Abstract
Background
Previous observational studies have suggested a potential link between depression and cervical spondylosis (CS). While it is known that depression and CS can coexist, the specific relationship between them is not fully understood. We hypothesize that there may be connections between the two conditions, but the independent causal relationship of depression as a risk factor for CS, remains uncertain. This particular study has important implications for the future clinical treatment of depression and cervical spondylosis because Mendelian randomization has not been widely used in this field. We obtained valuable results through big data analysis and have guiding significance for future research.
Methods
We conducted a two-sample Mendelian randomization (MR) study using data from genome-wide association studies to investigate the causal relationship between depression and CS in individuals of European ancestry. Additionally, we examined the impact of CS on susceptibility to depression using large population-level genetic data (number of depression SNPs: 9,761,853; number of CS SNPs: 9,851,867). The primary approach for data analysis was the inverse-variance weighted (IVW) method to estimate potential causal effects. Furthermore, we performed sensitivity analyses utilizing methods such as Manhattan plot (CMplot), linkage disequilibrium (LD), F-filtering, removal of phenoscanner, MR-Egger, weighted median, MR-PRESSO simple mode weighted mode MR pleiotropy test MR heterogeneity assessment leave-one-out analysis to ensure result robustness.
Results
Our findings indicated that an elevated likelihood of CS was linked to depression [IVW odds ratio (OR): 1.322, 95% confidence interval (CI): 1.205–1.441, P=0.01243]. There was reciprocal evidence of causation, with the genetic predisposition to depression significantly heightening susceptibility to CS [IVW odds ratio (OR): 1.426, 95% confidence interval (CI): 1.236–1.651, P=0.01775].
Conclusion
This investigation provides genetic support for a bidirectional causal association between depression and CS. Specifically, individuals with depression are at greater risk of developing CS. Addressing depression may serve as an effective approach in mitigating or preventing the burden of CS and vice versa.
Keywords: depression, bidirectional Mendelian randomization, cervical spondylosis, mental health, genetic correlation analysis, quality of life
Introduction
Patients diagnosed with cervical spondylosis may present with symptoms such as neck discomfort, radiating pain in the upper extremities, and gait instability. Diagnosis is typically established through a combination of self-reported symptoms, X-ray imaging, computed tomography (CT), magnetic resonance imaging (MRI), and comprehensive physical examination.1 Conversely, depression can be identified based on subjective experiences that persist for two to three weeks—these include sustained low mood, anhedonia, fatigue, sleep disturbances, and appetite changes—as well as objective assessments of the patient’s behavior and psychological state. Standardized tools such as the Hamilton Depression Rating Scale and the Self-Rating Depression Scale are also employed in this diagnostic process. Furthermore, laboratory tests and thorough physical examinations are essential to exclude other potential underlying conditions.2
Depression is a mental condition linked to negative health consequences that may occur alongside CS. Despite this co-occurrence being acknowledged in clinical settings, the underlying connection between depression and cervical spondylosis remains poorly comprehended. Emotions, sleep patterns, inappropriate mechanical pressures, muscle rigidity, and the aging of the vertebral body are all factors associated with the onset of CS.
According to the World Health Organization (WHO) in 2019, CS is ranked as the second most prevalent chronic disease, following closely behind cardiovascular and cerebrovascular diseases.3,4 This condition is characterized by progressive degeneration of the cervical vertebral body and intervertebral discs, resulting in spinal imbalance and compression of cervical vessels, sympathetic nerves, spinal nerve roots, and spinal cord. It manifests as pain in the neck, shoulder, back, upper limb, head, chest or other symptoms and may even lead to loss of limb function. It is a chronic disease of middle-aged and older people, and its symptoms include various clinical neurological manifestations. The etiology and pathology of cervical spondylosis present a complex picture, leading to diverse clinical manifestations that significantly impact patients’ quality of life. According to the results of an epidemiological survey, 25% of individuals under 40 years old, 50% of those over 40, and 85% of those over 60 were found to have cervical degeneration.1 In patients with cervical spondylosis, a pronounced inflammatory response was observed during the advanced stage of disc herniation, characterized by infiltration of CD68-positive macrophages into the outer layer of annular fibers and a more diffuse expression of TNF-α in the inner layer of annular fibers. Recent studies have verified a significant occurrence of depression in individuals with cervical spine conditions such as cervical spondylosis.5 Additionally, scientists have established a link between the release of TNF-α in the brain and the onset of depression; patients displaying depressive symptoms exhibit higher levels of infiltrating CD68+ cells in the intestinal mucosal layer.6,7 The treatment of cervical spondylosis includes conservative treatment and surgical treatment. Surgical treatment can be considered after a comprehensive investigation of nerve root compression and progressive symptoms of Cervical spondylotic radiculopathy and Cervical spondylotic myelopathy.8,9 Symptomatic cervical spondylosis is a common cause of disability, mainly due to significant burden and reduced happiness, occupational characteristics, trauma, and so on.10
Depression is a prevalent mental health condition that significantly impacts the physical and psychological well-being of a considerable portion of the global population.11 It is characterized by persistent and notable low mood, influenced by both genetic and environmental factors.12 Furthermore, depression has been found to have a strong correlation with sleep disturbances,13 somatic symptoms,14 spinal lesions,15 cerebral small vessel disease (CSVD),16 heart disease,17 and Parkinson’s Disease.18 Additionally, it is linked to compromised physical health and accelerated bodily aging, both contributing to an increased risk of CS.19,20
Individuals suffering from depression may exhibit incorrect posture, leading to potential complications in the body. This can ultimately result in muscle strain and structural alterations in the spine.21 Consequently, individuals with depression may be susceptible to developing spinal conditions such as cervical spondylosis. Research has indicated that patients diagnosed with cervical spondylosis are at an elevated risk of experiencing symptoms of depression.22–24 A community-based cross-sectional study revealed that approximately 25% of individuals living with cervical spondylosis in a specific area displayed indications of anxiety or depression.25 Currently, it remains uncertain whether individuals affected by depression face an increased risk for developing cervical spondylosis. Due to limitations related to sample size, detecting a definitive association between depression and cervical spondylosis within clinical settings may prove challenging. Only through Mendelian randomization studies on larger samples will we be able to thoroughly evaluate this relationship.
