulations of the participating centers. After appropriate trial registration (ClinicalTrials.gov, ID: NCT05726188) and institutional review board approval (UniVR Prog. 3655CESC) by the promoting center, the study was approved by the institutional review board and ethic committee of each center.
Inclusion criteria were age equal to or greater than 18 years, CC up to rectosigmoid junction, histological diagnosis of adenocarcinoma, pT1 or pT2 according to the American Joint Committee on Cancer (AJCC)/Union International Contre Le Cancer TNM staging system,19 curative (R0) resection, elective and urgent surgery, minimally invasive or open surgery, completion surgery after endoscopic resection of pT1 cancer with high-risk features, and minimum follow-up of 24 months. Exclusion criteria were rectal cancer, high-grade dysplasia, histology other than adenocarcinoma, postoperative mortality, preoperative chemotherapy or radiotherapy, hereditary cancer (ie, familial adenomatous polyposis, hereditary non-polyposis colon cancer), history of inflammatory bowel disease, and previous history of CC or other malignant neoplasia. All consecutive cases operated on at participating centers between January 2010 and December 2019 and meeting the above criteria were included.
The primary outcome was the incidence of recurrence after potentially curative surgery for stage I CC. Secondary outcomes were the pattern of recurrence, clinical and pathological characteristics associated with an increased risk of recurrence, overall survival (OS), cancer-specific survival (CSS), and recurrence-free survival (RFS).
Preoperative Workup and Surgical Technique
All patients were staged with preoperative colonoscopy, chest-abdomen-pelvis computed tomography, and carcinoembryonic antigen (CEA) measurement. Other imaging modalities, such as magnetic resonance or positron emission tomography, were performed when indicated.
The main goal of surgery was complete tumor excision with clear longitudinal and circumferential margins. Anatomical resections with ligation of vessels at their origin were the procedures of choice to achieve an adequate lymphadenectomy. Partial resection was defined as the removal of a portion of the colon without ligation of the main feeding vessels.20
Histopathologic Staging
The eighth edition of the AJCC and the Union International Contre Le Cancer criteria were used for reporting the pTNM stage.19 Since the AJCC staging manual,19 the College of American Pathologist,21 and the National Comprehensive Cancer Network Guidelines for CC1 recommend examination of a minimum of 12 lymph nodes to accurately stage CC, the same cutoff was chosen to conduct our analysis. Histological grading according to the World Health Organization criteria, lymphatic invasion, vascular invasion, perineural invasion, and tumor budding were recorded when available.
Data Collection
Each center shared the anonymized data on the patient’s demographic and clinical features, surgical procedure, postoperative course, pathology, and follow-up with the promoting center. Patients who died within 30 days from surgery were excluded from the analysis. Follow-up was conducted according to each center’s protocol and national guidelines from the Italian Society of Medical Oncology, which recommends clinical, radiological, and endoscopic follow-up for 5 years after surgery.22 Retrieved data included time and mode of recurrence, survival status as well as time and cause of death. The type of recurrence was classified as follows: systemic if distant metastases were observed (ie, lung, liver, extra-regional lymph nodes); locoregional if the tumor was detected in the surgical anastomosis, tumor bed, or locoregional lymph nodes; peritoneal if tumor recurred as peritoneal carcinomatosis; multiple if the relapse was observed in more than one organ.20 OS was defined as the length of time between primary surgery and time of death from any cause, while CSS considered death from cancer as the endpoint. RFS was defined as the length of time between primary surgery and the time of cancer recurrence.
Statistical Analysis
Categorical data were compared using the chi-square test or Fisher exact test. Continuous data were analyzed using the ANOVA test or the Kruskal–Wallis test as appropriate.
Survival data were represented using Kaplan–Meier curves and Smoothed Hazard functions. Smoothing width was chosen through visual assessment and robustness checks. Survival analysis was accomplished by proportional hazards Cox regression model. The proportional hazards assumption of the Cox model was tested based on Schoenfeld residuals. In addition, the proportionality assumption was checked by graphic methods: it was verified whether −ln(−ln(survival)) curves for each category of risk factors were parallel when plotted versus ln(analysis time). To cope with missing data, a missing category was added to the variables Charlson score, tumor diameter, and preoperative CEA value. To verify that there was no center effect, the Cox model was replicated by stratifying for each center, confirming that the results were consistent with the main analysis.
Statistical analysis was performed using STATA software, version 18.
