Abstract
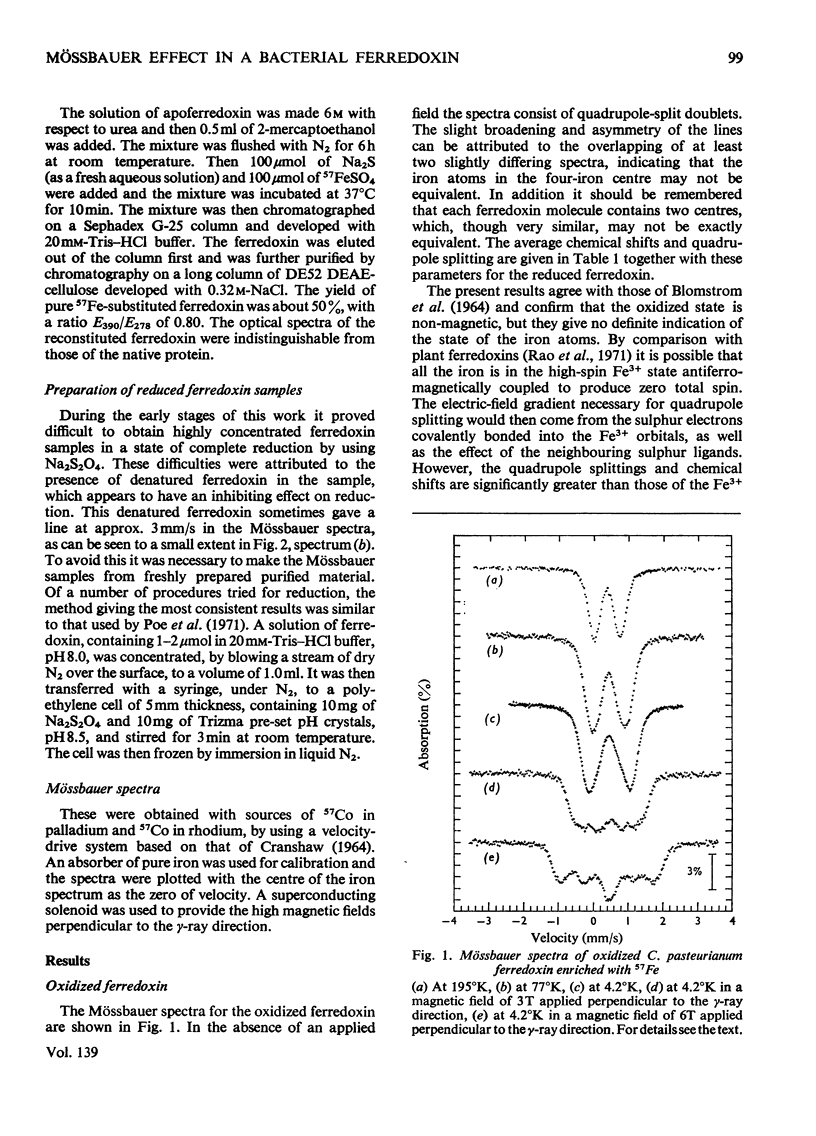
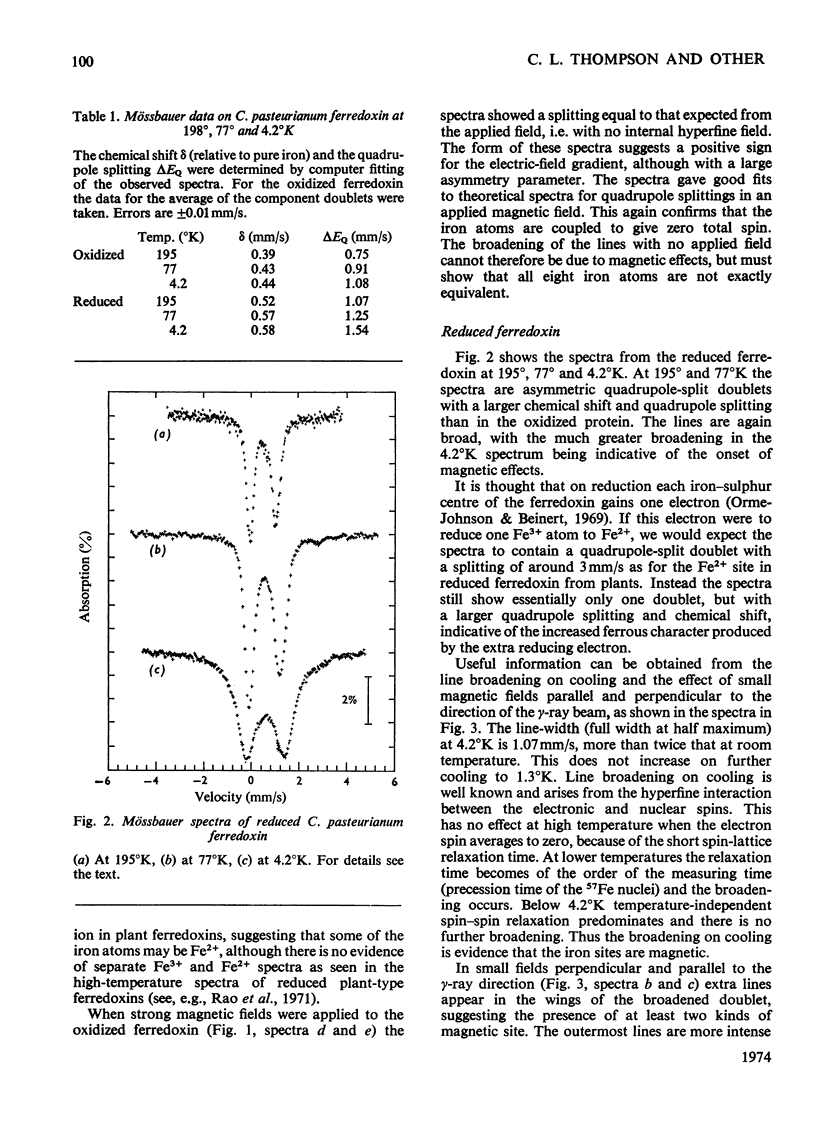
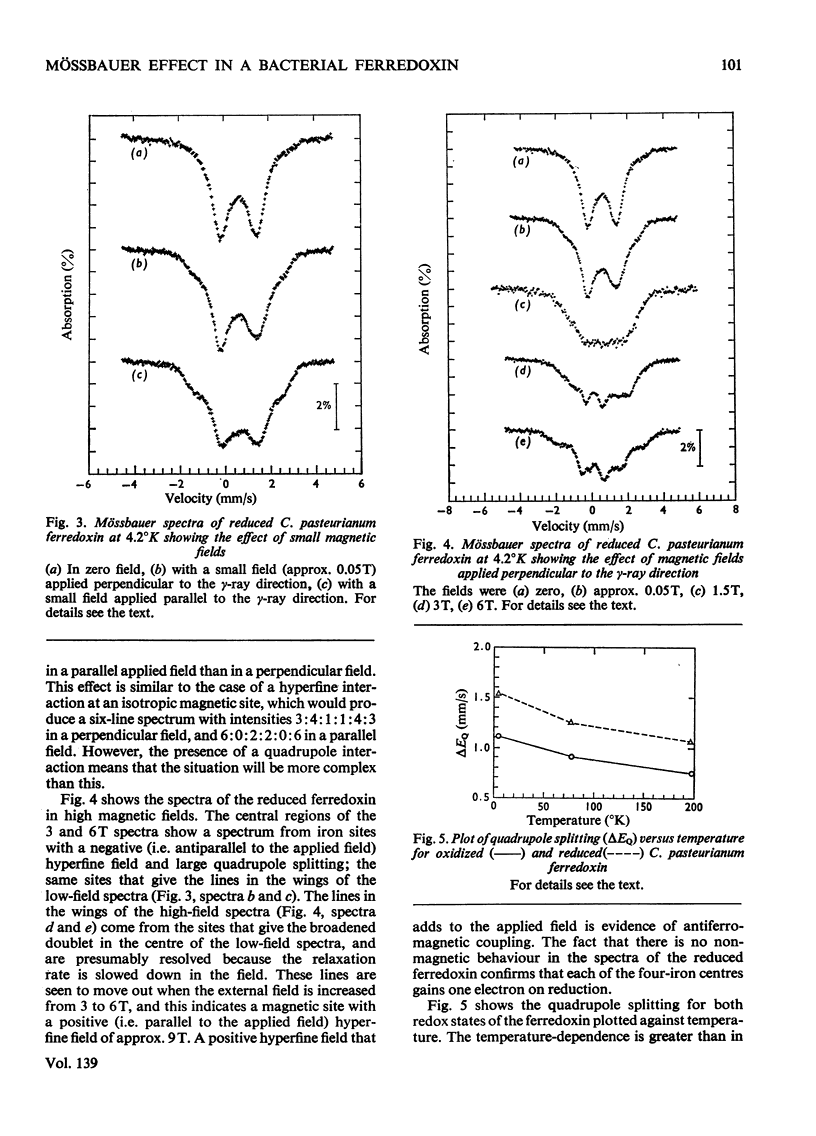
1. Mössbauer spectra of both redox states of the eight-iron ferredoxin from Clostridium pasteurianum were observed over a range of temperatures and in magnetic fields. 2. At high temperatures (77°K and above) the spectra of both states consist essentially of the superposition of two or more closely similar doublets. 3. The average chemical shift for the oxidized protein leads to the proposal that each of the two four-iron active centres consists formally of two Fe3+ and two Fe2+ atoms. 4. The average chemical shift and quadrupole splitting increase on reduction, consistent with there being one Fe3+ and three Fe2+ atoms per centre in the reduced molecule. 5. The spectral changes on reduction show that all the iron atoms are affected when one electron is added to each four-iron centre. 6. No separate Fe3+ and Fe2+ spectra were observed (as they were, for instance, in the reduced two-iron plant ferredoxins) suggesting that the d electrons are not localized on particular atoms, but are shared approximately equally by all four atoms in the four-iron centres. 7. At low temperatures (4°K and below) no magnetic hyperfine interaction was observed in the oxidized protein even in an applied magnetic field, confirming the non-magnetic nature of the molecule in the oxidized state, and suggesting that the four iron atoms in each centre are antiferromagnetically coupled together to give zero spin. 8. Magnetic hyperfine interaction was observed in the reduced protein at low temperatures, and showed that all the iron atoms were magnetic. This demonstrates that one electron goes to each centre on reduction. 9. On application of a large magnetic field to the reduced protein at low temperatures, both positive and negative hyperfine fields were shown to be present, thus directly showing that antiferromagnetic coupling exists between the iron atoms in the reduced state.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adman E. T., Sieker L. C., Jensen L. H. Structure of a bacterial ferredoxin. J Biol Chem. 1973 Jun 10;248(11):3987–3996. [PubMed] [Google Scholar]