Mendelian randomization (MR) is a method used in epidemiological research to assess causal inference by leveraging genetic variations closely linked to exposure factors as instrumental variables. It aims to investigate the causal relationship between exposure factors and outcomes. In bidirectional Mendelian randomization studies, it’s important to consider situations where various factors may mutually influence each other. The causal relationship between factors can be further analyzed by the bidirectional MR method. If both bidirectional MRs demonstrate statistical significance, it suggests that exposure serves as both the “cause” and the “effect”.26–29 In this study, we employed a bidirectional two-sample MR method to explore the potential bidirectional relationship between depression and CS.
Our goal is to use a two-sample Mendelian randomization method to explore the genetic link between CS and depression. The aim is to uncover hidden connections and offer strong theoretical support for clinical diagnosis and treatment.
Materials and Methods
We utilized previously published, unspecified of the population summary statistics of depression combined data from the IEU Open GWAS project (https://gwas.mrcieu.ac.uk/), we chose this data for the following reason: It is also could be made more clear for the justification for the SNP selection criteria--threshold adjustment for small samples size.
We also mined the basic data of the population summary statistics of CS from FINNGEN project (https://www.finngen.fi/en). Informed consent was acquired from all participating studies. The data were accessed for research purposes in 2018. The authors do not have access to information that could identify individual participants during or after data collection, as all personal information is currently not publicly available. The prejudice of the present study using the European population may some how limit the generalization of the present findings in the non-European population, Therefore, our study only targeted the European sample population, and the data reference has certain limitations.
The population summary statistics of depression were from the IEU OpenGWAS project with the largest sample size, involving 26,595 clinically diagnosed cases and 436,338 controls, the statistical population was European, and the recruited statistical population included both men and women, and the consortium was MRC-IEU. The population summary statistics of CS were from the FINNGEN project database, involving 3292 clinically diagnosed cases and 459,641 controls, the statistical population was European, and the recruited statistical population included both men and women.
We conducted Mendelian randomization (MR) analysis to investigate the causal link between the exposure phenotype and outcome phenotype, using germline genetic variation as the instrumental variable (IV) for exposure. Three fundamental principles of MR include: (1) SNPs are highly correlated with exposure factors. (2) SNPs are not influenced by confounding factors. (3) SNPs can only impact outcomes through exposure factors. Firstly, we selected independent SNPs that were closely related to depression and CS and whose P-value was less than 5×10−8. Due to the small sample size, we extended the threshold to 5×10−5 to select eligible instrumental variables.30,31 We performed association analysis on the data, selected SNPs strongly related to exposure factors, and plotted linear Manhattans and circular Manhattans (Figure 1A–D). 3572 eligible SNPs were screened out in the depression group (Supplement file 1). In the CS group, 449 SNPs were selected (Supplement file 2). Furthermore, we have removed SNPs that exhibit strong linkage disequilibrium (LD). We conducted the clumping procedure using R2 < 0.001 and a window size of 10,000 kb with individuals of European descent who have depression, but due to statistical differences, this setting is set when CS becomes R2< 0.01 and a window size = 5000 kb, and the depression SNPs are finally selected as 148 (Supplement file 3) and CS SNPs as 59 (Supplement file 4). We utilized random effects inverse variance weighting (IVW) as the primary statistical approach to assess the potential bidirectional causal relationship between depression and CS, IVW was the primary method for causal estimation because it was the most precise and robust method.32 In terms of the correlation hypothesis, the R2 value indicates the proportion of variance in the exposed variable attributed to genetic variation. We employed phenoscanner to identify potential confounding factors, and no such factors were found in our final results. Additionally, we applied MR-Egger regression intercept and its 95% confidence interval (CI) to investigate bias resulting from pleiotropy. MR-PRESSO outlier detection (biased SNPs) was performed, MR-PRESSO was a widely used method for detecting widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases, which was published in the May 2018 issue of the journal “Nature Genetics”. Its core consisted of three parts: (1) detecting the presence of horizontal pleiotropy using the “MR-PRESSO global test”; (2) removing outlier SNPs (outliers) and estimating the corrected results (this result excludes horizontal pleiotropy) using the “MR-PRESSO outlier test”; and (3) using the “MR-PRESSO test” to test whether there is a difference between the corrected and uncorrected results.33 The Heterogeneity test and Pleiotropy test were used to detect the differences between IV and test whether there is horizontal pleiotropy among IVs, Heterogeneity test: This test primarily assesses the differences among various instrumental variables (IVs). A substantial difference between these IVs indicates a high level of heterogeneity; Pleiotropy test: This assessment focuses on determining whether multiple IVs exhibit horizontal pleiotropy, which was typically represented by the intercept term in MR Egger’s method. A significant deviation of this intercept from zero suggests the presence of horizontal pleiotropy.34 We also conducted a Leave-one-out sensitivity test to assess the stability and reliability of the findings, leave-one-out analysis was used to determine whether the causal association signal was driven by a single SNPs.35 Subsequently, we created scatter plot, forest plot, and funnel plot; Forest plots were made to visualize the results, and gene pleiotropy and sensitivity were analyzed by scatter plots and funnel plots.36 All p-values are two-tailed. The analyses were carried out using the TwoSampleMR and MendelianRandomization packages in R (version 4.3.1, www.r-project.org/).
