Abstract
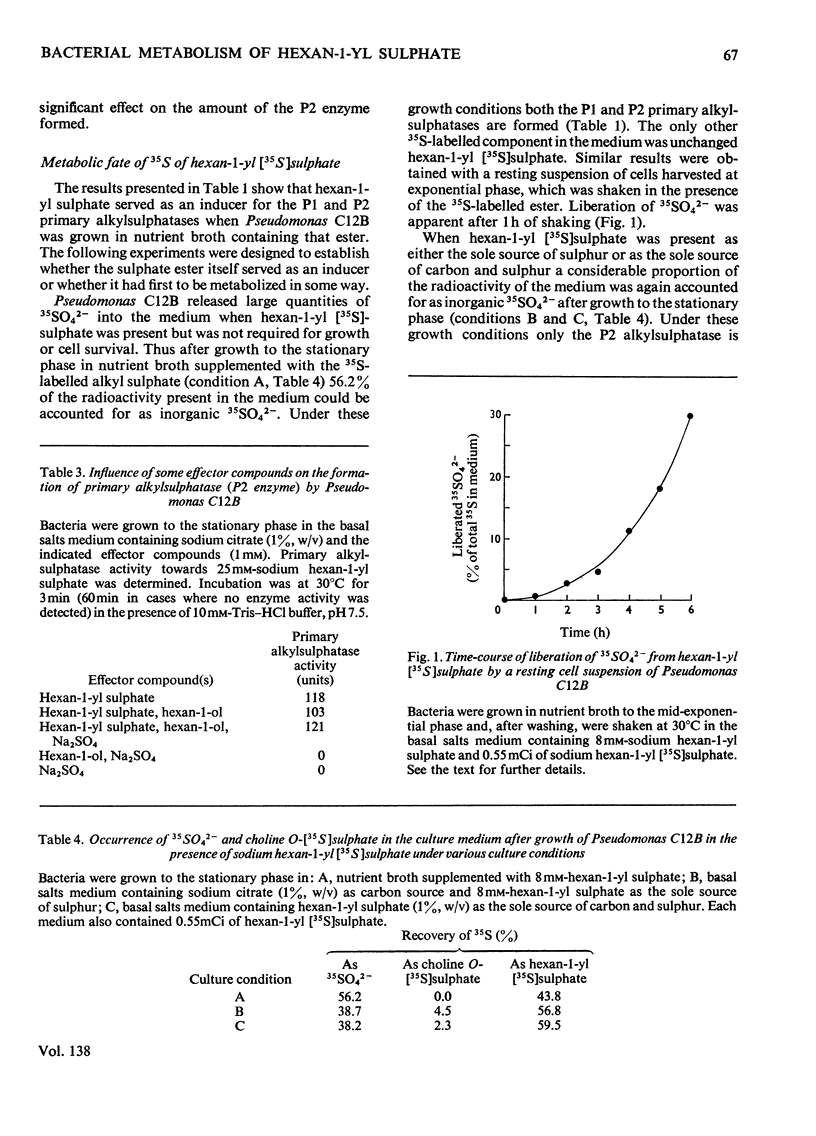
Sodium hexan-1-yl sulphate and certain related alkyl sulphate esters have been shown to serve as inducers of the formation of primary alkylsulphatases (designated as P1 and P2) in Pseudomonas C12B. When the organism is grown on sodium hexan-1-yl [35S]sulphate as the sole source of sulphur or as the sole source of carbon and sulphur only the P2 alkylsulphatase is formed and inorganic 35SO42− is liberated into the media. Cell extracts contain this anion as the major 35S-labelled metabolite although two unidentified labelled metabolites as well as choline O-[35S]sulphate occur in trace quantities in some extracts. Dialysed cell extracts are capable of liberating inorganic 35SO42− from sodium hexan-1-yl [35S]sulphate without the need to include cofactors known to be required for the bacterial degradation of n-alkanes. The collective results suggest that sodium hexan-1-yl sulphate can act as an inducer of P1 alkylsulphatase formation without the need for prior metabolic modification of the carbon moiety of the ester.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DODGSON K. S. Determination of inorganic sulphate in studies on the enzymic and non-enzymic hydrolysis of carbohydrate and other sulphate esters. Biochem J. 1961 Feb;78:312–319. doi: 10.1042/bj0780312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DODGSON K. S., LLOYD A. G., TUDBALL N. O-sulphate esters of L-serine, L-threonine and L-hydroxyproline. Biochem J. 1961 Apr;79:111–117. doi: 10.1042/bj0790111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denner W. H., Olavesen A. H., Powell G. M., Dodgson K. S. The metabolism of potassium dodecyl [35-S]sulphate in the rat. Biochem J. 1969 Jan;111(1):43–51. doi: 10.1042/bj1110043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodgson K. S., Fitzgerald J. W., Payne W. J. Chemically defined inducers of alkylsulphatases present in Pseudomonas C12B. Biochem J. 1974 Jan;138(1):53–62. doi: 10.1042/bj1380053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Dodgson K. S. Carbon and sulphur utilization during growth of Pseudomonas fluorescens on potassium D-glucose 6-O-sulphate as the sole sulphur source. Biochem J. 1971 Apr;122(3):277–283. doi: 10.1042/bj1220277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Dodgson K. S. Sulphur utilization during growth of pseudomonas fluorescens on potassium D-glucose 6-O-sulphate. Biochem J. 1971 Feb;121(3):521–528. doi: 10.1042/bj1210521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. W., Payne W. J. Induction in a Pseudomonas species of sulphatases active on short chain alkylsulphates. Microbios. 1972 Mar-Apr;5(18):87–100. [PubMed] [Google Scholar]
