Abstract
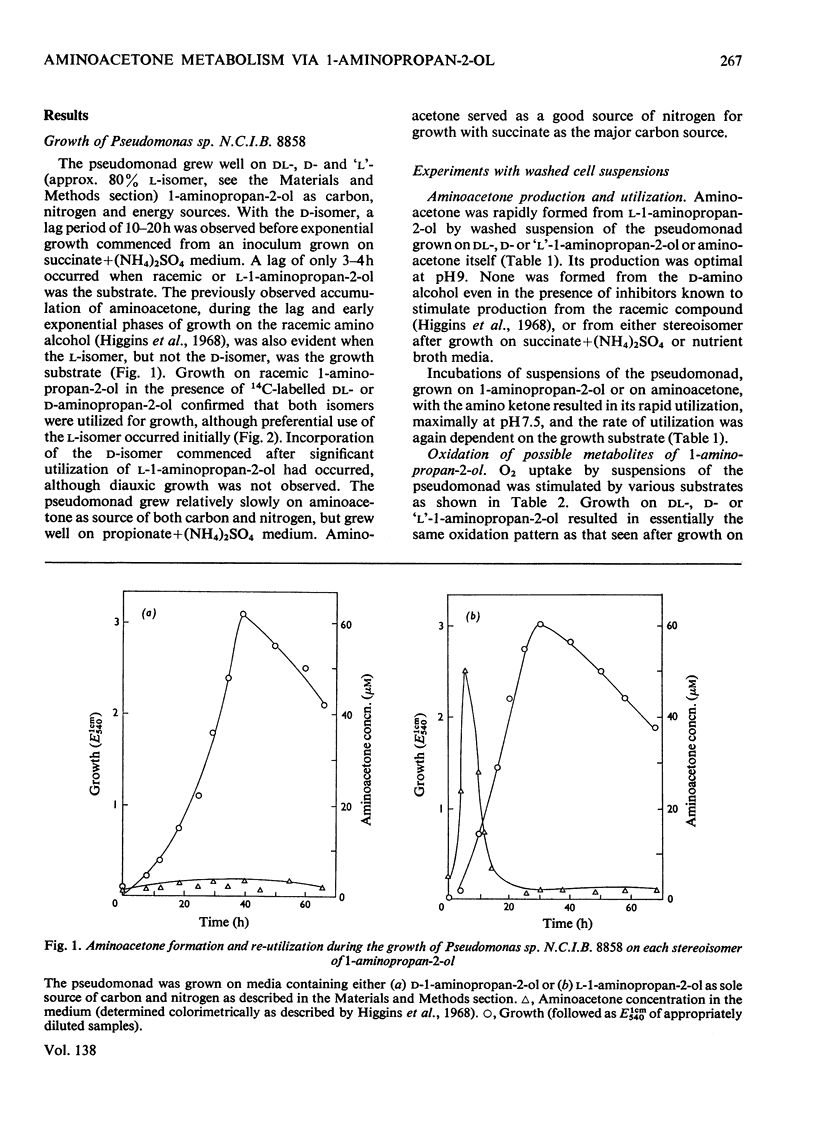
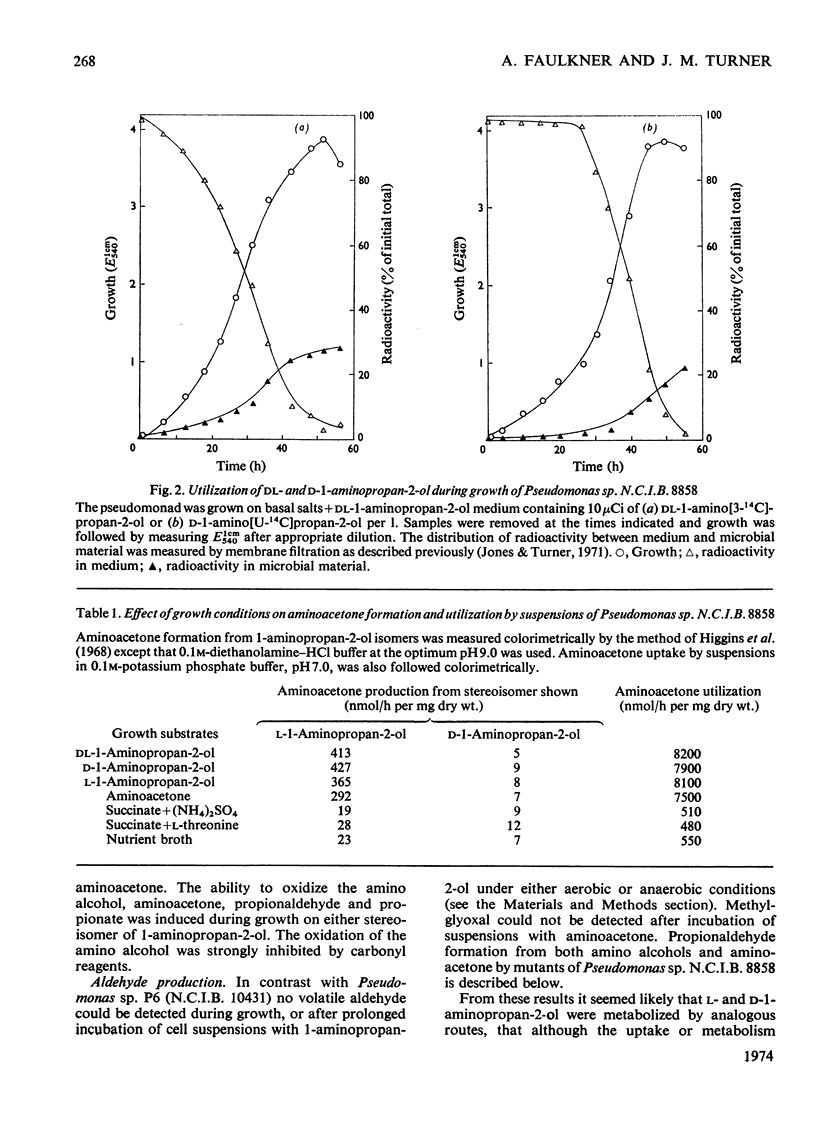
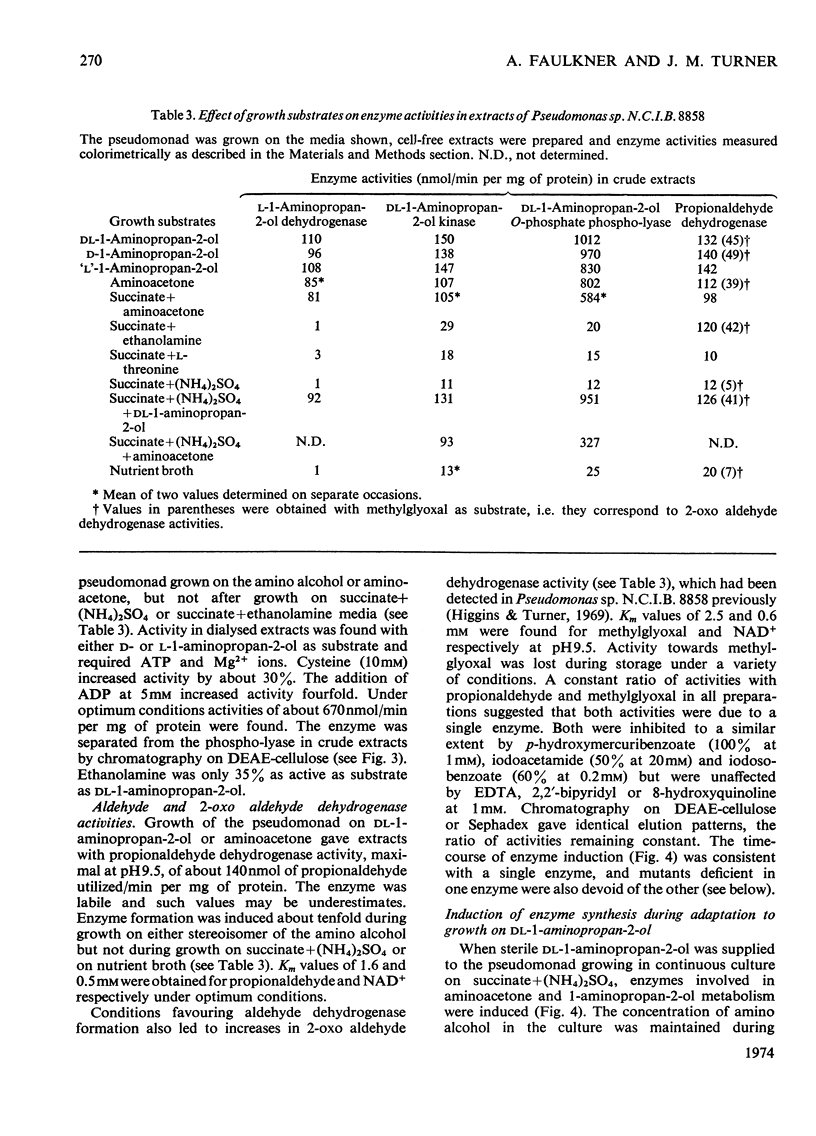
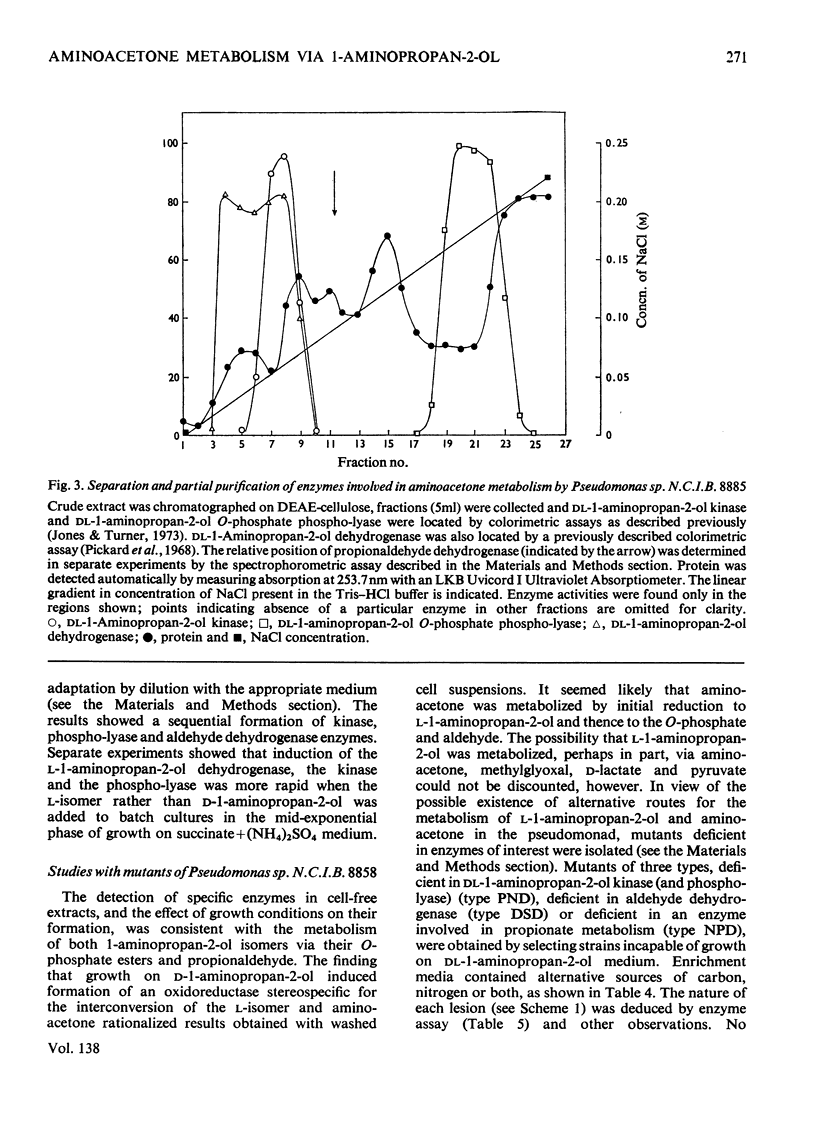
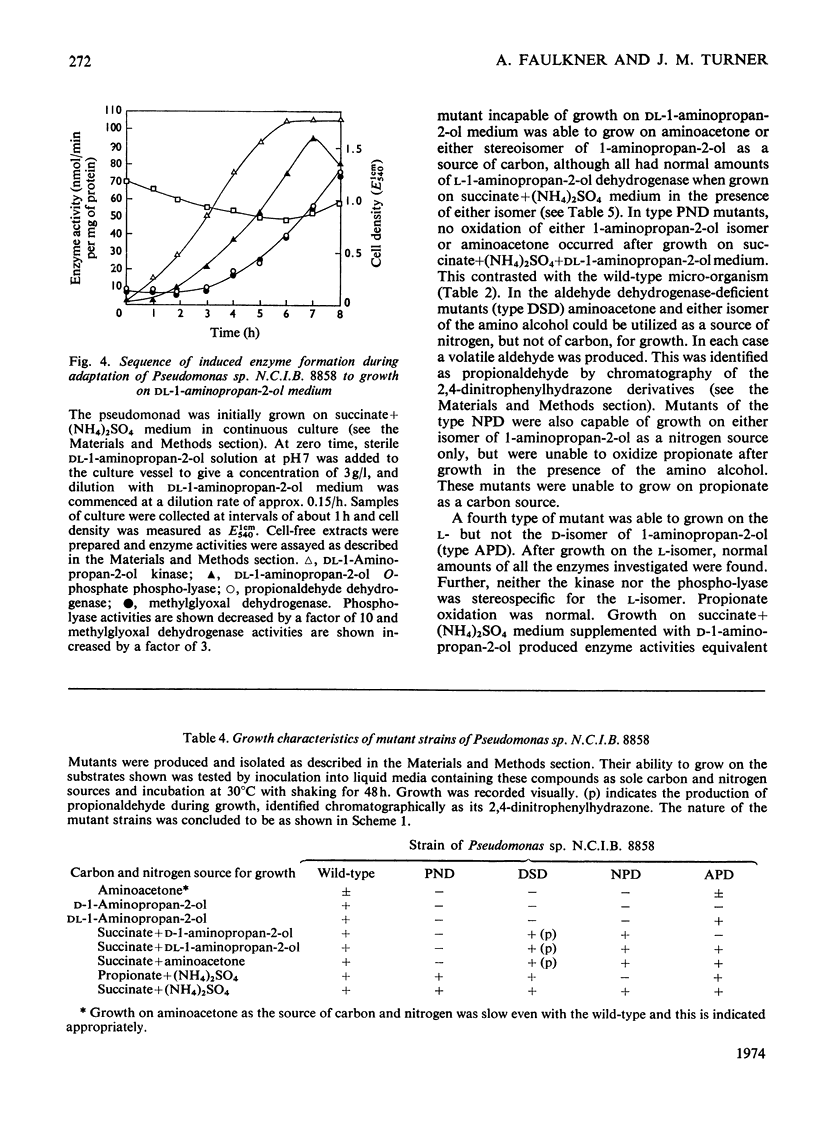
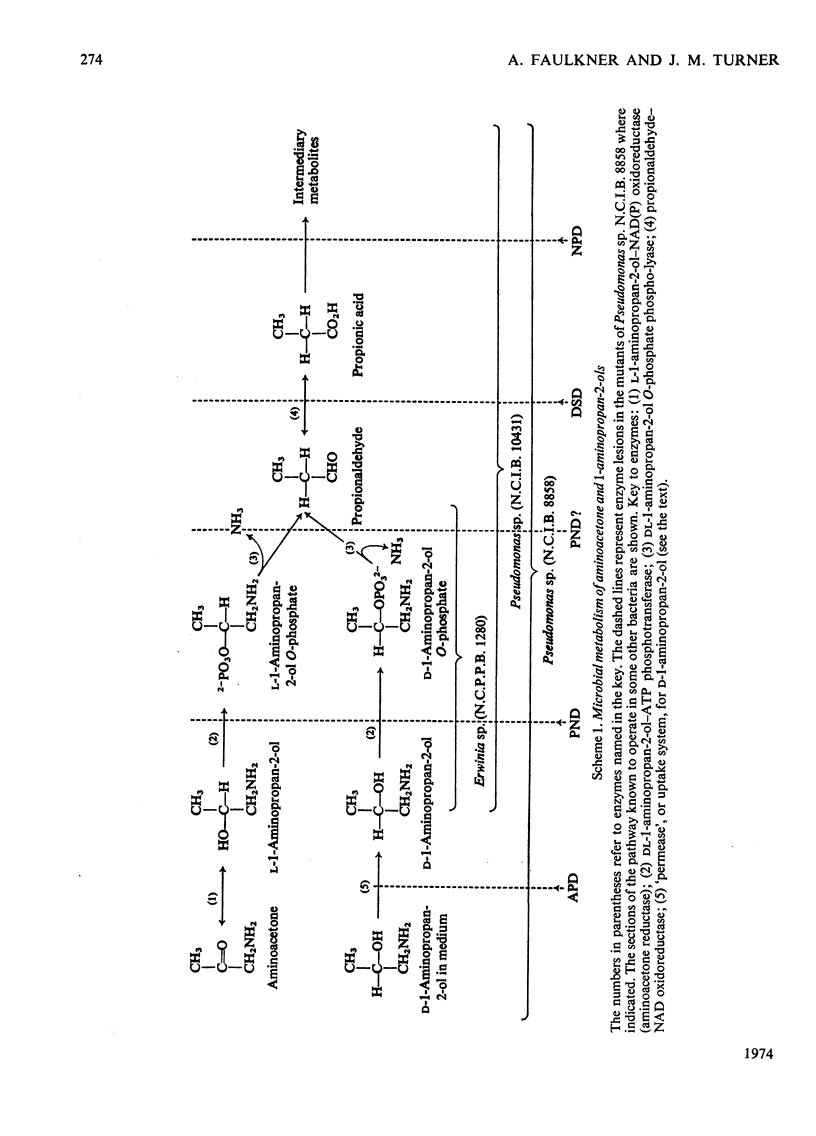
1. Pseudomonas sp. N.C.I.B. 8858 grew well on d- and l-1-aminopropan-2-ol and on aminoacetone. 2. Cell-free extracts possessed high activities of inducibly formed l-1-aminopropan-2-ol-NAD+ oxidoreductase, amino alcohol–ATP phosphotransferase, dl-1-aminopropan-2-ol O-phosphate phospho-lyase and aldehyde–NAD+ oxidoreductase, but no 1-aminopropan-2-ol racemase or d-1-aminopropan-2-ol-NAD+ oxidoreductase. 3. The amino alcohol kinase (activated by ADP) was non-stereospecific towards 1-aminopropan-2-ol and was one-third as active with ethanolamine. The phospho-lyase was active with l- and d-1-aminopropan-2-ol O-phosphate, but ethanolamine O-phosphate was only one-tenth as active as its higher homologues. The purified aldehyde dehydrogenase was active with propionaldehyde, acetaldehyde and also with methylglyoxal. The previously observed 2-oxo aldehyde dehydrogenase activity was considered to be due to the broadly specific aldehyde dehydrogenase. 