Figure 1.
Selected SNPs strongly related to exposure factors, and plotted linear Manhattans and circular Manhattans (A–D).
Results
The Impact of Depression on CS
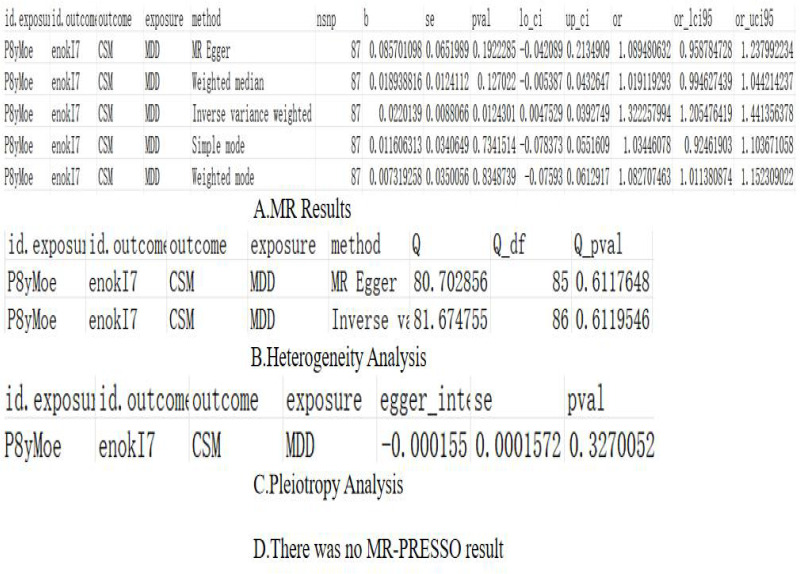
The results of IVW showed that the prevalence of depression may increase the risk of CS, and we believed that this association had a certain causal relationship. Furthermore, the OR values for MR Egger, Weighted median, Simple mode, and Weighted mode exceeded 1, aligning with the findings of the IVW assessment. The p-value results of the heterogeneity test and pleiotropy test were >0.05, there was no significant heterogeneity, horizontal pleiotropy, or outliers.
The detailed MR results and sensitivity analysis for the previous results are presented in Figure 2A–D and Supplement file 5 (the final results of SNPs output after comparison). Figure 3 are forest plot and funnel plot, Figure 4 are leave-one-out plot and scatter plot.
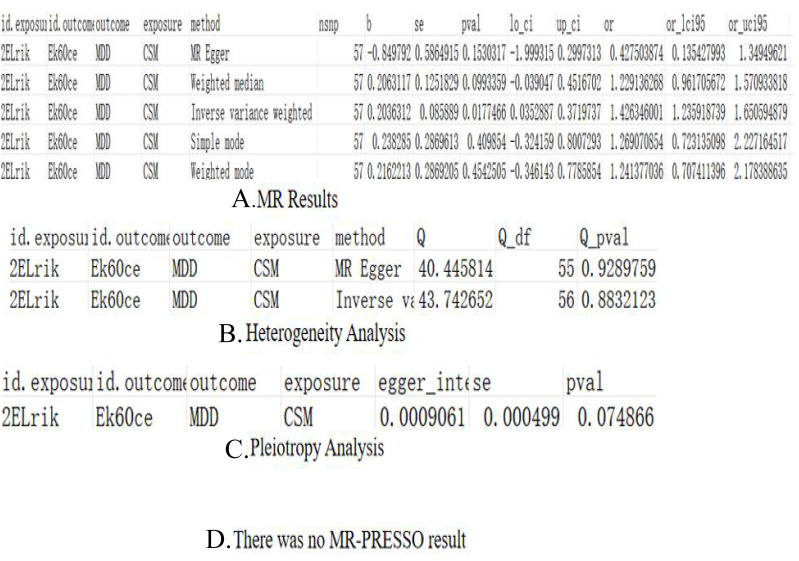
Figure 2.
Depression on CS (A) Mendelian analysis results, (B) heterogeneity analysis, (C) pleiotropy analysis, (D) MR-PRESSO results.
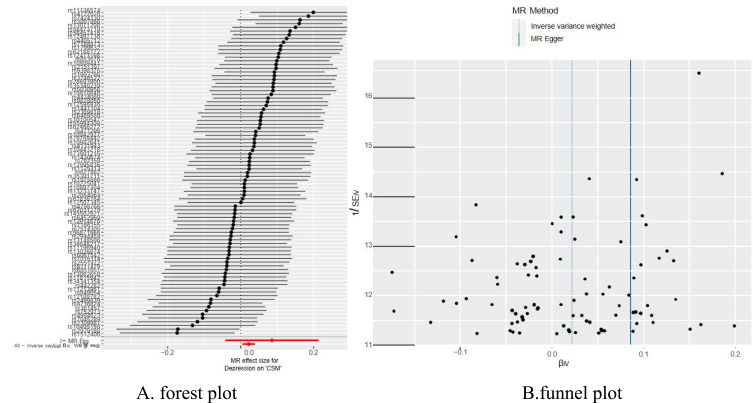
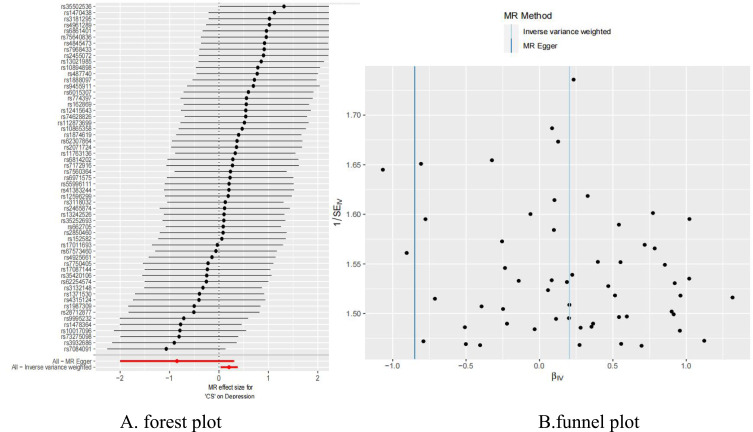
Figure 3.