RESULTS
Patients’ Selection
Of the 1883 patients who underwent surgery for stage I CC at the participating centers, 258 were excluded due to failure to meet the inclusion criteria (n = 59), incomplete data (n = 28), or lack of adequate follow-up (n = 171). Moreover, 14 patients (0.9%) died in the postoperative period and were excluded (Supplemental Figure 1, http://links.lww.com/AOSO/A427). After the application of inclusion and exclusion criteria, 1611 patients were analyzed.
Clinical and Pathological Characteristics and Recurrence
Table 1 presents the demographics and tumor characteristics of the whole population and in relation to the development of recurrence. Mean (SD) age in the cohort was 68.8 (11.5) years and 54.9% of patients were males. Cases were equally distributed between midgut (50.7%) and hindgut (49.3%) with most of the tumors invading beyond the submucosa (61.7%). Mean (SD) tumor diameter was 31.2 (16.8) mm. Mucinous or signet ring cell histology was observed in 8.0% of the cases, and poorly differentiated (G3) tumors represented 9.6%. Lymphovascular and perineural invasion was demonstrated in 20.6% and 3.7% of the cases, respectively.
TABLE 1.
Clinical and Pathological Characteristics of the Whole Cohort and Patients Who Developed Recurrence
| Characteristics | N | Total | Recurrence | P | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Age, yr, mean (SD) | 1611 | 68.8 (11.5) | 67.1 (11.8) | 70.7 (10.9) | 0.263 |
| Gender | 1611 | 0.021 | |||
| Male | 883 (54.9%) | 54 (6.1%) | 829 (93.9%) | ||
| Female | 728 (45.2%) | 26 (3.6%) | 702 (45.9%) | ||
| BMI | 1097 | 0.234 | |||
| Underweight/normal | 502 (45.8%) | 20 (4.0%) | 481 (96%) | ||
| Overweight/obese | 595 (54.2%) | 37 (6.2%) | 558 (93.8%) | ||
| Charlson Comorbidity Index | 1516 | 0.160 | |||
| ≤4 | 758 (50.0%) | 32 (4.2%) | 726 (95.8%) | ||
| >4 | 758 (50.0%) | 45 (5.9%) | 713 (94.1%) | ||
| Site | 1611 | 0.305 | |||
| Midgut | 816 (50.7%) | 36 (4.4%) | 780 (95.6%) | ||
| Hindgut | 795 (49.3%) | 44 (5.5%) | 751 (94.5%) | ||
| CEA, median (IQR) | 1006 | 2 (2) | 2.5 (3.5) | 1.8 (1.8) | 0.007 |
| pT | 1611 | 0.013 | |||
| pT1 | 617 (38.3%) | 20 (3.2%) | 597 (96.8%) | ||
| pT2 | 994 (61.7%) | 60 (6.0%) | 934 (94%) | ||
| Tumor diameter, mm, mean (SD) | 1190 | 31.2 (16.8) | 40.2 (19.1) | 30.6 (16.7) | <0.001 |
| Macroscopic type | 885 | 0.931 | |||
| Sessile | 470 (53.1%) | 16 (3.4%) | 454 (96.6%) | ||
| Pedunculated | 307 (34.7%) | 10 (3.3%) | 297 (96.7%) | ||
| LST | 108 (12.2%) | 4 (3.7%) | 104 (96.3%) | ||
| Histology | 1611 | 0.400 | |||
| Adenocarcinoma | 1483 (92.0%) | 76 (5.1%) | 1407 (94.9%) | ||
| Mucinous/SR | 128 (8.0%) | 4 (3.1%) | 124 (96.9%) | ||
| Grading | 1576 | 0.552 | |||
| G1–G2 | 1425 (90.4%) | 69 (4.8%) | 1356 (95.2%) | ||
| G3 | 151 (9.6%) | 9 (6.0%) | 142 (94%) | ||
| LVI | 1478 | 0.01 | |||
| Present | 305 (20.6%) | 22 (7.2%) | 283 (92.8%) | ||
| Absent | 1173 (79.4%) | 42 (3.6%) | 1131 (96.4%) | ||
| PNI | 1426 | 1.00 | |||
| Present | 53 (3.7%) | 2 (3.8%) | 51 (96.2%) | ||
| Absent | 1373 (96.3%) | 56 (4.1%) | 1317 (95.9%) | ||
P value refers to the comparison between patients who developed recurrence and patients who did not. Categorical variables are reported as count (%) while quantitative variable as mean (SD) or median (IQR) as appropriate.