- BLOMSTROM D. C., KNIGHT E., Jr, PHILLIPS W. D., WEIHER J. F. THE NATURE OF IRON IN FERREDOXIN. Proc Natl Acad Sci U S A. 1964 Jun;51:1085–1092. doi: 10.1073/pnas.51.6.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan B. B., Arnon D. I. Ferredoxins: chemistry and function in photosynthesis, nitrogen fixation, and fermentative metabolism. Adv Enzymol Relat Areas Mol Biol. 1970;33:119–176. doi: 10.1002/9780470122785.ch3. [DOI] [PubMed] [Google Scholar]
- Cammack R. "Super-reduction" of chromatium high-potential iron-sulphur protein in the presence of dimethyl sulphoxide. Biochem Biophys Res Commun. 1973 Sep 18;54(2):548–554. doi: 10.1016/0006-291x(73)91457-5. [DOI] [PubMed] [Google Scholar]
- Cammack R., Rao K. K., Hall D. O., Johnson C. E. Mössbauer studies of adrenodoxin. The mechanism of electron transfer in a hydroxylase iron-sulphur protein. Biochem J. 1971 Dec;125(3):849–856. doi: 10.1042/bj1250849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter C. W., Jr, Freer S. T., Xuong N. H., Alden R. A., Kraut J. Structure of the iron-sulfur cluster in the Chromatius iron protein at 2.25 Angstrom resolution. Cold Spring Harb Symp Quant Biol. 1972;36:381–385. doi: 10.1101/sqb.1972.036.01.049. [DOI] [PubMed] [Google Scholar]
- Carter C. W., Jr, Kraut J., Freer S. T., Alden R. A., Sieker L. C., Adman E., Jensen L. H. A comparison of Fe 4 S 4 clusters in high-potential iron protein and in ferredoxin. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3526–3529. doi: 10.1073/pnas.69.12.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunham W. R., Bearden A. J., Salmeen I. T., Palmer G., Sands R. H., Orme-Johnson W. H., Beinert H. The two-iron ferredoxins in spinach, parsley, pig adrenal cortex, Azotobacter vinelandii, and Clostridium pasteurianum: studies by magnetic field Mössbauer spectroscopy. Biochim Biophys Acta. 1971 Nov 2;253(1):134–152. doi: 10.1016/0005-2728(71)90240-4. [DOI] [PubMed] [Google Scholar]