4. Mutants of Pseudomonas sp. N.C.I.B. 8858 deficient in 1-aminopropan-2-ol kinase, 1-aminopropan-2-ol O-phosphate phospho-lyase, aldehyde dehydrogenase or an enzyme involved in propionate metabolism were incapable of growth on aminoacetone or 1-aminopropan-2-ol as carbon source, although all except the kinase- or phospho-lyasedeficient mutants could use these compounds and ethanolamine as nitrogen sources. The aldehyde dehydrogenase-deficient mutants produced copious amounts of propionaldehyde and acetaldehyde during growth on the corresponding amino alcohols. 5. The path of aminoacetone metabolism in Pseudomonas sp. N.C.I.B. 8858 was concluded to involve l-1-aminopropan-2-ol, the O-phosphate ester of this compound, propionaldehyde and propionate as obligatory intermediates. d-1-Aminopropan-2-ol was metabolized by the same route as the l-isomer, gratuitously inducing formation of the stereospecific l-1-aminopropan-2-ol dehydrogenase. 6. Extracts of the pseudomonad grown with ethanolamine as the nitrogen source were devoid of 1-aminopropan-2-ol dehydrogenase, the kinase and the phospho-lyase, but exhibited cobamide coenzyme-dependent deaminase activity. Mutants deficient in kinase or phospho-lyase (deaminating) grew well on ethanolamine as the nitrogen source. Ethanolamine deaminase was inactive with, but inhibited by, 1-aminopropan-2-ol.