(A) Forest plot of the casual effect of depression on CS; (B) funnel plot of the casual effect of depression on CS.
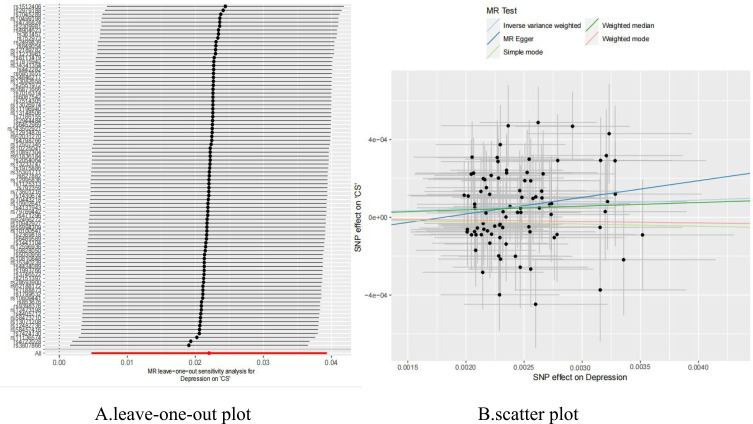
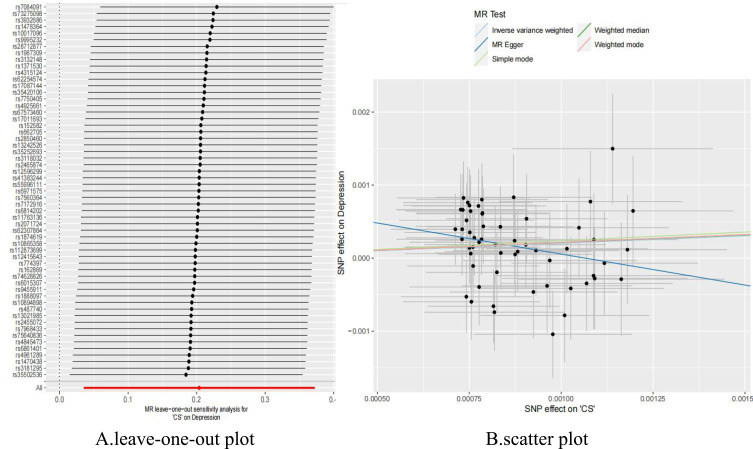
Figure 4.
(A) Leave-one-out plot of the casual effect of depression on CS; (B) scatter plot of the casual effect of depression on CS.
The Impact of CS on Depression
The findings from the IVW analysis (p=0.018) indicated a potential association between the prevalence of CS and an elevated likelihood of experiencing depression. Additionally, the OR values for Weighted median, Simple mode, and Weighted mode were all greater than 1, aligning with the outcomes of the IVW assessment. The value results of the heterogeneity test and pleiotropy test were >0.05, indicating that there was no pleiotropy and heterogeneity, and the results were dependable. When using CS as an exposure factor, the OR of MR Egger suggested that there may be active correlation factors, and further research was needed. The outcome direction still indicated that CS increases the risk of depression, The occurrence of these events may be attributed to the statistical error in the study population and variations in lifestyle habits among the European population. It is imperative to further conduct a meticulous screening of the database and analyze factors such as dietary patterns, sleep quality, and body composition for a comprehensive understanding of the population, thus mitigating these occurrences.
The detailed MR results and sensitivity analysis for the previous results are presented in Figure 5A–D and Supplement file 6 (the final results of SNPs output after comparison). Figure 6 are forest plot and funnel plot, Figure 7 are leave-one-out plot, and scatter plot.
Figure 5.
CS on Depression (A) Mendelian analysis results, (B) heterogeneity analysis, (C) pleiotropy analysis, (D) MR-PRESSO results.
Figure 6.
(A) Forest plot of the casual effect of CS on depression; (B) funnel plot of the casual effect of CS on depression.
Figure 7.
(A) Leave-one-out plot of the casual effect of CS on depression; (B) scatter plot of the casual effect of CS on depression.