- Eisenstein K. K., Wang J. H. Conversion of light to chemical free energy. I. Porphyrin-sensitized photoreduction of ferredoxin by glutathione. J Biol Chem. 1969 Apr 10;244(7):1720–1728. [PubMed] [Google Scholar]
- Evans M. C., Hall D. O., Bothe H., Whatley F. R. The stoicheiometry of electron transfer by bacterial and plant ferredoxins. Biochem J. 1968 Dec;110(3):485–489. doi: 10.1042/bj1100485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans M. C., Hall D. O., Johnson C. E. Hyperfine structure of (57Fe) iron in the Mössbauer spectrum of the high-potential iron protein from Chromatium. Biochem J. 1970 Sep;119(2):289–291. doi: 10.1042/bj1190289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J. F., Hall D. O., Thornley J. H., Whatley F. R. The iron complex in spinach ferredoxin. Proc Natl Acad Sci U S A. 1966 Sep;56(3):987–990. doi: 10.1073/pnas.56.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskovitz T., Averill B. A., Holm R. H., Ibers J. A., Phillips W. D., Weiher J. F. Structure and properties of a synthetic analogue of bacterial iron--sulfur proteins. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2437–2441. doi: 10.1073/pnas.69.9.2437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong J. S., Rabinowitz J. C. Molar extinction coefficient and iron and sulfide content of clostridial ferredoxin. J Biol Chem. 1970 Oct 10;245(19):4982–4987. [PubMed] [Google Scholar]
- Hong J., Rabinowitz J. C. Preparation and properties of clostridial apoferredoxins. Biochem Biophys Res Commun. 1967 Oct 26;29(2):246–252. doi: 10.1016/0006-291x(67)90595-5. [DOI] [PubMed] [Google Scholar]
- Johnson C. E., Cammack R., Rao K. K., Hall D. O. The interpretation of the EPR and Mössbauer spectra of two-iron, one-electron, iron-sulphur proteins. Biochem Biophys Res Commun. 1971 May 7;43(3):564–571. doi: 10.1016/0006-291x(71)90651-6. [DOI] [PubMed] [Google Scholar]
