Abstract
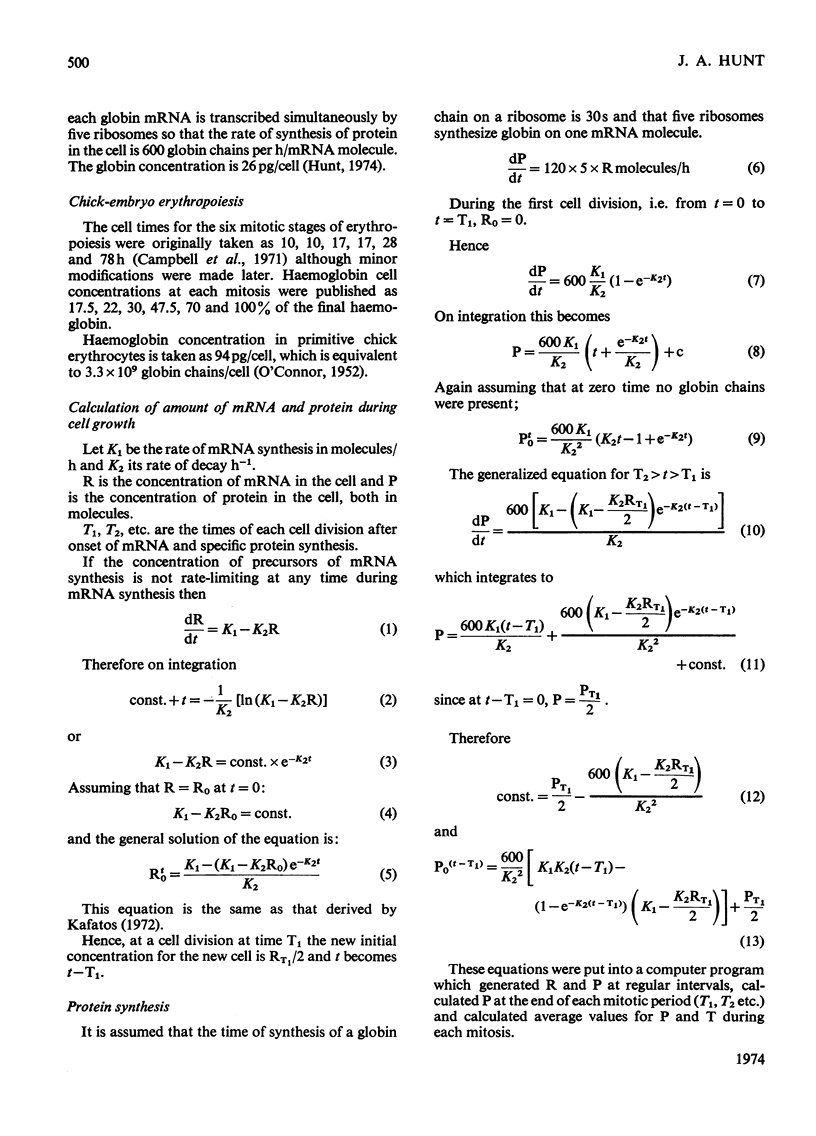
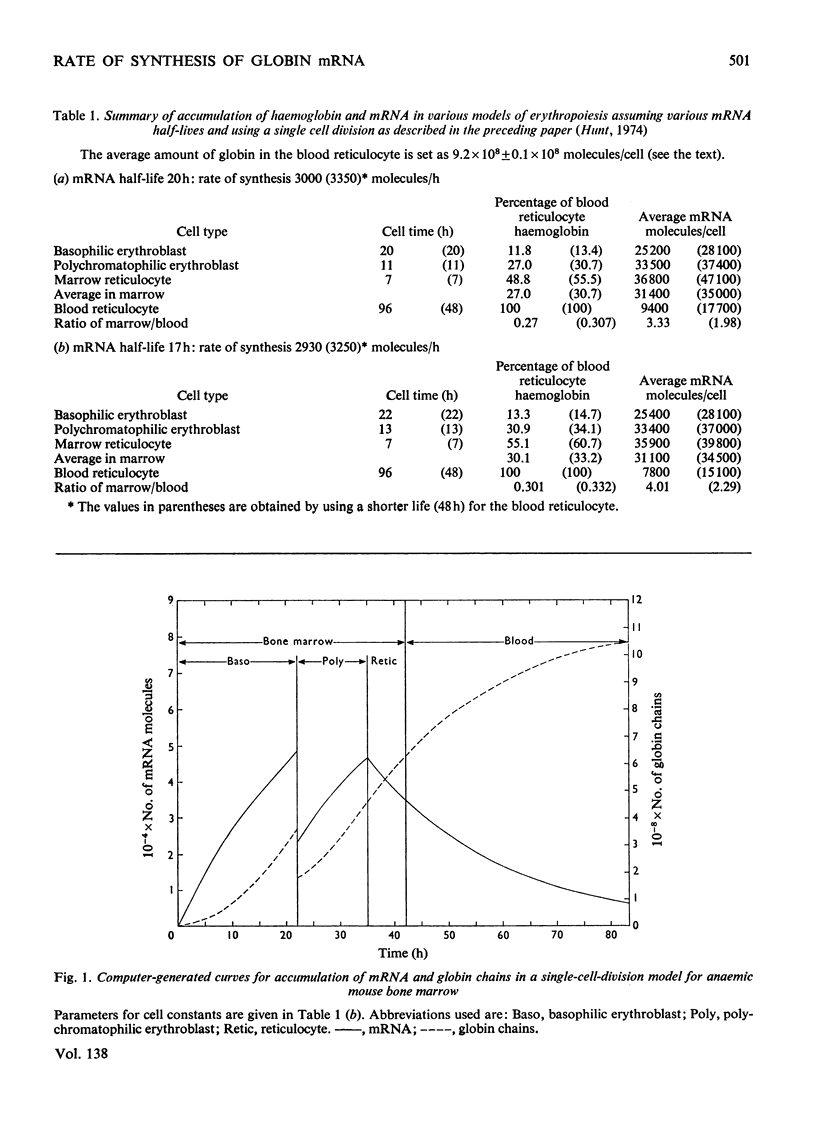
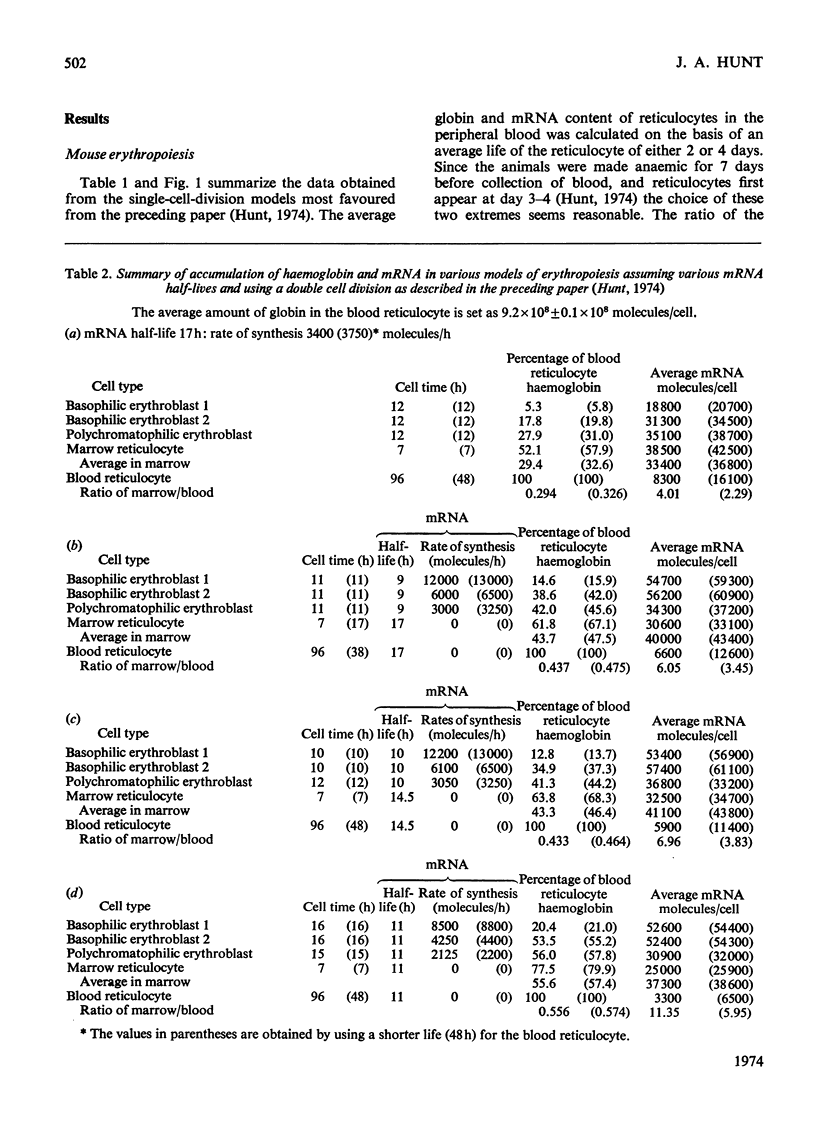
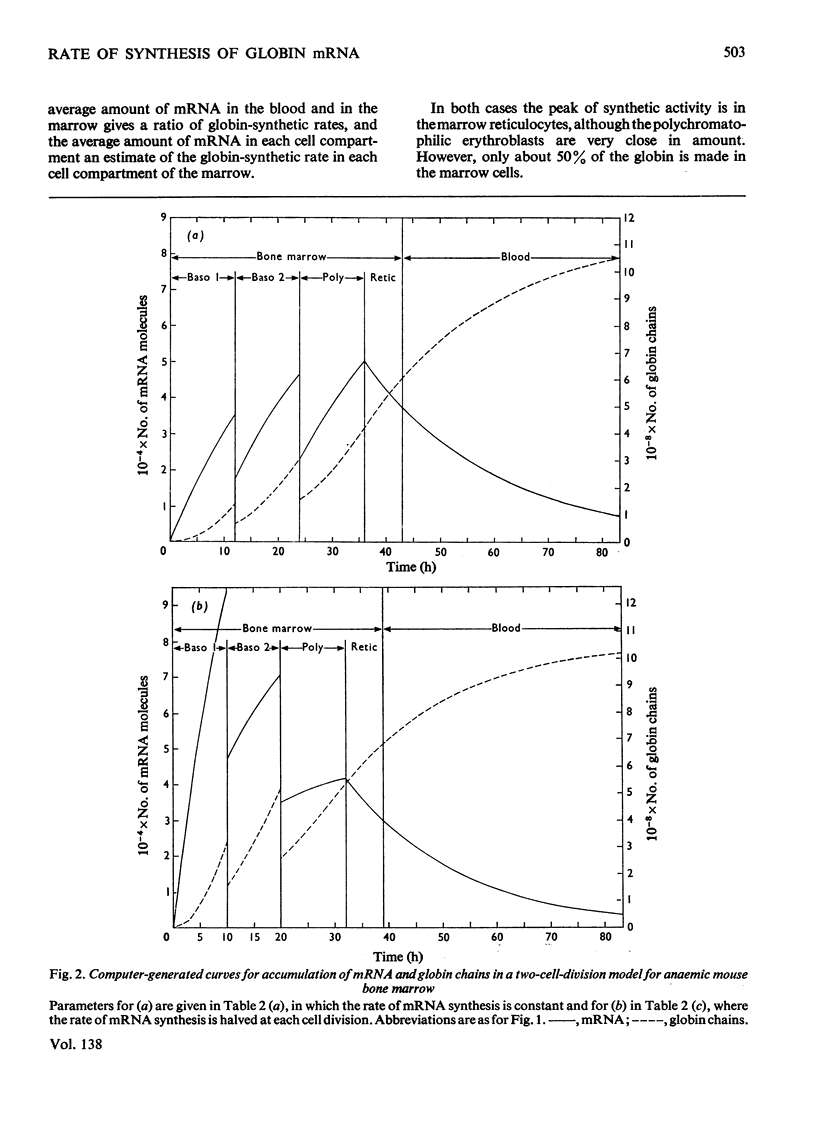
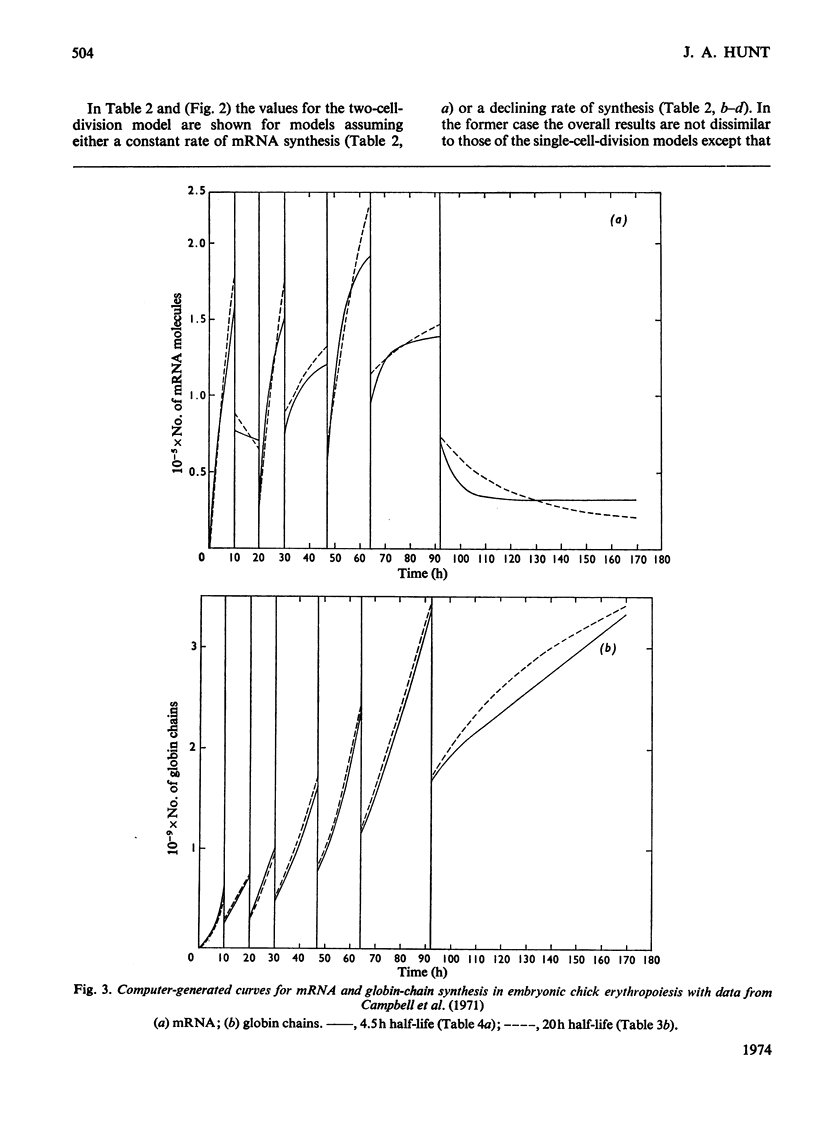
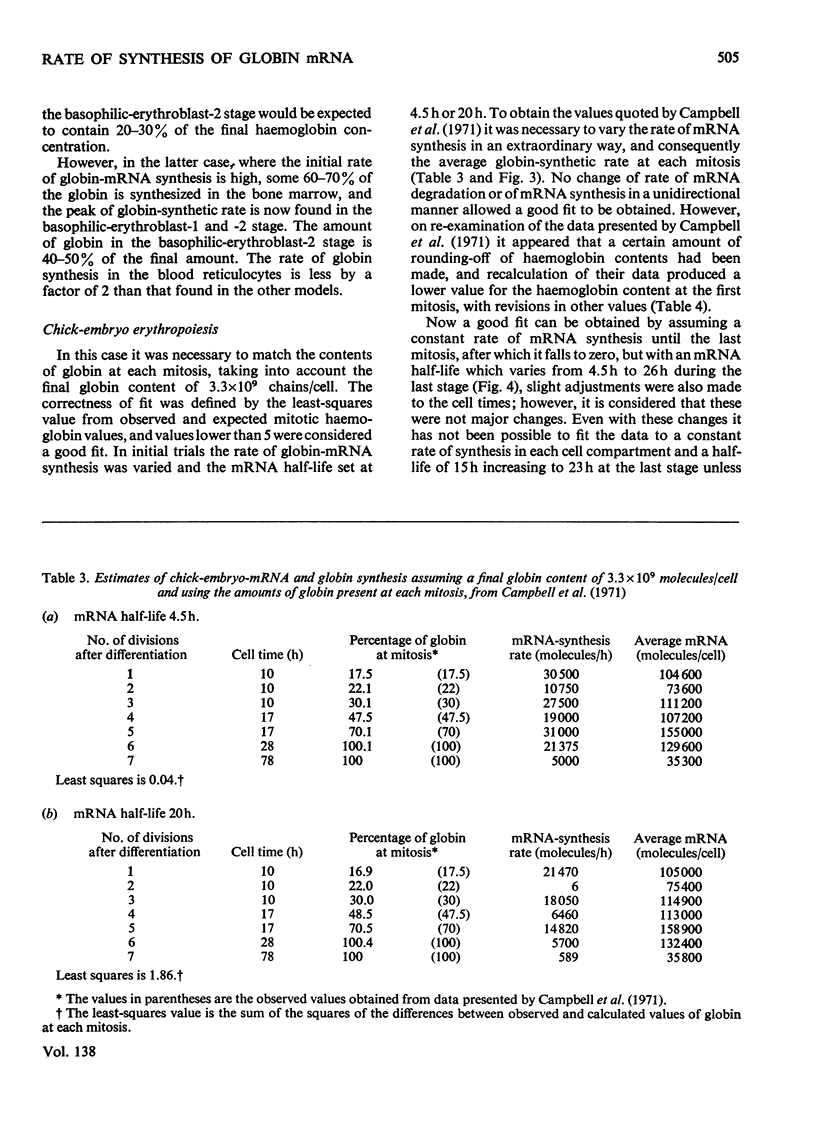
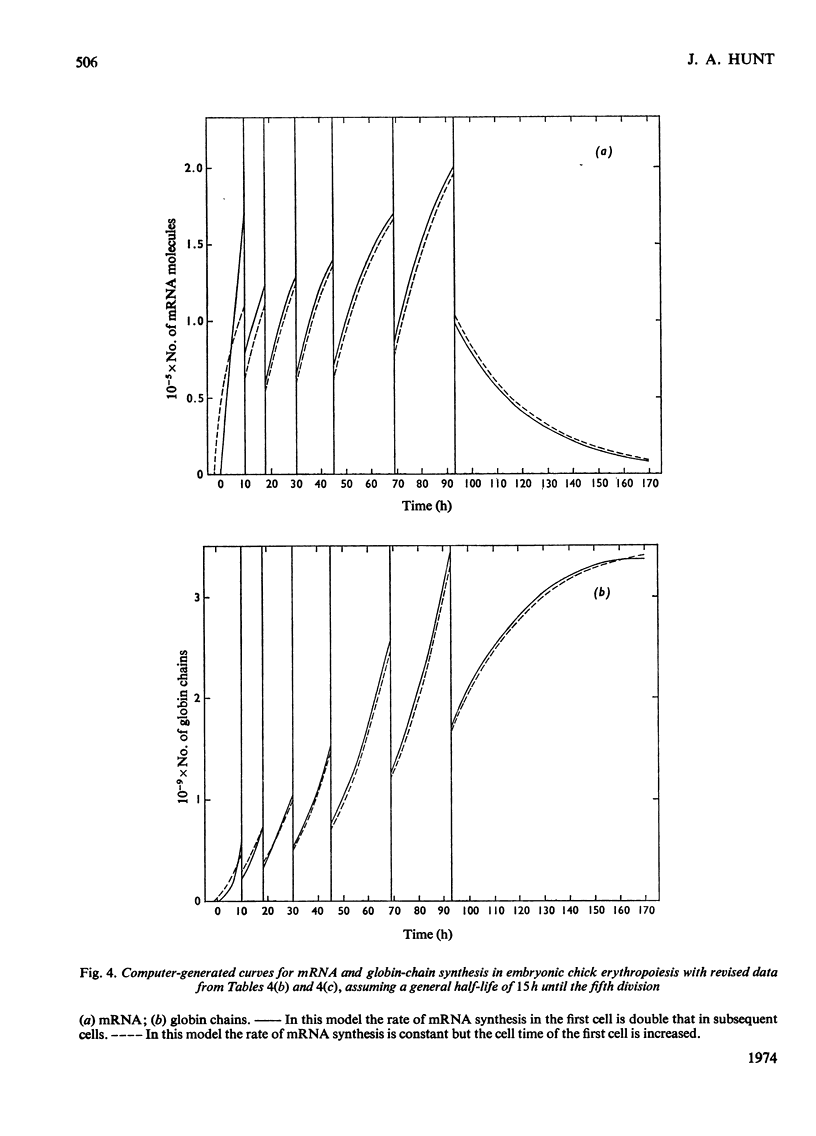
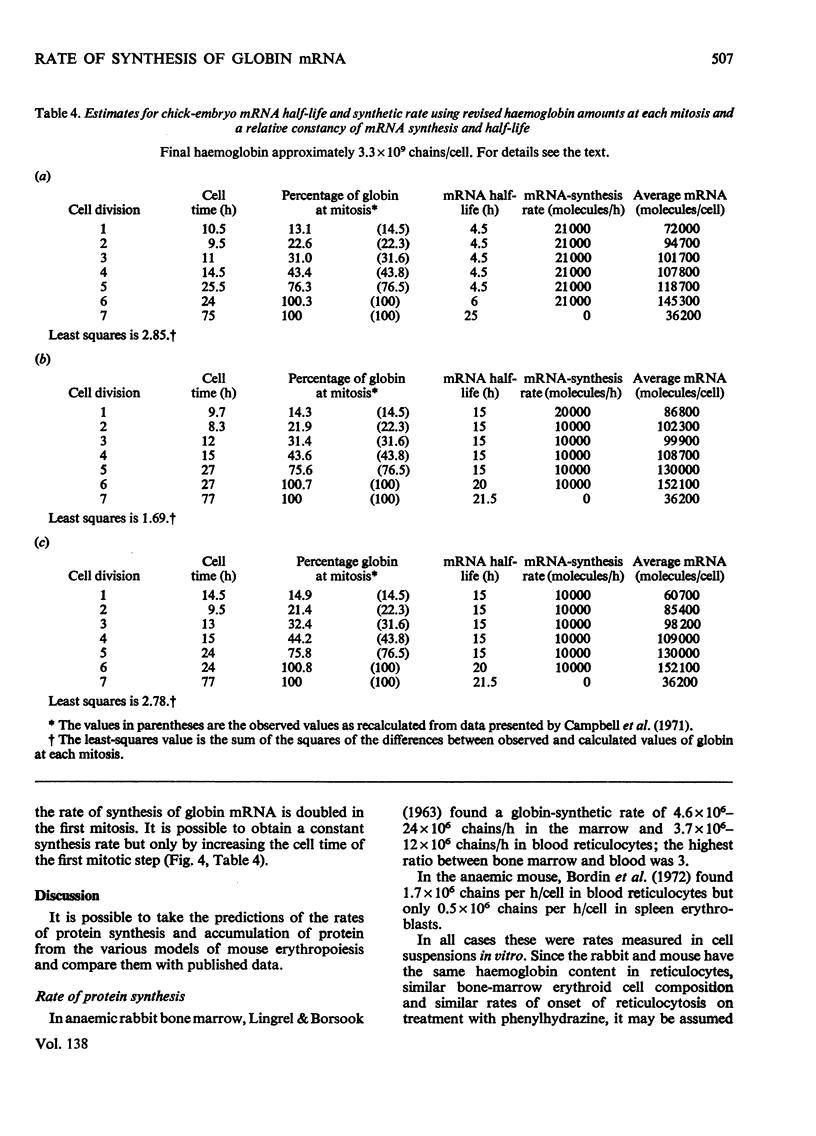
By the use of the favoured models defining mRNA synthesis and half-life from the preceding paper (Hunt, 1974) and the known content of globin in a reticulocyte it is possible to estimate the absolute rate of mRNA and globin synthesis and the mRNA and globin content in each type of erythroid cell. The best model requires an mRNA-synthetic rate of 3000 molecules per h/cell. This rate compares favourably