Abstract
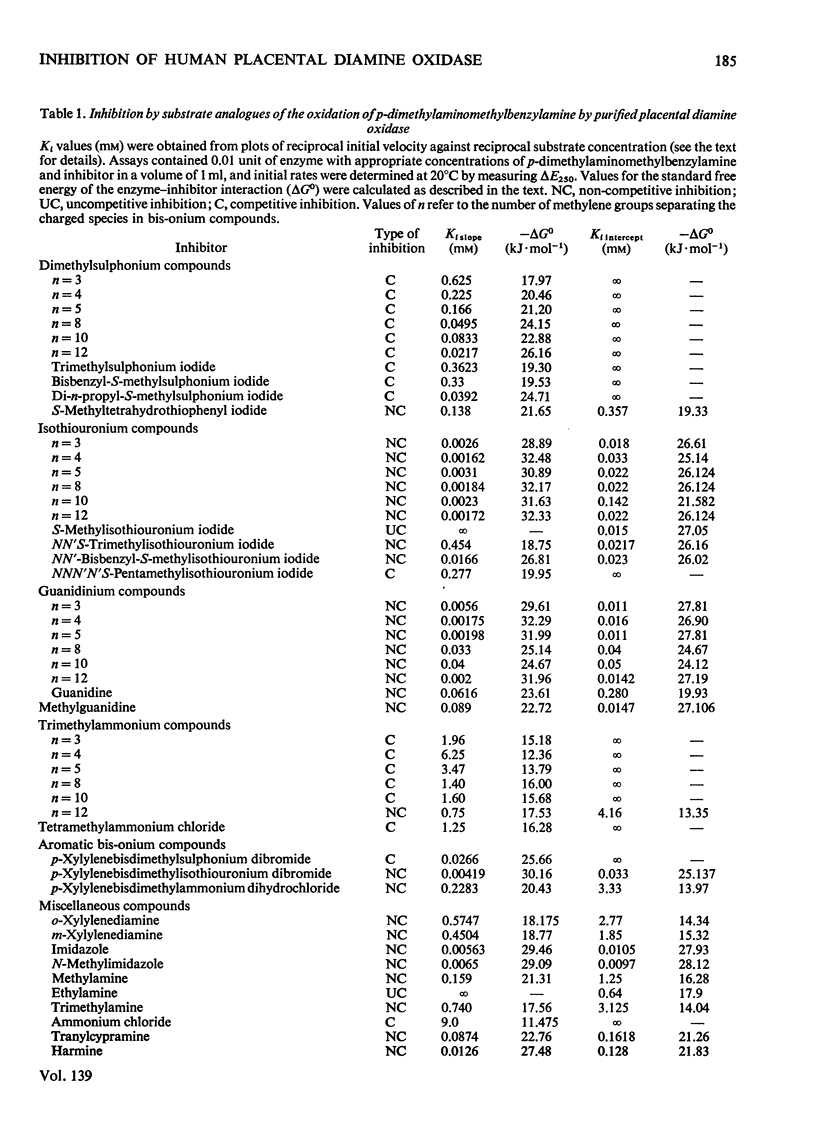
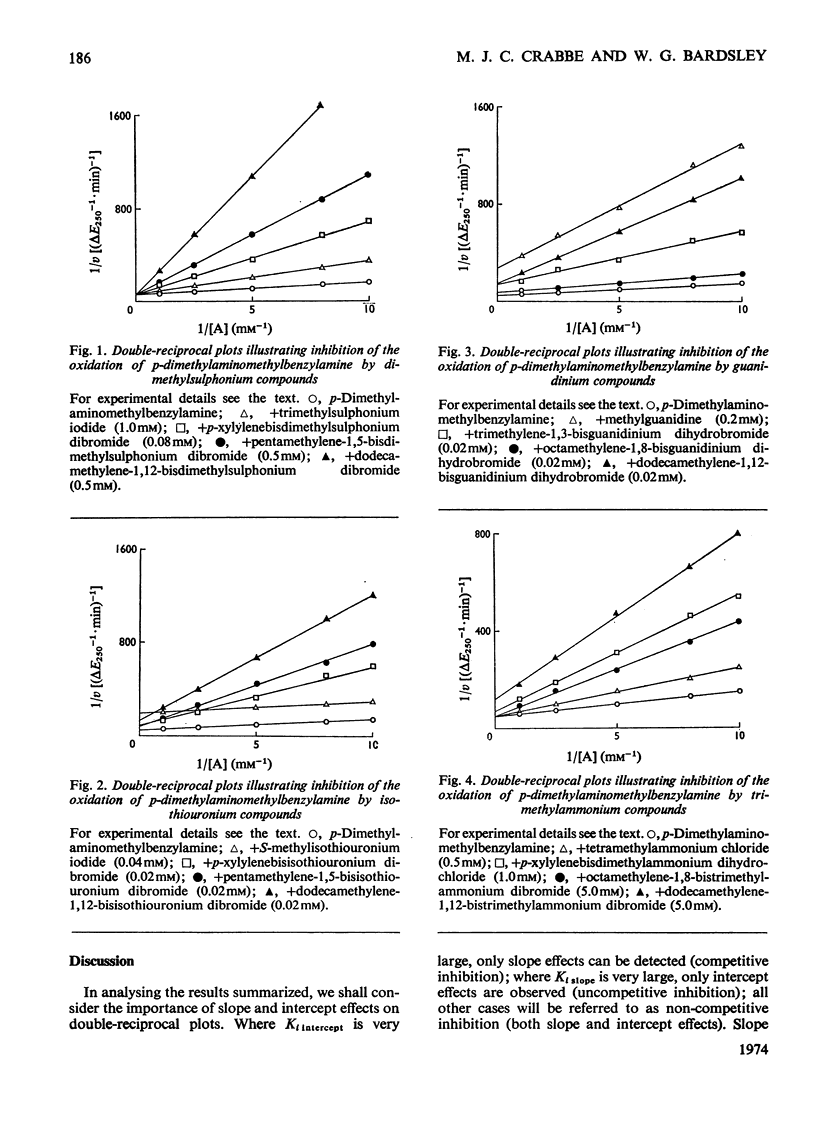
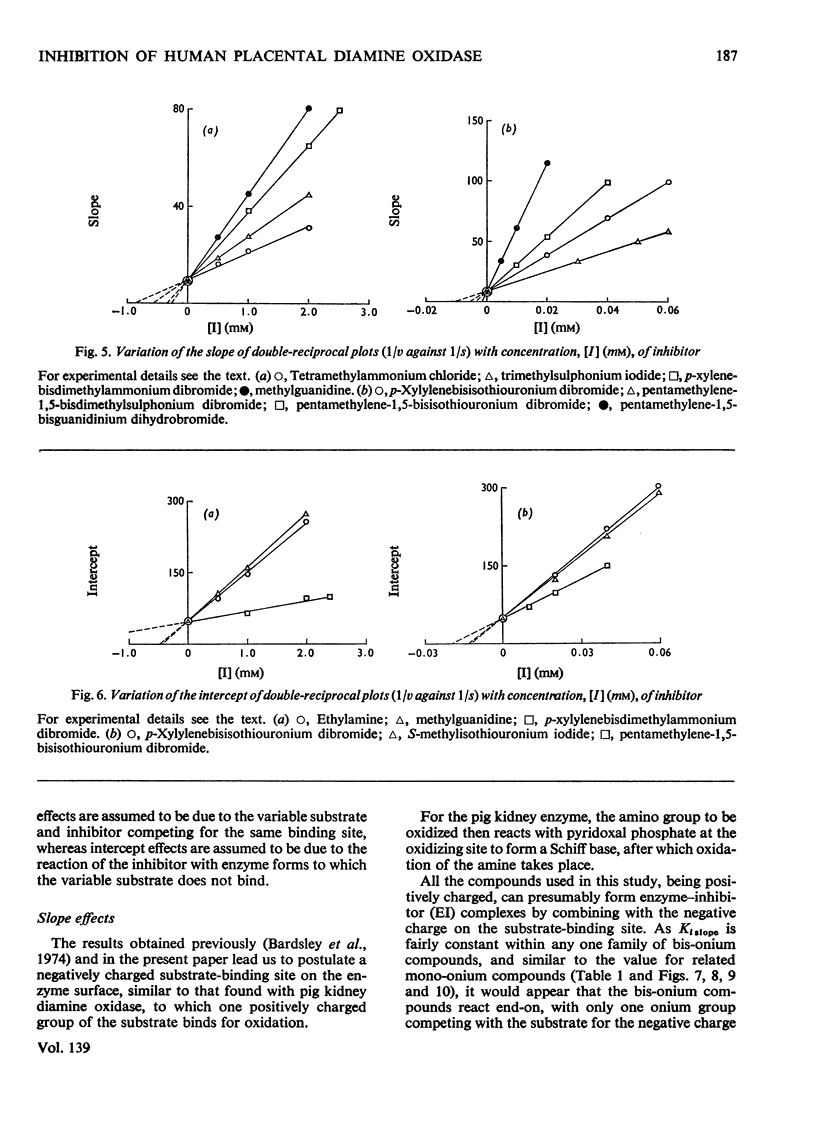
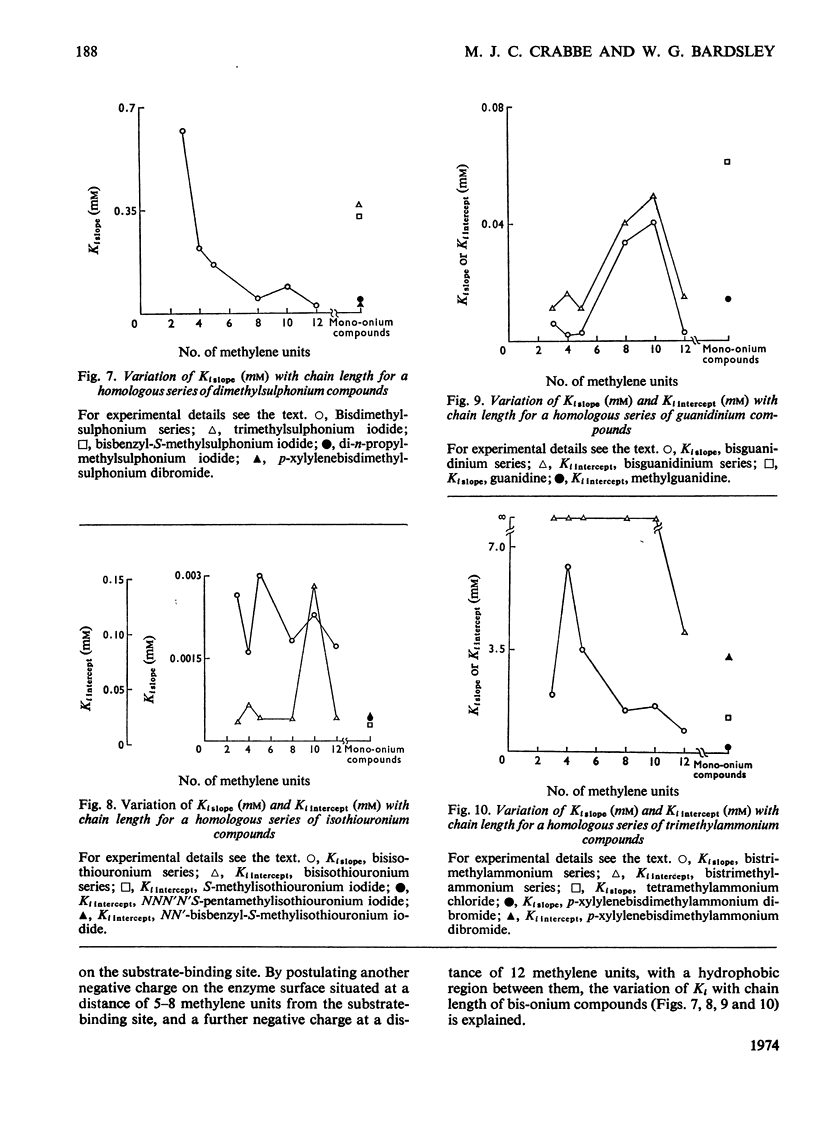
1. The oxidation of p-dimethylaminomethylbenzylamine by purified placental diamine oxidase was followed by measuring the change in E250 caused by the production of p-dimethylaminomethylbenzaldehyde. 2. The inhibition of this reaction by substrate analogues such as isothiouronium, guanidinium, dimethylsulphonium and trimethylammonium compounds was extensively studied. 3. The type and degree of inhibition by mono- and bis-onium compounds is described, and a theory is developed to explain the type of inhibition produced.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bardsley W. G., Ashford J. S., Hill C. M. Synthesis and oxidation of aminoalkyl-onium compounds by pig kidney diamine oxidase. Biochem J. 1971 May;122(4):557–567. doi: 10.1042/bj1220557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Ashford J. S. Inhibition of pig kidney diamine oxidase by substrate analogues. Biochem J. 1972 Jun;128(2):253–263. doi: 10.1042/bj1280253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Crabbe M. J., Shindler J. S., Ashford J. S. Oxidation of p-dimethylaminomethylbenzylamine by pig kidney diamine oxidase. A new method for spectrophotometric assay. Biochem J. 1972 May;127(5):875–879. doi: 10.1042/bj1270875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardsley W. G., Hill C. M., Lobley R. W. A reinvestigation of the substrate specificity of pig kidney diamine oxidase. Biochem J. 1970 Mar;117(1):169–176. doi: 10.1042/bj1170169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradsley W. G., Crabbe M. J., Scott I. V. The amine oxidases of human placenta and pregnancy plasma. Biochem J. 1974 Apr;139(1):169–181. doi: 10.1042/bj1390169. [DOI] [PMC free article] [PubMed] [Google Scholar]


