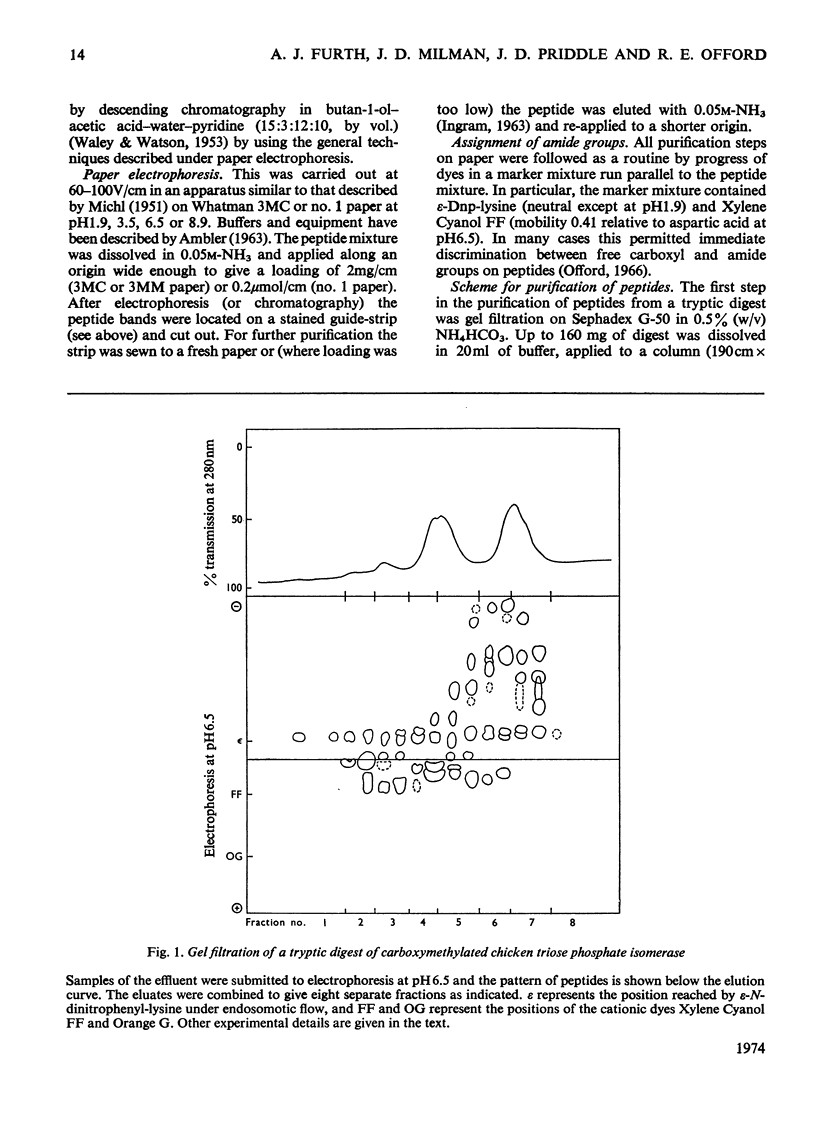
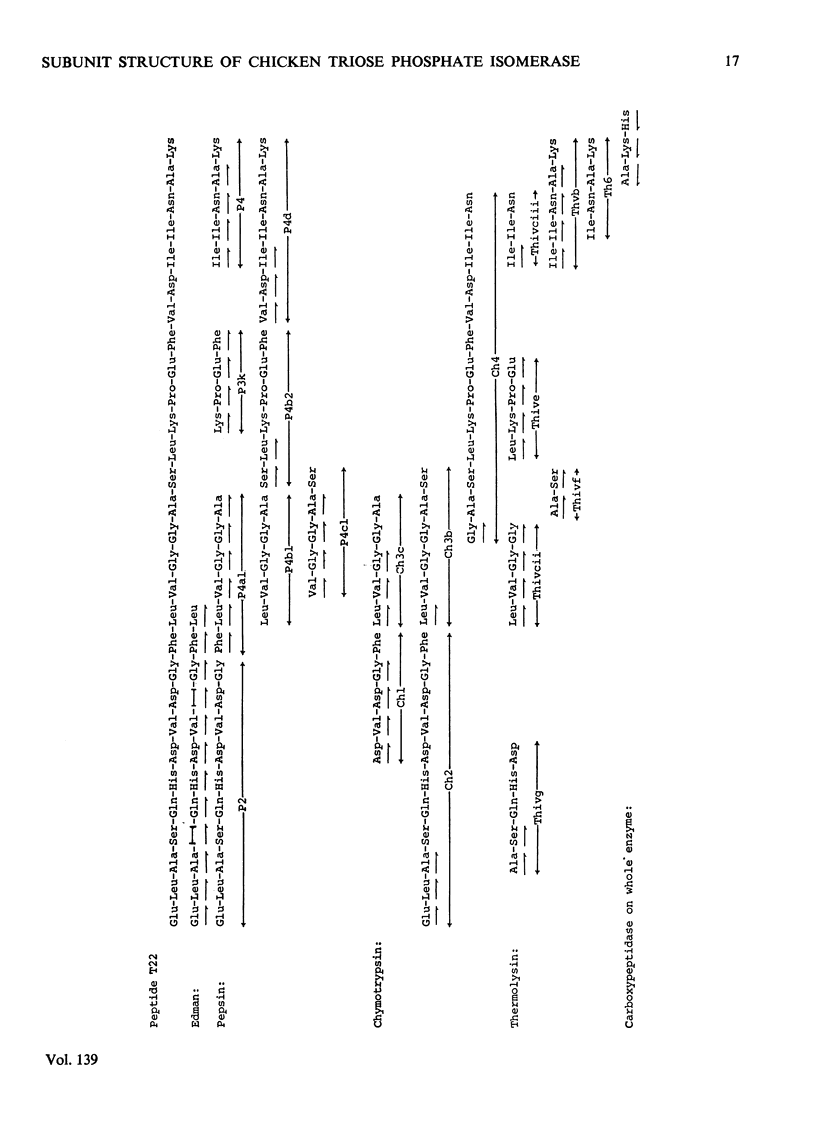
Abstract
1. Triose phosphate isomerase was prepared by chromatography on DEAE-cellulose of an (NH4)2SO4 fraction of an extract of homogenized chicken breast muscle. The product is homogeneous on gel electrophoresis and is suitable for growing crystals for X-ray work. The specific activity is 10000 units/mg and the value for E0.1%280 is 1.20. 2. Comparison between the sum of the amino acid compositions of the tryptic peptides of the protein and the amino acid composition obtained on total hydrolysis of the protein indicates that the relative subunit mass is about 27000. 3. These data, together with the results of the examination of the amino acid compositions of a number of minor peptides, the number of peptides in the tryptic digest and the complete amino acid sequences of the tryptic peptides (the determination of which is described here), give no indication that the subunits are dissimilar. 4. A tentative amino acid sequence is presented for the protein, in which the ordering of the tryptic peptides is derived by homology with the sequence of the rabbit muscle enzyme (Corran & Waley, 1973). 5. An appendix describes the use that was made of mass spectrometry in the determination of some of the sequences. Mass-spectrometric data have been obtained for 35 residues, that is about 15% of the total sequence of the protein. 6. An extended version of the present paper has been deposited as Supplementary Publication SUP 50025 at the British Library, Lending Division (formerly the National Lending Library for Science and Technology), Boston Spa, Yorks. LS23 7BQ, U.K., from whom copies may be obtained on the terms given in Biochem. J. (1973) 131, 5.