Abstract
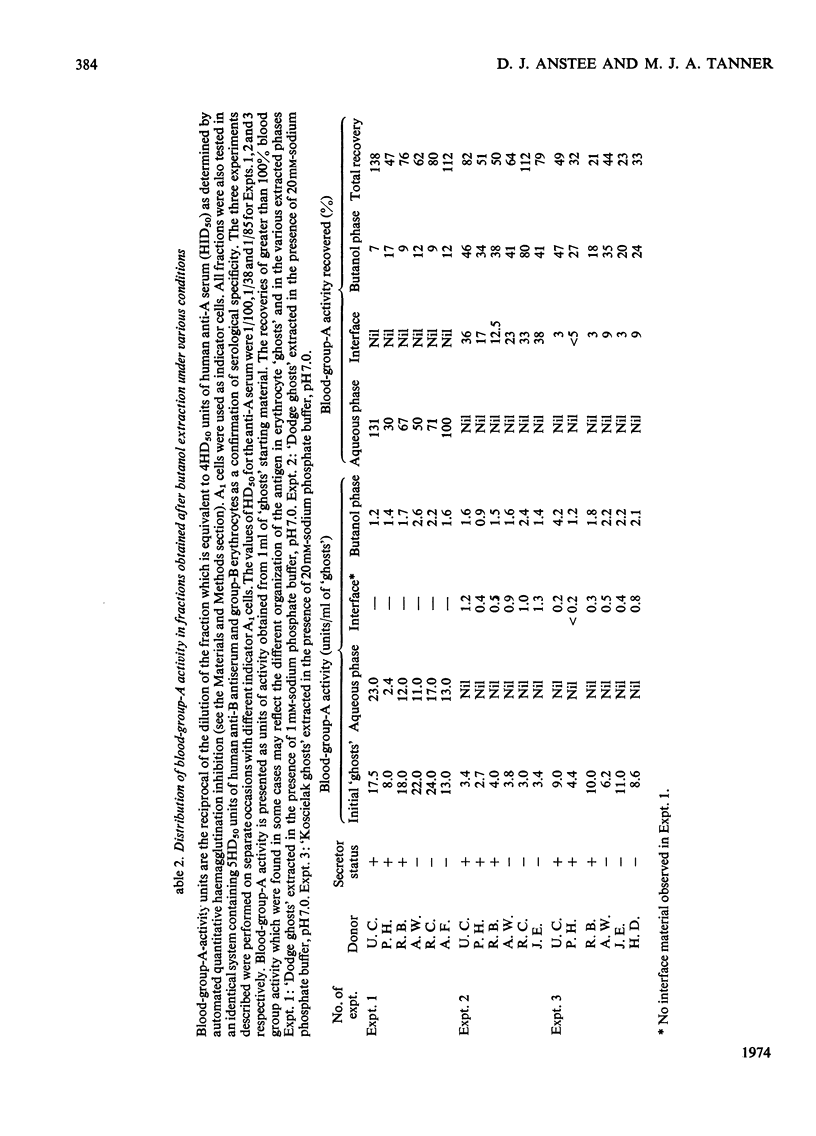
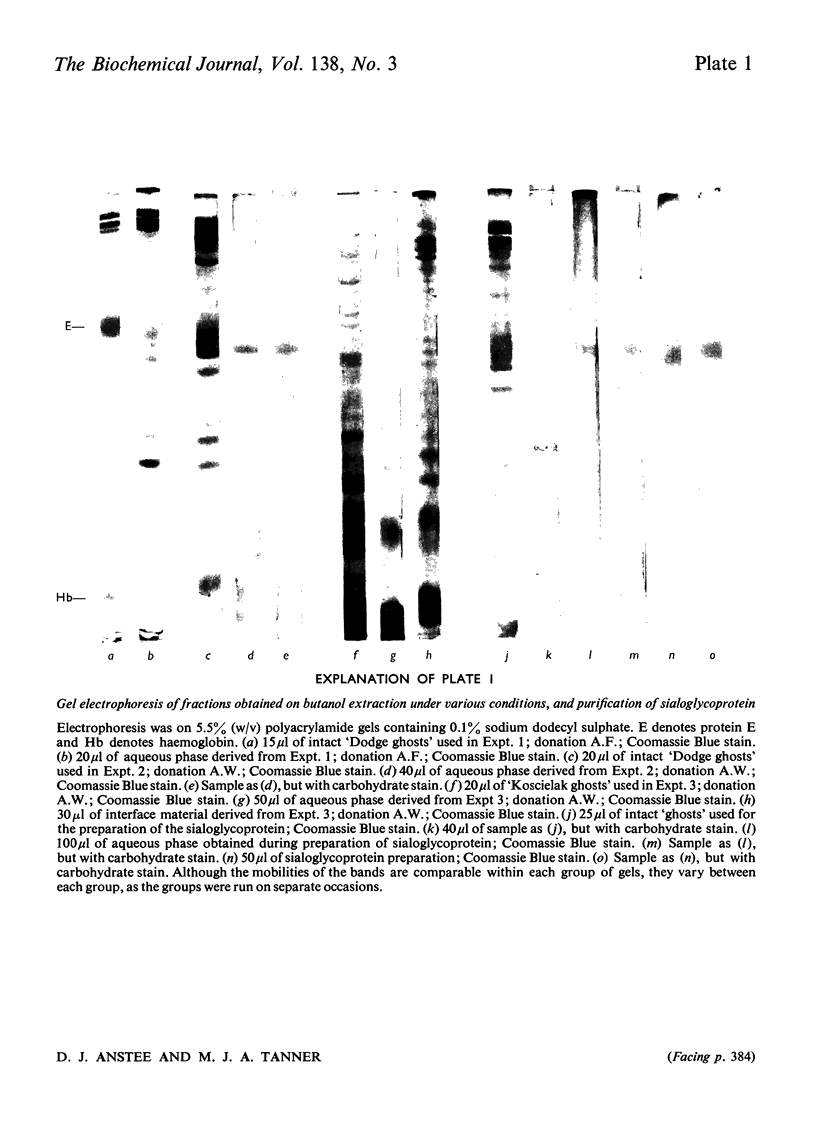
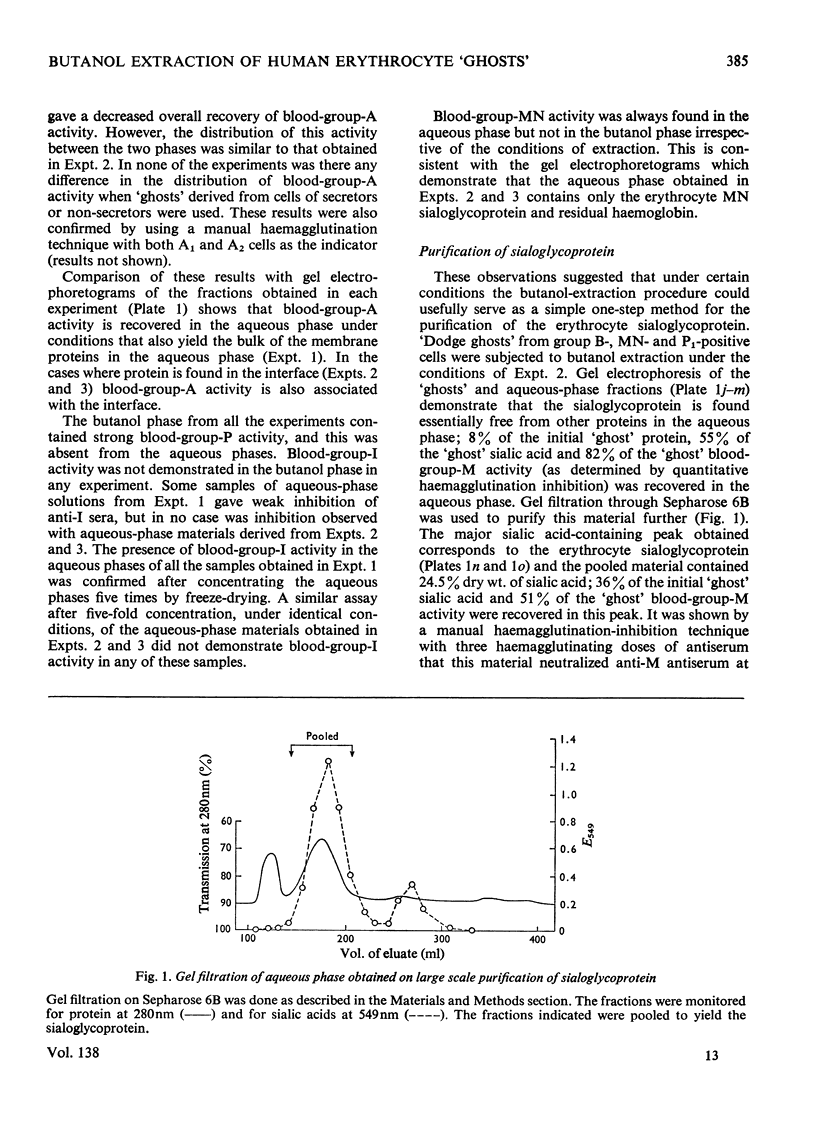
The distribution of protein and blood-group-antigen activity obtained after butanol extraction of erythrocyte `ghosts' under various conditions is described. Butanol extraction under low-ionic strength conditions results in the recovery of membrane protein in high yield in the aqueous phase. Blood-group-A activity is found in both the aqueous and butanol phases, whereas blood-group-P activity is confined to the butanol phase and blood-group-I and blood-group-MN activity are restricted to the aqueous phase. Much lower yields of protein are obtained in the aqueous phase when high-ionic-strength conditions are used. An appreciable amount of material is precipitated at the interface. Under these conditions blood-group-P activity is found only in the butanol phase, blood group-A activity in the butanol phase and interface material and only blood-group-MN activity in the aqueous phase. In contrast with previous reports no correlation could be demonstrated between the secretor status of the donors and the presence of blood-group-A activity in the aqueous phase after butanol extraction under any of the extraction conditions used. By using butanol extraction under high-ionic-strength conditions it is possible to isolate the blood-group-MN-active sialoglycoprotein in high yield from erythrocyte `ghosts' by a simple procedure.