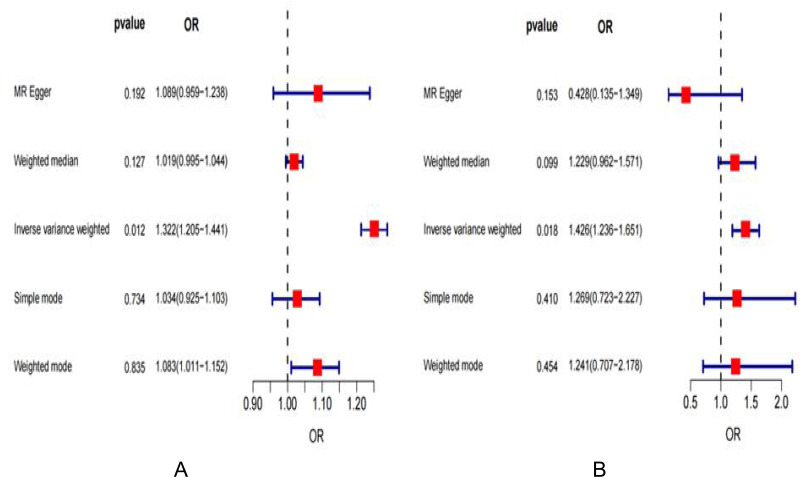
The two figures are visual forest plots of the Mendelian randomization analysis of CS and depression (Figure 8A and B). Table 1 shows the results of bidirectional MR analyses.
Figure 8.
The two figures are visual forest plots of the Mendelian randomization analysis of CS and depression (A and B); (A) the casual effect of depression on CS (forest); (B) the casual effect of CS on depression (forest).
Table 1.
The Results of Bidirectional MR Analyses
| Exposure | Outcome | No. of SNPs | Methods | OR (95% CI) | β (SE) | p | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| The forward MR analyses | |||||||||||
| Depression | CS | 148 | IVW MR‐Egger Weighted median Weighted mode Simple mode |
1.322257994 (1.205–1.441) 1.089480632 (0.959–1.238) 1.019119293 (0.995–1.044) 1.082707463 (1.011–1.152) 1.03446078 (0.925–1.104) |
0.0220139 (0.008806643) 0.085701098 (0.065198879) 0.018938816 (0.012411153) -0.007319258 (0.035005598) -0.011606313 (0.034064884) |
0.012430116 0.192228459 0.127021989 0.83487385 0.734151358 |
|||||
| The reverse MR analyses | |||||||||||
| CS | Depression | 58 | IVW MR‐Egger Weighted median Weighted mode Simple mode |
1.426346001 (1.236–1.650) 0.427503874 (0.135–1.349) 1.229136267 (0.962–1.571) 0.061291714 (0.707–2.178) 0.05516086 (0.723–2.227) |
0.203631218 (0.085889038) -0.84979192 (0.586491465) 0.206311701 (0.125182923) 0.216221276 (0.286920494) 0.238285021 (0.286961345) |
0.017746563 0.153031696 0.099335881 0.454250535 0.409853987 |
|||||
Abbreviations: CI, confidence interval; IVW, inverse variance weighted; LTL, leukocyte telomere length; MR, Mendelian randomization; OR, odds ratio; SNPs, single‐nucleotide polymorphisms.
Discussion
In this research, we conducted a comprehensive MR analysis to evaluate the connection between cervical spondylosis (CS) and the likelihood of experiencing depression. Our findings indicate that there is an association between depression and an elevated risk of CS, potentially suggesting a causal relationship. Similarly, in the opposite direction, CS and depression may also have a causal link. Studies have indicated that individuals with low moods and depression are at higher risk for developing cervical spondylosis, and these mood states can impact overall well-being and sense of fulfillment, ultimately leading to negative health outcomes and reduced quality of life.37 Studies have found that older patients with vertebral-related diseases and depression face higher preoperative disability risk and lower postoperative quality of recovery.15,38 Meanwhile, patients with cervical spondylosis face health-related economic burdens, which increases the risk of depression. Canales et al found that depressed patients exhibited changes in body posture during depressive episodes, such as neck, and chest kyphosis and neck stiffness.39 Rosario et al found that long-term postural inaccuracy led to cone structure remodeling and osteophyte formation. Depressed patients with long-term postural inaccuracy due to various factors lead to poor development of the shoulder and neck and eventually become deformities.40 Some research has indicated that individuals with depression may be more inclined to seek improved medical attention, resulting in a higher likelihood of detecting cervical spondylosis compared to non-depressed individuals.41,42 Given that cervical spondylosis is diagnosed through clinical imaging and pathological anatomy descriptions, it could potentially predate the diagnosis of depression. Additionally, the discomfort associated with cervical spondylosis may contribute to the development of depression, which could account for the disparities observed in the statistical results between IVW and OR of MR Egger. It may also need a larger and more accurate sample for analysis. Depression may increase the discomfort of CS, but not directly lead to cervical deformity. Depression could result in a decrease in physical activity and exercise, potentially leading to muscle stiffness, spinal pain, and deformity. As a result, patients with depression may be more prone to being diagnosed with symptomatic cervical spondylosis. There is no basic research supporting a definite link between depression and CS, but our MR results showed that depression is a risk factor for CS, and the results are consistent.
Diseases such as cervical spondylosis are characterized by inflammatory destruction and bone proliferation. The primary clinical manifestations include joint mobility impairments and pain, with severe cases potentially leading to limb paralysis, thereby significantly impacting the patient’s quality of life and mental health.43 Depression and inflammation are mutually reinforcing processes. In certain patients, inflammation is a critical factor in the pathogenesis of depression; conversely, depression can elicit an exaggerated cytokine response to stressors and pathogens that are typically considered non-threatening.44 Certain disease-related behaviors (such as cervical pain and sleep disturbances) along with detrimental health habits (including poor dietary practices and sedentary lifestyles) may exacerbate ongoing systemic inflammation and depressive disorders. Inflammatory responses that are more severe, frequent, or prolonged as a consequence of depression can adversely affect both physical and mental health, resulting in significantly diminished treatment adherence and exacerbating the symptoms of CS. The bidirectional relationship among depression, inflammation, and disease (CS) indicates that effective treatment of depression may significantly influence mood, inflammatory processes, and overall health.45,46 Consequently, we conclude that mental state significantly influences the progression of pain and inflammatory diseases, with patients’ negative moods directly exacerbating poor dietary choices, unhealthy life habits, and inadequate sleep quality.