- MORTENSON L. E., VALENTINE R. C., CARNAHAN J. E. An electron transport factor from Clostridium pasteurianum. Biochem Biophys Res Commun. 1962 Jun 4;7:448–452. doi: 10.1016/0006-291x(62)90333-9. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H., Beinert H. Heterogeneity of paramagnetic species in two iron-sulfur proteins: Clostridium pasteurianum ferredoxin and milk xanthine oxidase. Biochem Biophys Res Commun. 1969 Aug 7;36(3):337–344. doi: 10.1016/0006-291x(69)90569-5. [DOI] [PubMed] [Google Scholar]
- Orme-Johnson W. H. Iron-sulfur proteins: structure and function. Annu Rev Biochem. 1973;42(0):159–204. doi: 10.1146/annurev.bi.42.070173.001111. [DOI] [PubMed] [Google Scholar]
- Poe M., Phillips W. D., McDonald C. C., Orme-Johnson W. H. PMR and magnetic susceptibility studies on Clostridium acidi-urici ferredoxin. Biochem Biophys Res Commun. 1971 Feb 19;42(4):705–713. doi: 10.1016/0006-291x(71)90545-6. [DOI] [PubMed] [Google Scholar]
- Rao K. K., Cammack R., Hall D. O., Johnson C. E. Mössbauer effect in Scenedesmus and spinach ferredoxins. The mechanism of electron transfer in plant-type iron-sulphur proteins. Biochem J. 1971 Apr;122(3):257–265. doi: 10.1042/bj1220257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao K. K., Evans M. C., Cammack R., Hall D. O., Thompson C. L., Jackson P. J., Johnson C. E. Mössbauer effect in rubredoxin. Determination of the hyperfine field of the iron in a simple iron-sulphur protein. Biochem J. 1972 Oct;129(5):1063–1070. doi: 10.1042/bj1291063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargeant K., Ford J. W., Longyear V. M. Production of Clostridium pasteurianum in a defined medium. Appl Microbiol. 1968 Feb;16(2):296–300. doi: 10.1128/am.16.2.296-300.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieker L. C., Adman E., Jensen L. H. Structure of the Fe-S complex in a bacterial ferredoxin. Nature. 1972 Jan 7;235(5332):40–42. doi: 10.1038/235040a0. [DOI] [PubMed] [Google Scholar]
- Thornley J. H., Gibson J. F., Whatley F. R., Hall D. O. Comment on a recent model of the iron complex in spinach ferredoxin. Biochem Biophys Res Commun. 1966 Sep 22;24(6):877–879. doi: 10.1016/0006-291x(66)90330-5. [DOI] [PubMed] [Google Scholar]