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BUFFONI F., BLASCHKO H. ENZYMIC OXIDATION OF AMINOKETONES IN MAMMALIAN BLOOD PLASMA. Experientia. 1963 Aug 15;19:421–421. doi: 10.1007/BF02171524. [DOI] [PubMed] [Google Scholar]
- Blackmore M. A., Turner J. M. Threonine metabolism via two-carbon compounds by Pseudomonas oxalaticus. J Gen Microbiol. 1971 Aug;67(2):243–246. doi: 10.1099/00221287-67-2-243. [DOI] [PubMed] [Google Scholar]
- DIXON M. A nomogram for ammonium sulphate solutions. Biochem J. 1953 Jun;54(3):457–458. doi: 10.1042/bj0540457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLIOTT W. H. A new threonine metabolite. Biochim Biophys Acta. 1958 Aug;29(2):446–447. doi: 10.1016/0006-3002(58)90215-4. [DOI] [PubMed] [Google Scholar]
- ELLIOTT W. H. Amino-acetone; its isolation and role in metabolism. Nature. 1959 Apr 11;183(4667):1051–1052. doi: 10.1038/1831051a0. [DOI] [PubMed] [Google Scholar]
- Fleshood H. L., Pitot H. C. O-phosphorylethanolamine ammonia lyase, a new pyridoxal phosphate-dependent enzyme. Biochem Biophys Res Commun. 1969 Jul 7;36(1):110–118. doi: 10.1016/0006-291x(69)90656-1. [DOI] [PubMed] [Google Scholar]