Statistically significant P values are highlighted in bold.
BMI indicates body mass index; IQR, interquartile range; LST, laterally spreading tumor; LVI, lymphovascular invasion; PNI, perineural invasion; SR, signet ring.
Recurrence was more frequent in male patients (6.1% vs 3.6%, P = 0.021), pT2 tumors (6.0% vs 3.2%, P = 0.013), and in the presence of lymphovascular invasion (7.2% vs 3.6%, P = 0.01). Preoperative CEA levels (P = 0.007) and tumor diameter (P < 0.001) were higher in the group who developed recurrence.
Surgical Outcomes and Recurrence
Most patients underwent elective surgery (99.3%), with minimally invasive surgery being the preferred option (67.7%) (Table 2). With regards to operative characteristics, only partial resection was associated with a higher incidence of recurrence (11.1% vs 4.6%, P = 0.011). No significant difference in recurrence rate was identified considering the setting of surgery (elective vs urgent) or surgical approach (open vs minimally invasive).
TABLE 2.
Surgical Characteristics of the Whole Cohort and Patients Who Developed Recurrence
| Characteristics | N | Total | Recurrence | P | |
|---|---|---|---|---|---|
| Yes | No | ||||
| Type of surgery | 1611 | 1.000 | |||
| Urgent | 12 (0.7%) | 0 (0.0%) | 12 (100%) | ||
| Elective | 1599 (99.3%) | 80 (5.0%) | 1519 (95%) | ||
| Surgical approach | 1611 | 0.142 | |||
| Open | 521 (32.3%) | 32 (6.1%) | 489 (93.9%) | ||
| Minimally invasive | 1090 (67.7%) | 48 (4.4%) | 1042 (93.6%) | ||
| Type of resection | 1611 | 0.011 | |||
| Anatomic | 1521 (94.4%) | 70 (4.6%) | 1451 (94.8%) | ||
| Partial | 90 (5.6%) | 10 (11.1%) | 80 (88.9%) | ||
| Proximal resection margin, mm, mean (SD) | 862 | 99.0 (82.9) | 88.3 (62.6) | 92.3 (64.4) | 0.550 |
| Distal resection margin, mm, mean (SD) | 973 | 85.3 (75.8) | 72.6 (62.2) | 73.7 (66.0) | 0.206 |
| Analyzed lymph nodes | 1559 | 0.300 | |||
| ≥12 | 1234 (79.2%) | 54 (4.4%) | 1180 (95.6%) | ||
| <12 | 325 (20.9%) | 19 (5.9%) | 306 (94.2%) | ||
| Analyzed nodes, median (IQR) | 1559 | 17 (11) | 15.0 (9) | 17 (10) | 0.090 |
P value refers to the comparison between patients who developed recurrence and patients who did not (not shown). Categorical variables are reported as count (%) while quantitative variable as mean (SD) or median (IQR) as appropriate.
Statistically significant P values are highlighted in bold.
IQR indicates interquartile range.
Despite a mean (SD) number of harvested lymph nodes of 19.1 (10.8) and adequate distal (mean ± SD: 85.3 ± 75.8 mm) and proximal resection margins (mean ± SD: 99 ± 82.9), 20.9% of cases had less than 12 analyzed lymph nodes.
Survival and Recurrence Data
At the time of analysis, 201 patients (12.5%) had died, 19 (1.2%) due to cancer recurrence or treatment and 180 (11.1%) due to causes unrelated to CC. The remaining 1412 patients presented a median (range) follow-up of 59.1 (24–154.4) months. The 5-year OS was 89.4% (95% confidence interval [CI]: 87.5–91.0, median not reached) and the 5-year CSS was 98.6% (95% CI: 97.8–99.2, median not reached).
