Abstract
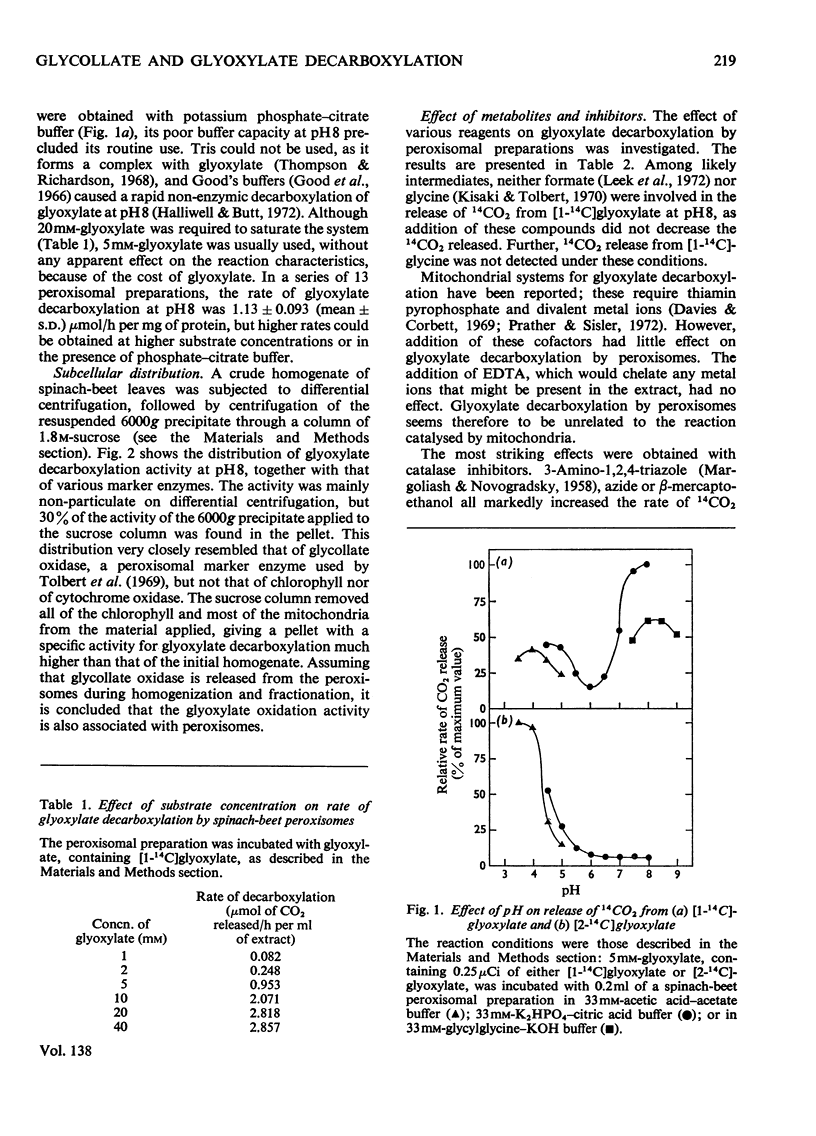
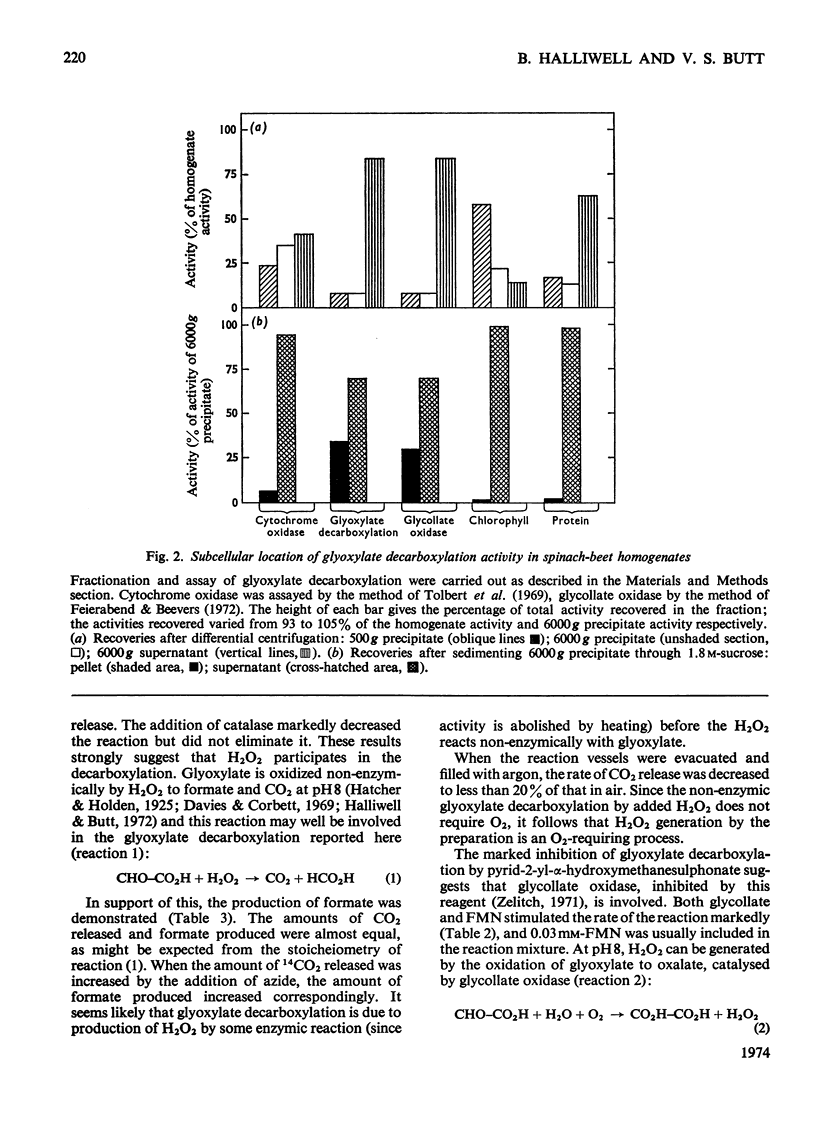
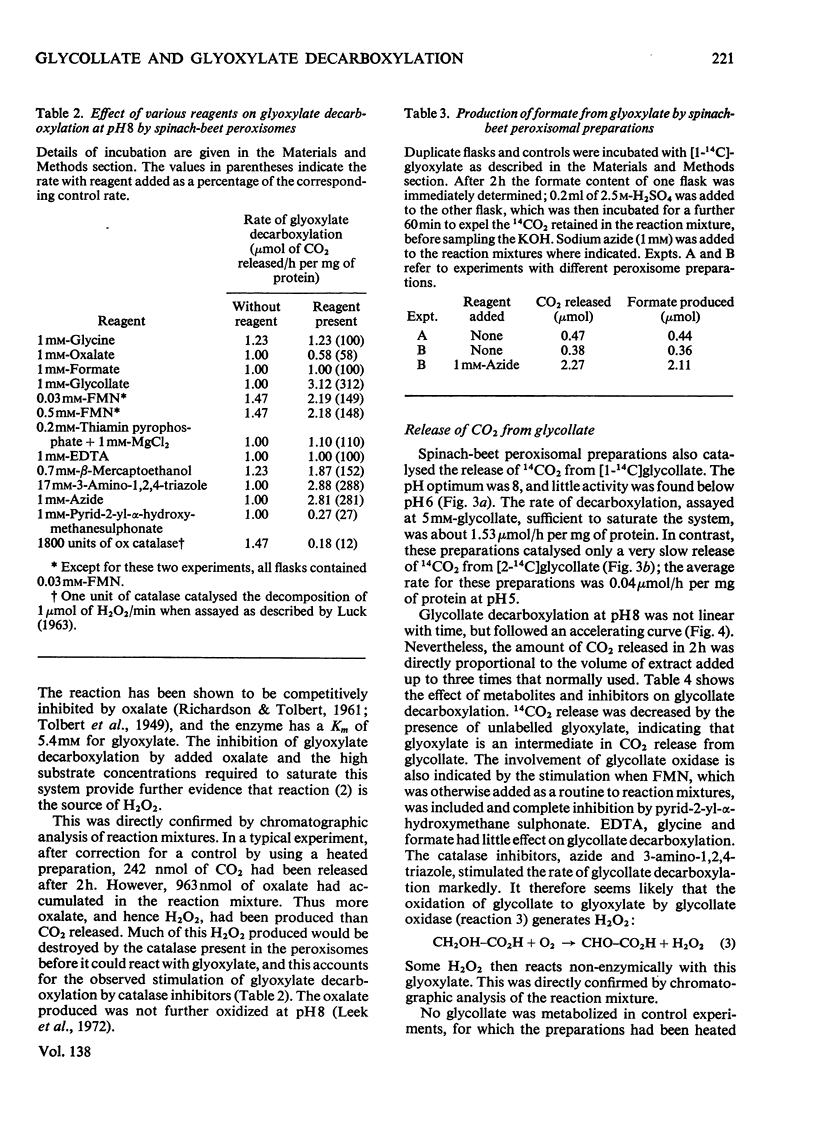
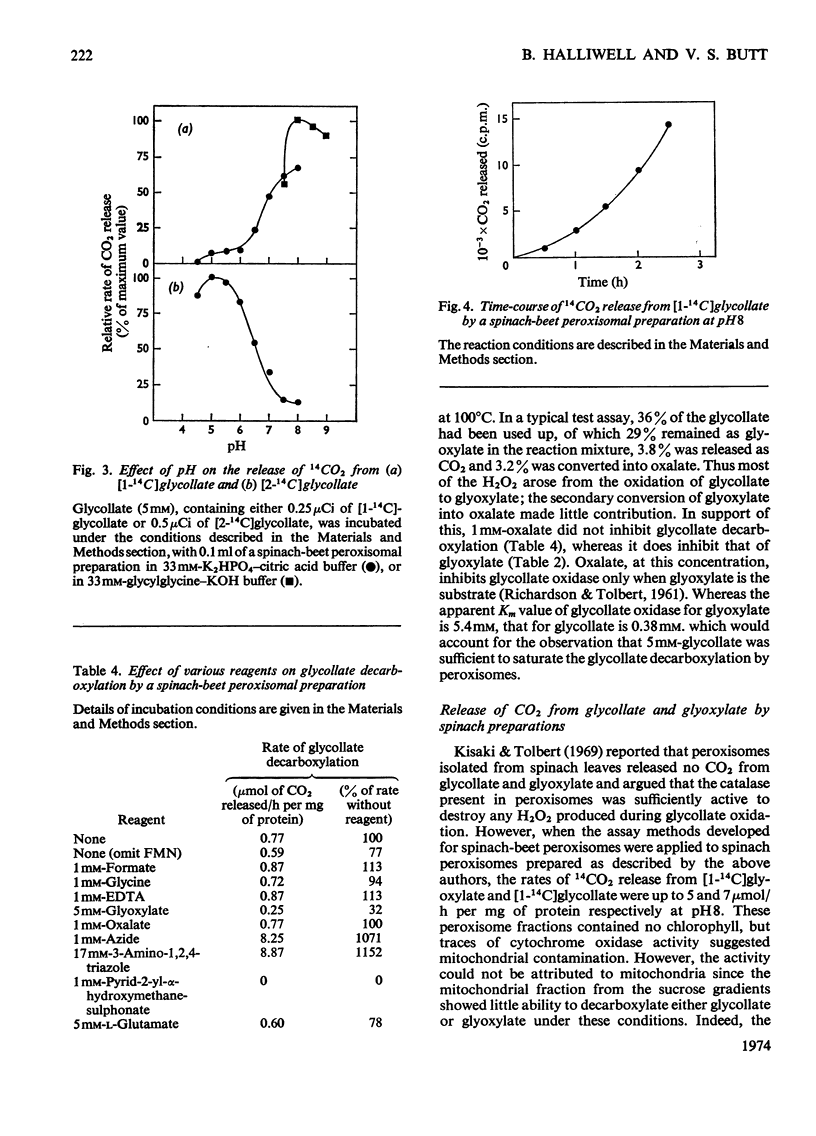
1. The conditions under which peroxisomal preparations from leaves of spinach beet and spinach catalyse the release of 14CO2 from [1-14C]glycollate and [1-14C]glyoxylate were investigated. 2. At pH8, 14CO2 production from [1-14C]glyoxylate was accompanied by equivalent quantities of formate. The accumulation of oxalate and the effects of various reagents, especially catalase inhibitors, show that glyoxylate is non-enzymically oxidized by H2O2, which is generated by the oxidation of glyoxylate to oxalate by the action of glycollate oxidase. 3. 14CO2 is shown to be generated from [1-14C]glycollate at pH8 by a similar reaction, but the H2O2 is generated mainly by the oxidation of glycollate to glyoxylate. 4. The physiological significance of these reactions is discussed, with special reference to their role in photorespiration.