- Fleshood H. L., Pitot H. C. The metabolism of O-phosphorylethanolamine in animal tissues. I. O-phosphorylethanolamine phospho-lyase: partial purification and characterization. J Biol Chem. 1970 Sep 10;245(17):4414–4420. [PubMed] [Google Scholar]
- Fleshood H. L., Pitot H. C. The metabolism of O-phosphorylethanolamine in animal tissues. II. Metabolic regulation of O-phosphorylethanolamine phospho-lyase in vivo. Arch Biochem Biophys. 1970 Dec;141(2):423–429. doi: 10.1016/0003-9861(70)90158-x. [DOI] [PubMed] [Google Scholar]
- Harder W., Quayle J. R. The biosynthesis of serine and glycine in Pseudomonas AM1 with special reference to growth on carbon sources other than C1 compounds. Biochem J. 1971 Mar;121(5):753–762. doi: 10.1042/bj1210753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heptinstall J., Quayle J. R. Pathways leading to and from serine during growth of Pseudomonas AM1 on C1 compounds or succinate. Biochem J. 1970 Apr;117(3):563–572. doi: 10.1042/bj1170563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins I. J., Pickard M. A., Turner J. M. Aminoacetone formation and utilization by pseudomonads grown on DL-1-aminopropan-2-ol. J Gen Microbiol. 