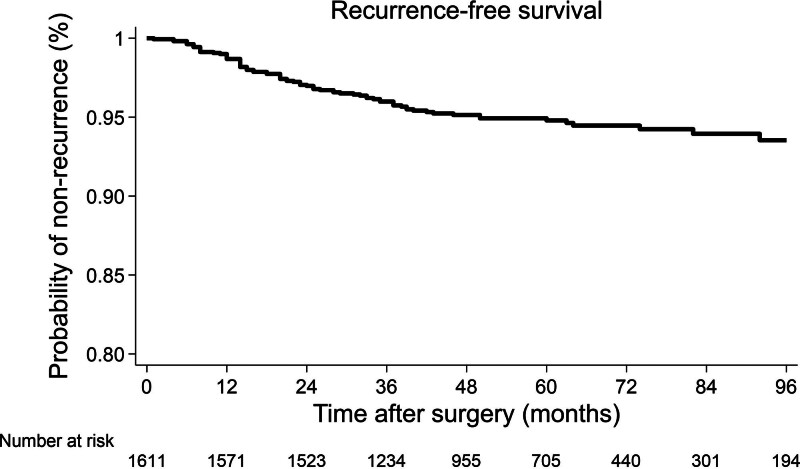
All recurrences were registered within the first 8 years after surgery. The 5-year cumulative risk of recurrence was 4.9%. RFS was 96.0% (95% CI: 94.9%–96.9%) at 3 years, 94.8% (95% CI: 93.4%–95.8%) at 5 years, and 93.5% (95% CI: 91.6%–95.0%) at 8 years (Fig. 1). Recurrences developed in 80 patients (5.0%) after a mean (±SD) time of 25.0 ± 18.2 months. An isolated cancer recurrence was observed in 90% of the cases (72/80 cases), while it was multiple in 10% (8/80 cases). The recurrence involved the liver in 43.8% of the cases and the lung in 16.3% of the cases, while it was locoregional in 10.0% of the cases. Two patients (2.5%) recurred at the peritoneum or the ovary. Five-year OS of patients who developed recurrence was 72.0% (95% CI: 58.5%–81.8%) months (Supplemental Figure 2, http://links.lww.com/AOSO/A427). Details regarding the treatment of recurrences were available for 62 (77.5%; 55 isolated, 7 multiple) patients. Almost all patients with multiple site recurrence received systemic chemotherapy as first-line treatment. Thirty-seven of 55 patients who developed isolated recurrence underwent surgery with curative intent (Supplemental Table 1, http://links.lww.com/AOSO/A427).
FIGURE 1.
Kaplan–Meier curve showing recurrence-free survival.
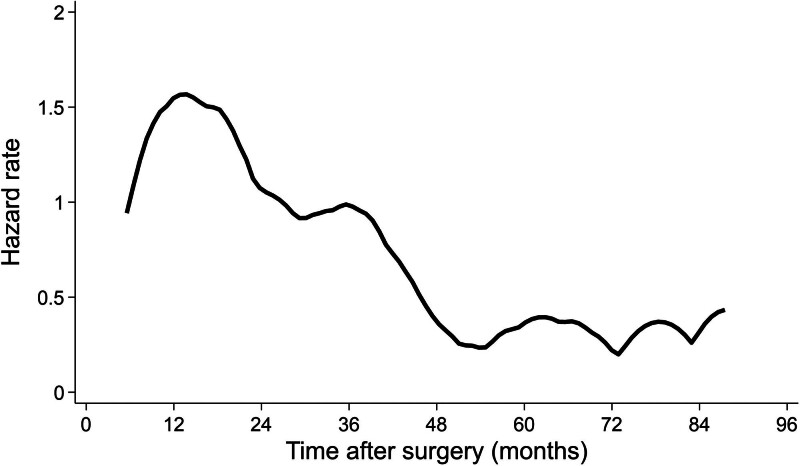
Hazard function analysis of cancer recurrence is depicted in Figure 2. Incidence of cancer recurrence peaked at 1.5 per 1000 person-years between 10 and 18 months after surgery and declined below 1.0 per 1000 person-years after 36 months. However, the incidence of cancer recurrence did not decrease to zero even after 60 months from surgery, oscillating between 0.3 and 0.4 cases per 1000 person-years. In fact, the cumulative incidence of recurrence was 77.5% (95% CI: 66.8%–86.1%) at 3 years and 93.8% (95% CI: 86.0%–97.9%) at 5 years.
FIGURE 2.
Hazard function analysis of cancer recurrence.
Risk Factors for Cancer Recurrence
Unadjusted and adjusted Cox regression analyses were performed (Table 3). The strongest independent predictor of RFS was tumor diameter, as the risk of recurrence increased 6-fold when cancer diameter increased from ≤20 mm to >40 mm. Other independent risk factors were CEA level, lymphovascular invasion, male gender, and less than 12 analyzed lymph nodes.
TABLE 3.