By conducting a bidirectional Mendelian randomization study using a large number of European population data, this study found that depression can lead to an increased risk of CS, and vice versa, CS can also lead to an increased risk of depression, but the statistical results are not as obvious and consistent as the positive MR results. Depression caused by CS can be well explained by quality of life, economic stress, long-term posture, sleep quality, and personal psychology, but it is rarely reported. We made a surprising discovery that individuals suffering from depression have an increased likelihood of developing cervical spondylosis. Research has shown no connection between the use of antidepressants and the onset of cervical spondylosis.47 Therefore, aside from medication, adopting postural training or making adjustments to mechanical stress may serve as a protective measure in reducing the risk of cervical spondylosis among patients with depression. Further investigation is necessary to assess the advantages of physiotherapy and postural training in lowering the risk of cervical spondylosis in individuals with depression. Clinicians should also take into account the self-rating depression scale (SDS) when interpreting somatization related to CS, in order to offer optimal professional diagnosis and treatment for patients dealing with depression.
Limitations
The study was unable to ascertain whether genetic differences exist among various races, countries, and regions due to its exclusive inclusion of participants of European descent; consequently, the findings are not generalizable to non-European populations. This investigation is observational in nature, and traditional observational studies are susceptible to bias stemming from residual confounding effects and sample overlap. Therefore, the causal relationship between CS and depression requires further validation. To mitigate bias in estimating causal effects, we conducted additional reverse analyses within this study.
Conclusions
Cervical spondylotic myelopathy (CSM) represents the most prevalent cause of non-traumatic spinal cord injury and associated disability globally. The Japanese Orthopaedic Association (JOA) scoring system is currently regarded as the primary outcome measure in both clinical research and practice for patients suffering from this form of cervical spondylosis.48 The findings of another study indicate that patients with concurrent cervical spondylotic myelopathy (CSM) and depression exhibited a comparatively poorer improvement in symptom severity, pain intensity, and disability scores following posterior decompression surgery when compared to patients without comorbid depression at any stage. In conclusion, our thorough examination revealed a significant causal link between depression and the likelihood of CS, while also suggesting that CS could potentially contribute to the progression of depression. This investigation provides genetic support for a bidirectional causal association between depression and CS. Specifically, individuals with depression are at greater risk of developing CS. Addressing depression may serve as an effective approach in mitigating or preventing the burden of CS and vice versa. Additional research is necessary to investigate the interplay between these two factors in order to advance clinical diagnosis and treatment methods. The inclusion of psychological tests and assessments in expanding CS treatment represents a highly advantageous step. Future research could potentially focus on interventions aimed at enhancing depressive symptoms among clinical patients during hospitalization, encompassing scale scoring, pharmacological intervention, behavioral intervention, and assistive device therapy.
Acknowledgments
We express our gratitude to the contributors of the IEU OpenGWAS project database and the MRC Integrative Epidemiology Unit (IEU) at the University of Bristol Consortium, as well as to the FINNGEN project online, for providing access to their combined statistical data for this research.
Funding Statement
This study did not receive any funding from external sources.
Data Sharing Statement
Additional content can be accessed through IEU OpenGWAS online and the FINNGEN project online. The information in the paper pertains to the European IEU OpenGWAS online and FINNGEN project from 2018, with detailed analysis data available in the accompanying materials.
Approval of Ethical Standards and Agreement to Take Part
The GWAS analytical research was approved in advance by the appropriate Institutional Review Board (Ethics Research Committee of Affiliated Hospital of Guizhou Medical University), we also contained an approval statement, and informed consent was acquired from all participants involved in the study. This current investigation utilized publicly accessible summary data, thus obviating the need for supplementary ethical clearance.
Disclosure
The entire group of individuals mentioned in the article have reached a consensus to make revisions and release the content in the journal for public access. Each and every author affirms that there are no conflicts of interest.