1968 Nov;54(1):105–114. doi: 10.1099/00221287-54-1-105. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Turner J. M. Enzymes of methylglyoxal metabolism in a Pseudomonad which rapidly metabolizes aminoacetone. Biochim Biophys Acta. 1969 Jul 30;184(2):464–467. doi: 10.1016/0304-4165(69)90052-x. [DOI] [PubMed] [Google Scholar]
- Higgins I. J., Turner J. M., Willetts A. J. Enzyme mechanism of aminoacetone metabolism by micro-organisms. Nature. 1967 Aug 19;215(5103):887–888. doi: 10.1038/215887a0. [DOI] [PubMed] [Google Scholar]
- Jones A., Faulkner A., Turner J. M. Microbial metabolism of amino alcohols. Metabolism of ethanolamine and 1-aminopropan-2-ol in species of Erwinia and the roles of amino alcohol kinase and amino alcohol o-phosphate phospho-lyase in aldehyde formation. Biochem J. 1973 Aug;134(4):959–968. doi: 10.1042/bj1340959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A., Turner J. M. Microbial metabolism of amino alcohols via aldehydes. J Gen Microbiol. 1971 Aug;67(3):379–381. doi: 10.1099/00221287-67-3-379. [DOI] [PubMed] [Google Scholar]