Influence of Individual, Tumor, and Surgical Characteristics on Tumor Recurrence
| Characteristics | Unadjusted Hazard Ratio (95% Confidence Interval) | Unadjusted P Value | Adjusted Hazard Ratio (95% Confidence Interval) | Adjusted P Value |
|---|---|---|---|---|
| Gender (women vs men) | 0.57 (0.35–0.90) | 0.017 | 0.54 (0.31–0.94) | 0.030 |
| Age | 0.99 (0.97–1.01) | 0.274 | 0.98 (0.96–1.01) | 0.229 |
| Charlson index | ||||
| Score<4 | 1 (reference) | 1 (reference) | ||
| Score>4 | 1.46 (0.93–2.30) | 0.100 | 1.21(0.64–2.32) | 0.558 |
| Missing | 0.78 (0.24–2.55) | 0.681 | 0.39 (0.11–1.42) | 0.155 |
| pT (pT2 vs pT1) | 1.92 (1.16–3.18) | 0.012 | 1.59 (0.84–3.02) | 0.157 |
| Tumor diameter | ||||
| ≤20 mm | 1 (reference) | 1 (reference) | ||
| 21–30 mm | 3.40 (1.23–9.45) | 0.019 | 2.94 (0.89–9.6) | 0.077 |
| 31–40 mm | 4.84 (1.77–13.2) | 0.002 | 5.83 (1.89–18.05) | 0.002 |
| >40 mm | 6.06 (2.24–16.43) | 0.000 | 6.00 (1.90–18.96) | 0.002 |
| Missing | 5.02 (1.94–13.00) | 0.001 | 4.09 (1.34–12.50) | 0.013 |
| LVI (yes vs no) | 2.07 (1.23–3.46) | 0.006 | 2.08 (1.90–3.62) | 0.010 |
| CEA level (>5 vs ≤5) | 2.12 (1.13–4.00) | 0.020 | 3.10 (1.48–6.49) | 0.003 |
| Missing | 1.24 (0.77–2.01) | 0.374 | 1.32 (0.73–2.40) | 0.363 |
| Analyzed lymph nodes (≥ 12 vs <12) | 0.75 (0.45–1.27) | 0.285 | 0.43 (0.24–0.77) | 0.004 |
| Type of resection (partial vs anatomic) | 2.44 (1.26–4.74) | 0.008 | 1.55 (0.47–5.10) | 0.467 |
Hazard ratios and P values were obtained by univariable and multivariable Cox regression model. A total of 1427 patients were included in the model.
Statistically significant P values are highlighted in bold.
LVI indicates lymphovascular invasion.
Depth of tumor invasion was significantly associated with recurrence in the univariable analysis, but not in multivariable analysis. However, when the analysis was restricted to the first 2 years of follow-up, pT remained significant even in multivariable analysis (hazard ratio of pT2 vs pT1 = 2.84, 95% CI: 1.05–7.71, P = 0.040) (Supplemental Table 2, http://links.lww.com/AOSO/A427), probably due to moderate collinearity between pT class and tumor diameter (Supplemental Table 3, http://links.lww.com/AOSO/A427). With regards to the incidence of cancer recurrence over time, the peak was reached between 12 and 18 months in pT2, while it was delayed to 30 to 36 months in pT1 (Supplemental Figures 3 and 4, http://links.lww.com/AOSO/A427).
DISCUSSION
In this article, we present the results of the largest multicenter study reporting the oncological outcomes of stage I CC treated with curative surgery. In fact, most of the available literature comes from population-based databases, including the Surveillance, Epidemiology, and End Results database and the Japanese Study Group database.3,23–25 Despite the advantage of analyzing a considerable number of patients, national cancer databases often lack accurate data on recurrence status and pathological details.26 On the other hand, some monocentric retrospective studies investigated the risk factors for recurrence after curative surgery for stage I CC,15–17,23,27 but the number of patients was limited or the population heterogeneous for the inclusion of rectal cancer patients.
Currently, the topic of follow-up after surgery for stage I CC remains controversial. Most Western guidelines suggest only endoscopic follow-up at 1 year after surgery1,4,5 due to the low risk of recurrence and the lack of strong evidence in favor of intensive follow-up. Present data suggest that some 5% of patients with stage I CC will recur over time, even if treated with potentially curative surgery. Most of these recurrences (90%) were at a single site, with the liver being the most frequent site of relapse. Postoperative follow-up should point toward the early identification of recurrence, in order to enable appropriate management. The site of recurrence also plays a significant role in the subsequent treatment, with most of isolated hepatic metastases being suitable for surgical resection.