References
- 1.Kuo DT, Tadi P. Cervical spondylosis. In: StatPearls. Treasure Island (FL): StatPearls Publishing; 2023. [PubMed] [Google Scholar]
- 2.Chao S. Overview of Depression. Emerg Med Clin N Am. 2024;42(1):105–113. doi: 10.1016/j.emc.2023.06.013 [DOI] [PubMed] [Google Scholar]
- 3.Waheed MA, Hasan S, Tan LA, et al. Cervical spine pathology and treatment: a global overview. J Spine Surg. 2020;6(1):340–350. doi: 10.21037/jss.2020.01.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shin DW, Shin JI, Koyanagi A, et al. regional, and national neck pain burden in the general population, 1990–2019: an analysis of the global burden of disease study 2019. Front Neurol. 2022;13:955367. doi: 10.3389/fneur.2022.955367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.He J, Xiong W, Li F, Luo W, Gao SC. Depression influences pain and function after cervical disc arthroplasty. J Neurosurg Sci. 2017;61(1):39–45. doi: 10.23736/S0390-5616.16.03032-0 [DOI] [PubMed] [Google Scholar]
- 6.Ma K, Zhang H, Baloch Z. Pathogenetic and Therapeutic Applications of Tumor Necrosis Factor-α (TNF-α) in major depressive disorder: a systematic review. Int J Mol Sci. 2016;17(5):733. doi: 10.3390/ijms17050733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cheng Y, Tang XY, Li YX, et al. Depression-induced neuropeptide Y secretion promotes prostate cancer growth by recruiting myeloid cells. Clin Cancer Res. 2019;25(8):2621–2632. doi: 10.1158/1078-0432.CCR-18-2912 [DOI] [PubMed] [Google Scholar]
- 8.Taso M, Sommernes JH, Kolstad F, et al. A randomized controlled trial comparing the effectiveness of surgical and nonsurgical treatment for cervical radiculopathy. BMC Musculoskelet Disord. 2020;21(1):171. doi: 10.1186/s12891-020-3188-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao Q, Qiang JH, Jin YL. Effectiveness of percutaneous neuromuscular electrical stimulation for neck pain relief in patients with cervical spondylosis. Medicine. 2018;97(26):e11080. doi: 10.1097/MD.0000000000011080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mowforth OD, Davies BM, Kotter MR. Quality of life among informal caregivers of patients with degenerative cervical myelopathy: cross-sectional questionnaire study. Interact J Med Res. 2019;8(4):e12381. doi: 10.2196/12381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu L, Wang H, Chen X, Zhang Y, Zhang H, Xie P. Gut microbiota and its metabolites in depression: from pathogenesis to treatment. EBioMedicine. 2023;90:104527. doi: 10.1016/j.ebiom.2023.104527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Penner-Goeke S, Binder EB. Epigenetics and depression. Dialogues Clin Neurosci. 2019;21(4):397–405. doi: 10.31887/DCNS.2019.21.4/ebinder [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelaye B, Addae G, Neway B, et al. Poor sleep quality, antepartum depression, and suicidal ideation among pregnant women. J Affect Disord. 2017;209:195–200. doi: 10.1016/j.jad.2016.11.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Y, Chen Y, Wu Y, et al. The common neural mechanism of somatic symptoms of depression and anxiety disorders: a resting-state functional magnetic resonance imaging study. Neuropsychobiology. 2023;82(1):51–60. doi: 10.1159/000527276 [DOI] [PubMed] [Google Scholar]
- 15.Bilal J, Berlinberg A, Trost J, Riaz IB, Bhattacharjee S. The influence of depression on health care expenditures among adults with spondylosis, intervertebral disc disorders, and other back problems in the United States. Pain Med. 2020;21(2):e45–e53. doi: 10.1093/pm/pny223 [DOI] [PubMed] [Google Scholar]
- 16.Castello JP, Pasi M, Kubiszewski P, et al. Cerebral small vessel disease and depression among intracerebral hemorrhage survivors. Stroke. 2022;53(2):523–531. doi: 10.1161/STROKEAHA.121.035488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raič M. Depression and heart diseases: leading health problems. Psychiatry Danub. 2017;29(Suppl 4):770–777. [PubMed] [Google Scholar]
- 18.Assogna F, Pellicano C, Savini C, et al. Drug choices and advancements for managing depression in Parkinson’s disease. Curr Neuropharmacol. 2020;18(4):277–287. doi: 10.2174/1570159X17666191016094857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahmed R, Ryan C, Christman S, et al. Structural MRI-based measures of accelerated brain aging do not moderate the acute antidepressant response in late-life depression. Am J Geriatr Psychiatry. 2022;30(9):1015–1025. doi: 10.1016/j.jagp.2021.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yang R, Xu D, Wang H, Xu J. Longitudinal trajectories of physical functioning among Chinese older adults: the role of depressive symptoms, cognitive functioning, and subjective memory. Age Ageing. 2021;50(5):1682–1691. doi: 10.1093/ageing/afab135 [DOI] [PubMed] [Google Scholar]
- 21.Jahanshahi M, Marsden CD. Body concept, disability, and depression in patients with spasmodic torticollis. Behav Neurol. 1990;3(2):117–131. doi: 10.1155/1990/764203 [DOI] [PubMed] [Google Scholar]
- 22.Zong Y, Xue Y, Zhao Y, et al. Depression contributed to an unsatisfactory surgery outcome among the posterior decompression of the cervical spondylotic myelopathy patients: a prospective clinical study. Neurol Sci. 2014;35(9):1373–1379. doi: 10.1007/s10072-014-1714-8 [DOI] [PubMed] [Google Scholar]
- 23.Kim EJ, Chotai S, Schneider BJ, Sivaganesan A, McGirt MJ, Devin CJ. Effect of depression on patient-reported outcomes following cervical epidural steroid injection for degenerative spine disease. Pain Med. 2018;19(12):2371–2376. doi: 10.1093/pm/pny196 [DOI] [PubMed] [Google Scholar]