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A., Oshino N., Chance B. The cellular production of hydrogen peroxide. Biochem J. 1972 Jul;128(3):617–630. doi: 10.1042/bj1280617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHANCE B. Effect of pH upon the reaction kinetics of the enzyme-substrate compounds of catalase. J Biol Chem. 1952 Feb;194(2):471–481. [PubMed] [Google Scholar]
- COHEN G., HOCHSTEIN P. GLUTATHIONE PEROXIDASE: THE PRIMARY AGENT FOR THE ELIMINATION OF HYDROGEN PEROXIDE IN ERYTHROCYTES. Biochemistry. 1963 Nov-Dec;2:1420–1428. doi: 10.1021/bi00906a038. [DOI] [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental Studies on Microbodies in Wheat Leaves : II. Ontogeny of Particulate Enzyme Associations. Plant Physiol. 1972 Jan;49(1):33–39. doi: 10.1104/pp.49.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E., Winget G. D., Winter W., Connolly T. N., Izawa S., Singh R. M. Hydrogen ion buffers for biological research. Biochemistry. 1966 Feb;5(2):467–477. doi: 10.1021/bi00866a011. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Butt V. S. Flavin mononucleotide-sensitized photo-oxidation of glyoxylate in Good's buffers. Biochem J. 1972 Oct;129(5):1157–1158. doi: 10.1042/bj1291157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D., Hartree E. F. Properties of catalase. Catalysis of coupled oxidation of alcohols. Biochem J. 1945;39(4):293–301. [PMC free article] [PubMed] [Google Scholar]