According to our data, 5-year OS drops from 90% to 72% after the diagnosis of recurrence, representing a critical step in the oncological history of patients with CC. We conducted unadjusted and adjusted Cox regression analysis to identify clinical and pathological factors associated with a higher risk of recurrence. The strongest independent predictor of cancer recurrence was tumor diameter, followed by preoperative CEA level, lymphovascular invasion, male gender, and less than 12 analyzed lymph nodes. Considering the different risk of recurrence between genders, our results are in line with previously published literature that identified worse OS, CSS, and RFS in male CC patients.7,28 Similarly, Shen and colleagues identified preoperative CEA as a surrogate of aggressive tumor biology in pT1N0M0 CC as well as a predictor of poor prognosis.25 Finally, though 94.4% of resections were defined as anatomic, 20.9% of them had less than 12 analyzed lymph nodes (Table 2). These findings prompt for a reflection since the extent of lymphadenectomy resulted in the only independent predictor of cancer recurrence that can be impacted by surgeons’ practice.
For what concerns the time to recurrence and the course of recurrence hazard after surgery, relapses developed after a median time of 20 months, and the incidence of cancer recurrence peaked at 1.5 per 1000 person-years between 10 and 18 months after surgery and declined below 1.0 per 1000 person-years after 36 months. The subanalysis based on the depth of tumor infiltration showed that the incidence of cancer recurrence in pT2 tumors peaked between 12 and 18 months after surgery, while the peak was delayed to 30 to 36 months in pT1 (Supplementary Figure 4 http://links.lww.com/AOSO/A427). These data are in line with the published data from the National Cancer Center Hospital in Japan.17 They retrospectively analyzed a population of 4330 stage I–III colorectal cancer patients including 1432 stage I and found a 4% recurrence rate and a peak at 19.1 months. Also, they found that the hazard rate peaked at 17.8 months for pT2, while the hazard curve for pT1 was relatively flat with a late peak at 37.2 months.
The main limitation of the present analysis is in the retrospective nature of the study leading to incomplete and missing data such as preoperative tumor markers and molecular characterization. Moreover, it was a multicenter study, and it was not possible to analyze all the processed data, with a significative reduction of the numerosity of the study sample making it difficult to compare present data with those coming from the larger population-based databases, including the Surveillance, Epidemiology, and End Results database and the Japanese Study Group database.3,23–25 Furthermore, data were collected from 25 different centers entailing some degree of heterogeneity in surgical treatments and follow-up, depending on centers and surgeons’ volume and experience.
Valuable strengths of this multicenter study reside in the fairly large cohort of carefully selected stage I CC patients, the application of strict inclusion criteria, and the minimum follow-up of 24 months, which warrant for reliable and reproducible results. Furthermore, the Cox model was replicated by stratifying for each center, confirming that the results were consistent with the main analysis.
CONCLUSIONS
This multicenter retrospective study showed that 5% of patients receiving curative surgery for stage I CC will eventually recur. Most patients relapse at a single site within the first 3 years after surgery. Independent predictors of cancer recurrence included tumor diameter, preoperative CEA level, lymphovascular invasion, male gender, and less than 12 analyzed lymph nodes. Based on current data, a call for a revision and standardization of the follow-up protocols for this group of patients, possibly identifying high-risk patients, seems to be necessary.
ACKNOWLEDGMENTS
The authors thank the following collaborators for participating in data management and collection: Adriana Gioia (UCO Clinica Chirurgica, Università di Trieste, Trieste, Italy), Cristina Vacca (UOC Chirurgia Generale 1, Fondazione Policlinico Universitario Agostino Gemelli – IRCCS, Roma, Italy), Dario Parini (Chirurgia Generale, Ospedale Santa Maria della Misericordia, Rovigo, Italy), Giovanni Tomasicchio (Chirurgia Generale e Mininvasiva, Ospedale San Camillo, Trento, Italy), Marcello Calabrò (S.C. Chirurgia, Ospedale E. Agnelli, ASL TO 3, Presidio Pinerolo, Italy), Bruno Sensi (Minimally Invasive and Gastrointestinal Surgery Unit, Department of General Surgery, University of Rome Tor Vergata, Rome, Italy), Filomena Misuriello (UOC Chirurgia Digestiva e del Colon-Retto - Ospedale Isola Tiberina Gemelli Isola, Roma, Italy), Carlo Alberto Schena (Unit of Digestive and HPB Surgery, Henri Mordor University Hospital, Paris, France), and Silvia Picotto (Chirurgia d’Urgenza e PS 3, AOU Città della Salute e della Scienza, Torino, Italy).