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMBLER R. P. THE AMINO ACID SEQUENCE OF PSEUDOMONAS CYTOCHROME C-551. Biochem J. 1963 Nov;89:349–378. doi: 10.1042/bj0890349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BENSON J. V., Jr, PATTERSON J. A. ACCELERATED AUTOMATIC CHROMATOGRAPHIC ANALYSIS OF AMINO ACIDS ON A SPHERICAL RESIN. Anal Chem. 1965 Aug;37:1108–1110. doi: 10.1021/ac60228a008. [DOI] [PubMed] [Google Scholar]
- Banner D. W., Bloomer A. C., Petsko G. A., Phillips D. C., Pogson C. I. Crystallographic studies of chicken triose phosphate isomerase. Cold Spring Harb Symp Quant Biol. 1972;36:151–155. doi: 10.1101/sqb.1972.036.01.021. [DOI] [PubMed] [Google Scholar]
- Burton P. M., Waley S. G. Studies on the sub-units of triose phosphate isomerase. Biochem J. 1968 May;107(6):737–744. doi: 10.1042/bj1070737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The amino acid sequence of rabbit muscle triose phosphate isomerase. FEBS Lett. 1973 Feb 15;30(1):97–99. doi: 10.1016/0014-5793(73)80627-1. [DOI] [PubMed] [Google Scholar]
- Corran P. H., Waley S. G. The tryptic peptides of rabbit muscle triose phosphate isomerase. Biochem J. 1974 Apr;139(1):1–10. doi: 10.1042/bj1390001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson A. F., Knowles J. R., Priddle J. D., Offord R. E. Uniquely labelled active site sequence in chicken muscle triose phosphate isomerase. Nature. 1970 Jul 11;227(5254):180–181. doi: 10.1038/227180a0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez G., Offord R. E. The subunit structure of prealbumin. Biochem J. 1971 Nov;125(1):309–317. doi: 10.1042/bj1250309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIRS C. H. The oxidation of ribonuclease with performic acid. J Biol Chem. 1956 Apr;219(2):611–621. [PubMed] [Google Scholar]