with the estimated chain-extension rate of 43 nucleotides/s in Escherichia coli (Manor et al., 1969) provided that the four α- and β-chain cistrons per cell are transcribed by polymerases spaced 50 nucleotide base pairs apart. Similar calculations can be made for erythropoiesis in the chick embryo, where cell times and relative globin content at each mitosis have been measured (Campbell et al., 1971), but where no reliable estimates of mRNA half-life have been made. In this case it was estimated that a constant rate of mRNA synthesis at 10000 molecules per h/cell through six cell divisions is necessary if the mRNA half-life is 15h; after the sixth mitosis the mRNA synthesis would stop and its half-life would increase to approx. 20h. If an mRNA half-life of 4.5h is used, the synthesis rate through the six mitoses would be 21000 molecules per h/cell, ceasing at the sixth mitosis, when the half-life would need to increase to 25h. The chain-elongation rate for the four α- and β-globin cistrons per cell would be 1–2 times higher than in E. coli and would either require a greater rate, polymerases spaced between 25 and 50 nucleotide base pairs apart on the DNA, or limited gene replication. These possibilities are discussed in the light of the low values found for globin cistron multiplicity in ducks and mice.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORSOOK H. DNA, RNA AND PROTEIN SYNTHESIS AFTER ACUTE, SEVERE BLOOD LOSS: A PICTURE OF ERYTHROPOIESIS AT THE