Supplementary Material
Footnotes
Disclosure: The authors declare that they have nothing to disclose.
Data will be made available upon reasonable request to the corresponding author.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.annalsofsurgery.com).
Contributor Information
Adriana Gioia, UCO Clinica Chirurgica, Università di Trieste, Trieste, Italy.
Cristina Vacca, UOC Chirurgia Generale 1, Fondazione Policlinico Universitario Agostino Gemelli – IRCCS, Roma, Italy.
Dario Parini, Chirurgia Generale, Ospedale Santa Maria della Misericordia, Rovigo, Italy.
Giovanni Tomasicchio, Chirurgia Generale e Mininvasiva, Ospedale San Camillo, Trento, Italy.
Marcello Calabrò, S.C. Chirurgia, Ospedale E. Agnelli, ASL TO 3, Presidio Pinerolo, Italy.
Bruno Sensi, Minimally Invasive and Gastrointestinal Surgery Unit, Department of General Surgery, University of Rome Tor Vergata, Rome, Italy.
Filomena Misuriello, UOC Chirurgia Digestiva e del Colon-Retto - Ospedale Isola Tiberina Gemelli Isola, Roma, Italy.
Carlo Alberto Schena, Unit of Digestive and HPB Surgery, Henri Mordor University Hospital, Paris, France.
Silvia Picotto, Chirurgia d’Urgenza e PS 3, AOU Città della Salute e della Scienza, Torino, Italy.
Collaborators: Adriana Gioia, Cristina Vacca, Dario Parini, Giovanni Tomasicchio, Marcello Calabrò, Bruno Sensi, Filomena Misuriello, Carlo Alberto Schena, and Silvia Picotto
REFERENCES
- 1.Benson AB, Veenok AP, Al-Hawari MM, et al. Colon Cancer, Version 2.2021, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2021;19:329–359. [DOI] [PubMed] [Google Scholar]
- 2.Argilés G, Tabernero J, Labianca R, et al. ; ESMO Guidelines Committee. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31:1291–1305. [DOI] [PubMed] [Google Scholar]
- 3.Hashiguchi Y, Muro K, Saito Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) Guidelines 2019 for the Treatment of Colorectal Cancer. Vol 25. Springer; Singapore; 2020. doi:10.1007/s10147-019-01485-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hardiman KM, Felder SI, Friedman G, et al. ; Prepared on Behalf of the Clinical Practice Guidelines Committee of the American Society of Colon and Rectal Surgeons. The American Society of Colon and Rectal Surgeons Clinical Practice guidelines for the surveillance and survivorship care of patients after curative treatment of colon and rectal cancer. Dis Colon Rectum. 2021;64:517–533. [DOI] [PubMed] [Google Scholar]
- 5.Meyerhardt JA, Mangu PB, Flynn PJ, et al. ; American Society of Clinical Oncology. Follow-up care, surveillance protocol, and secondary prevention measures for survivors of colorectal cancer: American Society of Clinical Oncology Clinical Practice Guideline endorsement. J Clin Oncol. 2013;31:4465–4470. [DOI] [PubMed] [Google Scholar]
- 6.Herzig D, Hardimann K, Weiser M, et al. The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the management of inherited polyposis syndromes. Dis Colon Rectum. 2017;60:881–894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Osterman E, Glimelius B. Recurrence risk after up-to-date colon cancer staging, surgery, and pathology: analysis of the entire Swedish population. Dis Colon Rectum. 2018;61:1016–1025. [DOI] [PubMed] [Google Scholar]
- 8.Weiser MR, Hsu M, Bauer PS, et al. Clinical calculator based on molecular and clinicopathologic characteristics predicts recurrence following resection of stage I-III colon cancer. J Clin Oncol. 2021;39:911–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Konishi T, Shimada Y, Lee LH, et al. Poorly differentiated clusters predict colon cancer recurrence. Am J Surg Pathol. 2018;42:705–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Turri G, Barresi V, Valdegamberi A, et al. Clinical significance of preoperative inflammatory markers in prediction of prognosis in node-negative colon cancer: correlation between neutrophil-to-lymphocyte ratio and poorly differentiated clusters. Biomedicines. 2021;9:94–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwaan MR. Postoperative CEA and other non-traditional risk factors for colon cancer recurrence: findings from Swedish population-based data. Ann Surg Oncol. 2020;27:971–972. [DOI] [PubMed] [Google Scholar]