- Fitzgerald J. W. The formation of choline O-sulphate by Pseudomonas C12B and other Pseudomonas species. Biochem J. 1973 Oct;136(2):361–369. doi: 10.1042/bj1360361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug M. J., Markovetz A. J. Utilization of aliphatic hydrocarbons by micro-organisms. Adv Microb Physiol. 1971;5:1–43. doi: 10.1016/s0065-2911(08)60404-x. [DOI] [PubMed] [Google Scholar]
- LLOYD A. G., TUDBALL N., DODGSON K. S. Infrared studies on sulphate esters. III. O-Sulphate esters of alcohols, amino alcohols and hydroxylated amino acids. Biochim Biophys Acta. 1961 Sep 30;52:413–419. doi: 10.1016/0006-3002(61)90397-3. [DOI] [PubMed] [Google Scholar]
- Lloyd A. G., Large P. J., Davies M., Olavesen A. H., Dodgson K. S. The glycosulphatase of Trichoderma viride. Biochem J. 1968 Jul;108(3):393–399. doi: 10.1042/bj1080393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas J. J., Burchiel S. W., Segel I. H. Choline sulfatase of Pseudomonas aeruginosa. Arch Biochem Biophys. 1972 Dec;153(2):664–672. doi: 10.1016/0003-9861(72)90385-2. [DOI] [PubMed] [Google Scholar]
- Ottery J., Olavesen A. H., Dodgson K. S. Metabolism of dodecyl sulphate in the rat: non-enzymic liberation of sulphate and gamma-butyroactone from the major metabolite, butyric acid 4-sulphate. Life Sci II. 1970 Dec 8;9(23):1335–1340. doi: 10.1016/0024-3205(70)90114-1. [DOI] [PubMed] [Google Scholar]
- Payne W. J., Painter B. G. Resolution by acrylamide gel electrophoresis of alkyl sulphatases and alcohol dehydrogenase. Microbios. 1971 Apr;3(12):199–206. [PubMed] [Google Scholar]
- Payne W. J., Williams J. P., Mayberry W. R. Primary alcohol sulfatase in a Pseudomonas species. Appl Microbiol. 1965 Sep;13(5):698–701. doi: 10.1128/am.13.5.698-701.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phibbs P. V., Jr, Eagon R. G. Transport and phosphorylation of glucose, fructose, and mannitol by Pseudomonas aeruginosa. Arch Biochem Biophys. 1970 Jun;138(2):470–482. doi: 10.1016/0003-9861(70)90371-1. [DOI] [PubMed] [Google Scholar]
- Scott J. M., Spencer B. Regulation of choline sulphatase synthesis and activity in Aspergillus nidulans. Biochem J. 1968 Jan;106(2):471–477. doi: 10.1042/bj1060471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAKEBE I. Isolation and characterization of a new enzyme choline sulfatase. J Biochem. 1961 Sep;50:245–255. doi: 10.1093/oxfordjournals.jbchem.a127440. [DOI] [PubMed] [Google Scholar]