- Knowles J. R., Leadlay P. F., Maister S. G. Triosephosphate isomerase: isotope studies on the mechanistic pathway. Cold Spring Harb Symp Quant Biol. 1972;36:157–164. doi: 10.1101/sqb.1972.036.01.022. [DOI] [PubMed] [Google Scholar]
- Miller J. C., Waley S. G. Amino acid sequences around the cysteine residues of rabbit muscle triose phosphate isomerase. Biochem J. 1971 Apr;122(2):209–218. doi: 10.1042/bj1220209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norton I. L., Pfuderer P., Stringer C. D., Hartman F. C. Isolation and characterization of rabbit muscle triose phosphate isomerase. Biochemistry. 1970 Dec 8;9(25):4952–4958. doi: 10.1021/bi00827a019. [DOI] [PubMed] [Google Scholar]
- Offord R. E. Electrophoretic mobilities of peptides on paper and their use in the determination of amide groups. Nature. 1966 Aug 6;211(5049):591–593. doi: 10.1038/211591a0. [DOI] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. Halogenation of tyrosine during acid hydrolysis. Biochim Biophys Acta. 1963 May 14;71:468–471. doi: 10.1016/0006-3002(63)91108-9. [DOI] [PubMed] [Google Scholar]
- Trentham D. R., McMurray C. H., Pogson C. I. The active chemical state of D-glyceraldehyde 3-phosphate in its reactions with D-glyceraldehyde 3-phosphate dehydrogenase, aldolase and triose phosphate isomerase. Biochem J. 1969 Aug;114(1):19–24. doi: 10.1042/bj1140019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALEY S. G., WATSON J. The action of trypsin on polylysine. Biochem J. 1953 Sep;55(2):328–337. doi: 10.1042/bj0550328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods K. R., Wang K. T. Separation of dansyl-amino acids by polyamide layer chromatography. Biochim Biophys Acta. 1967 Feb 21;133(2):369–370. doi: 10.1016/0005-2795(67)90078-5. [DOI] [PubMed] [Google Scholar]