- Kisaki T., Tolbert N. E. Glycolate and glyoxylate metabolism by isolated peroxisomes or chloroplasts. Plant Physiol. 1969 Feb;44(2):242–250. doi: 10.1104/pp.44.2.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leek A. E., Halliwell B., Butt V. S. Oxidation of formate and oxalate in peroxisomal preparations from leaves of spinach beet (Beta vulgaris L.). Biochim Biophys Acta. 1972 Dec 29;286(2):299–311. doi: 10.1016/0304-4165(72)90266-8. [DOI] [PubMed] [Google Scholar]
- MARGOLIASH E., NOVOGRODSKY A. A study of the inhibition of catalase by 3-amino-1:2:4:-triazole. Biochem J. 1958 Mar;68(3):468–475. doi: 10.1042/bj0680468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RICHARDSON K. E., TOLBERT N. E. Oxidation of glyoxylic acid to oxalic acid by glycolic acid oxidase. J Biol Chem. 1961 May;236:1280–1284. [PubMed] [Google Scholar]
- Rehfeld D. W., Tolbert N. E. Aminotransferases in peroxisomes from spinach leaves. J Biol Chem. 1972 Aug 10;247(15):4803–4811. [PubMed] [Google Scholar]
- TOLBERT N. E., CLAGETT C. O., BURRIS R. H. Products of the oxidation of glycolic acid and L-lactic acid by enzymes from tobacco leaves. J Biol Chem. 1949 Dec;181(2):905–914. [PubMed] [Google Scholar]
- Thompson J. S., Richardson K. E. Determination of pyruvate in enzyme-catalyzed reactions in the presence of glyoxylate. Anal Biochem. 1968 Aug;24(2):197–201. doi: 10.1016/0003-2697(68)90170-x. [DOI] [PubMed] [Google Scholar]
- Tolbert N. E., Oeser A., Yamazaki R. K., Hageman R. H., Kisaki T. A survey of plants for leaf peroxisomes. Plant Physiol. 1969 Jan;44(1):135–147. doi: 10.1104/pp.44.1.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. Increased rate of net photosynthetic carbon dioxide uptake caused by the inhibition of glycolate oxidase. Plant Physiol. 1966 Dec;41(10):1623–1631. doi: 10.1104/pp.41.10.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelitch I. The photooxidation of glyoxylate by envelope-free spinach chloroplasts and its relation to photorespiration. Arch Biochem Biophys. 1972 Jun;150(2):698–707. doi: 10.1016/0003-9861(72)90088-4. [DOI] [PubMed] [Google Scholar]