COMBINED MORPHOLOGICAL AND MOLECULAR LEVELS. Ann N Y Acad Sci. 1964 Oct 7;119:523–539. doi: 10.1111/j.1749-6632.1965.tb54053.x. [DOI] [PubMed] [Google Scholar]
- Bishop J. O., Pemberton R., Baglioni C. Reiteration frequency of haemoglobin genes in the duck. Nat New Biol. 1972 Feb 23;235(60):231–234. doi: 10.1038/newbio235231a0. [DOI] [PubMed] [Google Scholar]
- Bordin S., Farace M. G., Fantoni A. Rate of hemoglobin synthesis controlled at the translational level in differentiating erythroid cells from adult mice. Biochim Biophys Acta. 1972 Oct 11;281(2):277–288. doi: 10.1016/0005-2787(72)90180-3. [DOI] [PubMed] [Google Scholar]
- Borsook H., Teigler D., Gunderson A. Studies on erythropoiesis. 3. Different patterns of protein and hemoglobin synthesis before and after terminal differentiation in adult rabbit marrow. Arch Biochem Biophys. 1968 May;125(2):429–435. doi: 10.1016/0003-9861(68)90599-7. [DOI] [PubMed] [Google Scholar]
- Campbell G. le M., Weintraub H., Mayall B. H., Holtzer H. Primitive erythropoiesis in early chick embryogenesis. II. Correlation between hemoglobin synthesis and the mitotic history. J Cell Biol. 1971 Sep;50(3):669–681. doi: 10.1083/jcb.50.3.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRASSO J. A., WOODARD J. W., SWIFT H. Cytochemical studies of nucleic acids and proteins in erythrocytic development. Proc Natl Acad Sci U S A. 1963 Jul;50:134–140. doi: 10.1073/pnas.50.