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anstee D. J., Holt P. D., Pardoe G. I. Agglutinins from fish ova defining blood groups B and P. Vox Sang. 1973 Oct;25(4):347–360. doi: 10.1111/j.1423-0410.1973.tb04383.x. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Steck T. L., Wallach D. F. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochemistry. 1971 Jun 22;10(13):2606–2617. doi: 10.1021/bi00789a030. [DOI] [PubMed] [Google Scholar]
- GIBBS M. B., COLLINS W. S., AKEROYD J. H. Quantitative hemagglutination inhibition studies of blood group substances. I. Assay of the blood group A activity of substances. J Immunol. 1961 Oct;87:396–404. [PubMed] [Google Scholar]
- Gardas A., Kościelak J. A, B and H blood group specificities in glycoprotein and glycolipid fractions of human erythrocyte membrane. Absence of blood group active glycoproteins in the membranes of non-secretors. Vox Sang. 1971 Feb;20(2):137–149. doi: 10.1111/j.1423-0410.1971.tb00546.x. [DOI] [PubMed] [Google Scholar]
- Hamaguchi H., Cleve H. Solubilization of human erythrocyte membrane glycoproteins and separation of the MN glycoprotein from a glycoprotein with I, S, and A activity. Biochim Biophys Acta. 1972 Sep 29;278(2):271–280. doi: 10.1016/0005-2795(72)90232-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liotta I., Quintiliani M., Quintiliani L., Buzzonetti A., Giuliani E. Extraction and partial purification of blood group substances A,B and H from erythrocyte stroma. Vox Sang. 1972;22(2):171–182. doi: 10.1111/j.1423-0410.1972.tb05123.x. [DOI] [PubMed] [Google Scholar]
- Maddy A. H. The properties of the protein of the plasma membrane of ox erythrocytes. Biochim Biophys Acta. 1966 Mar 28;117(1):193–200. doi: 10.1016/0304-4165(66)90166-8. [DOI] [PubMed] [Google Scholar]
- Rosse W. F., Lauf P. K. Reaction of cold agglutinins with I antigen solubilized from human red cells. Blood. 1970 Dec;36(6):777–784. [PubMed] [Google Scholar]
- Tanner M. J., Boxer D. H. Separation and some properties of the major proteins of the human erythrocyte membrane. Biochem J. 1972 Sep;129(2):333–347. doi: 10.1042/bj1290333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Whittemore N. B., Trabold N. C., Reed C. F., Weed R. I. Solubilized glycoprotein from human erythrocyte membranes possessing blood group A, B and H activity. Vox Sang. 1969 Oct;17(4):289–299. doi: 10.1111/j.1423-0410.1969.tb00398.x. [DOI] [PubMed] [Google Scholar]