- Jones A., Turner J. M. Microbial metabolism of amino alcohols. 1-Aminopropan-2-ol and ethanolamine metabolism via propionaldehyde and acetaldehyde in a species of Pseudomonas. Biochem J. 1973 May;134(1):167–182. doi: 10.1042/bj1340167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KORNBERG H. L. The metabolism of C2 compounds in micro-organisms. I. The incorporation of [2-14C] acetate by Pseudomonas fluorescens, and by a Corynebacterium, grown on ammonium acetate. Biochem J. 1958 Mar;68(3):535–542. doi: 10.1042/bj0680535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. A., Turner J. M. Enzymic oxidation of D-1-aminopropan-2-ol by diol dehydrogenases of microbial origin. Biochim Biophys Acta. 1968 Dec 23;170(2):455–456. doi: 10.1016/0304-4165(68)90034-2. [DOI] [PubMed] [Google Scholar]
- Lowe D. A., Turner J. M. Microbial metabolism of amino ketones: D-1-aminopropan-2-ol and aminoacetone metabolism in Escherichia coli. J Gen Microbiol. 1970 Sep;63(1):49–61. doi: 10.1099/00221287-63-1-49. [DOI] [PubMed] [Google Scholar]
- Müller G., Gross R., Siebke G. Zur Frage der Bildung des Isopropanolamin-Anteiles im vitamin B 12. Hoppe Seylers Z Physiol Chem. 1971 Dec;352(12):1720–1722. [PubMed] [Google Scholar]