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison P. R., Hell A., Birnie G. D., Paul J. Evidence for single copies of globin genes in the mouse genome. Nature. 1972 Sep 22;239(5369):219–221. doi: 10.1038/239219a0. [DOI] [PubMed] [Google Scholar]
- Hunt J. A. Half-life and rate of synthesis of globin messenger ribonucleic acid. Determination of half-life of messenger ribonucleic acid and its relative synthetic rate in erythroid cells. Biochem J. 1974 Mar;138(3):487–498. doi: 10.1042/bj1380487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KNOPF P. M., LAMFROM H. CHANGES IN THE RIBOSOME DISTRIBUTION DURING INCUBATION OF RABBIT RETICULOCYTES IN VITRO. Biochim Biophys Acta. 1965 Mar 15;95:398–407. doi: 10.1016/0005-2787(65)90186-3. [DOI] [PubMed] [Google Scholar]
- Kafatos F. C. The cocoonase zymogen cells of silk moths: a model of terminal cell differentiation for specific protein synthesis. Curr Top Dev Biol. 1972;7:125–191. doi: 10.1016/s0070-2153(08)60071-x. [DOI] [PubMed] [Google Scholar]
- Manor H., Goodman D., Stent G. S. RNA chain growth rates in Escherichia coli. J Mol Biol. 1969 Jan 14;39(1):1–29. doi: 10.1016/0022-2836(69)90329-5. [DOI] [PubMed] [Google Scholar]
- Miller O. L., Jr, Bakken A. H. Morphological studies of transcription. Acta Endocrinol Suppl (Copenh) 1972;168:155–177. doi: 10.1530/acta.0.071s155. [DOI] [PubMed] [Google Scholar]
- O'CONNOR R. J. Growth and differentiation in the red blood cells of the chicken embryo. J Anat. 1952 Jul;86(3):320–325. [PMC free article] [PubMed] [Google Scholar]