- 12.Osterman E, Mezheyeuski A, Sjöblom T, et al. Beyond the NCCN risk factors in colon cancer: an evaluation in a Swedish population-based cohort. Ann Surg Oncol. 2020;27:1036–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ammendola S, Turri G, Marconi I, et al. The presence of poorly differentiated clusters predicts survival in stage II colorectal cancer. Virchows Arch. 2020;478:241–248. [DOI] [PubMed] [Google Scholar]
- 14.Weiser MR, Gon̈en M, Chou JF, et al. Predicting survival after curative colectomy for cancer: individualizing colon cancer staging. J Clin Oncol. 2011;29:4796–4802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim S, Huh JW, Lee WY, et al. Lymphovascular invasion, perineural invasion, and tumor budding are prognostic factors for stage I colon cancer recurrence. Int J Colorectal Dis. 2020;35:881–885. [DOI] [PubMed] [Google Scholar]
- 16.Kim C, Kim WR, Kim KY, et al. Predictive nomogram for recurrence of stage I colorectal cancer after curative resection. Clin Colorectal Cancer. 2018;17:e513–e518. [DOI] [PubMed] [Google Scholar]
- 17.Kudose Y, Shida D, Ahiko Y, et al. Evaluation of recurrence risk after curative resection for patients with stage I to III colorectal cancer using the hazard function: retrospective analysis of a single-institution large cohort. Ann Surg. 2022;275:727–734. [DOI] [PubMed] [Google Scholar]
- 18.Agha RA, Borrelli MR, Vella-Baldacchino M, et al. ; STROCSS Group. The STROCSS statement: strengthening the reporting of cohort studies in surgery. Int J Surg. 2017;46:198–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amin M, Edge SB, Greene F. AJCC Cancer Staging Manual, 8th Edition. Springer; 2018. [Google Scholar]
- 20.Pedrazzani C, Turri G, Park SY, et al. Clinical–pathologic characteristics and long-term outcomes of left flexure colonic cancer: a retrospective analysis of an international multicenter cohort. Dis Colon Rectum. 2020;63:1593–1601. [DOI] [PubMed] [Google Scholar]
- 21.Sepulveda AR, Hamilton SR, Allegra CJ, et al. Molecular biomarkers for the evaluation of colorectal cancer: guideline from the American Society for Clinical Pathology, College of American Pathologists, Association for Molecular Pathology, and American Society of Clinical Oncology. J Mol Diagn. 2017;19:187–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Associazione Italiana di Oncologia Medica (AIOM). Linee Guida tumori del colon retto. AIOM; 2020:1–47. Available at: https://www.aiom.it/linee-guida-aiom-2020-tumori-del-colon/. Accessed March 2024. [Google Scholar]
- 23.Kobayashi H, Mochizuki H, Morita T, et al. Characteristics of recurrence after curative resection for T1 colorectal cancer: Japanese multicenter study. J Gastroenterol. 2011;46:203–211. [DOI] [PubMed] [Google Scholar]
- 24.Hines RB, Jiban MJH, Specogna AV, et al. Surveillance colonoscopy in older stage I colon cancer patients and the association with colon cancer-specific mortality. Am J Gastroenterol. 2020;115:924–933. [DOI] [PubMed] [Google Scholar]
- 25.Shen F, Cui J, Hong X, et al. Preoperative serum carcinoembryonic antigen elevation in stage I colon cancer: improved risk of mortality in stage T1 than in stage T2. Int J Colorectal Dis. 2019;34:1095–1104. [DOI] [PubMed] [Google Scholar]
- 26.In H, Bilimoria KY, Stewart AK, et al. Cancer recurrence: an important but missing variable in national cancer registries. Ann Surg Oncol. 2014;21:1520–1529. [DOI] [PubMed] [Google Scholar]
- 27.Jung HS, Ryoo SB, Lim HK, et al. Tumor size and harvested LNs <12 are the risk factors for recurrence in stage I colon and rectal cancer after radical resection. Cancers (Basel). 2021;13. doi:10.3390/cancers13215294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karim S, Brennan K, Nanji S, et al. Association between prognosis and tumor laterality in early-stage colon cancer. JAMA Oncol. 2017;3:1386–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]