- 24.Chu Y, Zhang Y, Wang S, Dai H. Resilience mediates the influence of hope, optimism, social support, and stress on anxiety severity among Chinese patients with cervical spondylosis. Front Psychiatry. 2022;13:997541. doi: 10.3389/fpsyt.2022.997541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chu Y, Wang X, Dai H. Prevalence and risk factors for anxiety and depression among community-dwelling patients with cervical spondylosis during the COVID-19 pandemic. Heliyon. 2023;9(2):e13497. doi: 10.1016/j.heliyon.2023.e13497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zheng J, Baird D, Borges MC, et al. Recent developments in Mendelian randomization studies. Curr Epidemiol Rep. 2017;4(4):330–345. doi: 10.1007/s40471-017-0128-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katan MB. Apolipoprotein E isoforms, serum cholesterol, and cancer. Lancet. 1986;1(8479):507–508. doi: 10.1016/S0140-6736(86)92972-7 [DOI] [PubMed] [Google Scholar]
- 28.Smith GD, Ebrahim S. ‘Mendelian randomization’: can genetic epidemiology contribute to understanding environmental determinants of disease? IntJ Epidemiol. 2003;32(1):1–22. doi: 10.1093/ije/dyg070 [DOI] [PubMed] [Google Scholar]
- 29.Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA. 2017;318(19):1925‐1926. doi: 10.1001/jama.2017.17219 [DOI] [PubMed] [Google Scholar]
- 30.Liu M, Park S. A causal relationship between vitamin C intake with hyperglycemia and metabolic syndrome risk: a two-sample Mendelian randomization study. Antioxidants. 2022;11(5):857. doi: 10.3390/antiox11050857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S. A causal and inverse relationship between plant-based diet intake and in a two-sample Mendelian randomization study. Foods. 2023;12(3):545. doi: 10.3390/foods12030545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Yu X, Wu X, Chai K, Wang S. Causal relationships between gut microbiota, immune cell, and Non-small cell lung cancer: a two-step, two-sample Mendelian randomization study. J Cancer. 2024;15(7):1890–1897. doi: 10.7150/jca.92699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Verbanck M, Chen CY, Neale B, Do R. Detection of widespread horizontal pleiotropy in causal relationships inferred from Mendelian randomization between complex traits and diseases. Nat Genet. 2018;50(8):1196. doi: 10.1038/s41588-018-0164-2 [DOI] [PubMed] [Google Scholar]
- 34.Yang X, Wei J, Sun L, et al. Causal relationship between iron status and preeclampsia-eclampsia: a Mendelian randomization analysis. Clin Exp Hypertens. 2024;46(1):2321148. doi: 10.1080/10641963.2024.2321148 [DOI] [PubMed] [Google Scholar]
- 35.Yuan Z, Kang Y, Mo C, et al. Causal relationship between gut microbiota and tuberculosis: a bidirectional two-sample Mendelian randomization analysis. Respir Res. 2024;25(1):16. doi: 10.1186/s12931-023-02652-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang MC, Lin A, Sang ZC, Ge L. Causality between atopic diseases and osteoarthritis: a Mendelian randomization study. Zhongguo Gu Shang. 2024;37(9):904–909. doi: 10.12200/j.issn.1003-0034.20230868 [DOI] [PubMed] [Google Scholar]
- 37.Keenan-Miller D, Hammen CL, Brennan PA. Health outcomes related to early adolescent depression. J Adolesc Health. 2007;41:256–262. doi: 10.1016/j.jadohealth.2007.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu HF, Hsu TL, Hung SH, Tseng YL, Liu CL, Wang TJ. Preoperative disability and its influencing factors in patients with lumbar spondylolisthesis. Hu Li Za Zhi. 2018;65(1):33–41. doi: 10.6224/JN.201802_65(1).06 [DOI] [PubMed] [Google Scholar]
- 39.Canales JZ, Cordás TA, Fiquer JT, Cavalcante AF, Moreno RA. Posture and body image in individuals with major depressive disorder: a controlled study. Rev Bras Psiquiatr. 2010;32:375–380. doi: 10.1590/S1516-44462010000400010 [DOI] [PubMed] [Google Scholar]
- 40.Rosario JL, Bezerra Diógenes MS, Mattei R, Leite JR. Differences similarities in postural alterations caused by sadness and depression. J Bodyw Mov Ther. 2014;14:540–544. doi: 10.1016/j.jbmt.2013.12.010 [DOI] [PubMed] [Google Scholar]
- 41.Kumaresan S, Yoganandan N, Pintar FA, Maiman DJ, Goel VK. Contribution of disc degeneration to osteophyte formation in the cervical spine: a biomechanical investigation. J Orthop Res. 2001;19:977–984. doi: 10.1016/S0736-0266(01)00010-9 [DOI] [PubMed] [Google Scholar]
- 42.Hall CA, Reynolds-Iii CF. Late-life depression in the primary care setting: challenges, collaborative care, and prevention. Maturitas. 2014;79(2):147–152. doi: 10.1016/j.maturitas.2014.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, Zhou Q, Pu X, et al. Association between vertebral endplate defects and patient-reported symptoms: an immunohistochemical study investigating the COX-2/PGE-2/EP-4 axis. Spine J. 2024;24(8):1407–1415. doi: 10.1016/j.spinee.2024.04.003 [DOI] [PubMed] [Google Scholar]
- 44.Kiecolt-Glaser JK, Derry HM, Fagundes CP. Inflammation: depression fans the flames and feasts on the heat. Am J Psychiatry. 2015;172(11):1075–1091. doi: 10.1176/appi.ajp.2015.15020152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Du X, Liu ZY, Tao XX, et al. Research progress on the pathogenesis of knee osteoarthritis. Orthop Surg. 2023;15(9):2213–2224. doi: 10.1111/os.13809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Johnston JN, Greenwald MS, Henter ID, et al. Inflammation, stress and depression: an exploration of ketamine’s therapeutic profile. Drug Discov Today. 2023;28(4):103518. doi: 10.1016/j.drudis.2023.103518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Al-Tamimi YZ, Guilfoyle M, Seeley H, Laing RJ. Measurement of long-term outcome in patients with cervical spondylotic myelopathy treated surgically. Eur Spine J. 2013;22:2552–2557. doi: 10.1007/s00586-013-2965-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Furlan JC, Catharine Craven B. Psychometric analysis and critical appraisal of the original, revised, and modified versions of the Japanese Orthopaedic Association score in the assessment of patients with cervical spondylotic myelopathy. Neurosurg Focus. 2016;40(6):E6. doi: 10.3171/2016.3.FOCUS1648 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Additional content can be accessed through IEU OpenGWAS online and the FINNGEN project online. The information in the paper pertains to the European IEU OpenGWAS online and FINNGEN project from 2018, with detailed analysis data available in the accompanying materials.