- PAZ M. A., BLUMENFELD O. O., ROJKIND M., HENSON E., FURFINE C., GALLOP P. M. DETERMINATION OF CARBONYL COMPOUNDS WITH N-METHYL BENZOTHIAZOLONE HYDRAZONE. Arch Biochem Biophys. 1965 Mar;109:548–559. doi: 10.1016/0003-9861(65)90400-5. [DOI] [PubMed] [Google Scholar]
- Pickard M. A., Higgins I. J., Turner J. M. Purification and properties of l-1-aminopropan-2-ol. NAD oxidoreductase from a pseudomonad grown on DL-1-aminopropan-2-ol. J Gen Microbiol. 1968 Nov;54(1):115–126. doi: 10.1099/00221287-54-1-115. [DOI] [PubMed] [Google Scholar]
- Tuner J. M. Microbial metabolism of amino ketones. Aminoacetone formation from 1-aminopropan-2-ol by a dehydrgenase in Escerichia coli. Biochem J. 1966 May;99(2):427–433. doi: 10.1042/bj0990427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner J. M. Microbial metabolism of amino ketones. L-1-aminopropan-2-ol dehydrogenase and L-threonine dehydrogenase in Escherichia coli. Biochem J. 1967 Jul;104(1):112–121. doi: 10.1042/bj1040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Aminoacetone formation and decomposition in liver. Biochem Biophys Res Commun. 1961 Feb 24;4:96–100. doi: 10.1016/0006-291x(61)90354-0. [DOI] [PubMed] [Google Scholar]
- URATA G., GRANICK S. Biosynthesis of alpha-aminoketones and the metabolism of aminoacetone. J Biol Chem. 1963 Feb;238:811–820. [PubMed] [Google Scholar]


