Abstract
Perennial ryegrass (Lolium perenne) is a widely cultivated forage and turf grass species. Salt stress can severely damage the growth of grass plants. The genome-wide molecular mechanisms of salt tolerance have not been well understood in perennial grass species. In this study, the salt sensitive genotype P1 (PI265351, Chile) and the salt tolerant genotype P2 (PI368892, Algeria) of perennial ryegrass were subjected to 200 mM NaCl, and transcriptomics and metabolomics analyses were performed. A total of 5,728 differentially expressed genes (DEGs) were identified through pairwise comparisons. Antioxidant enzyme encoding genes (LpSOD1, LpCAT1), ion channel gene LpCaC1 and transcription factors (LpERFs, LpHSF1 and LpMYB1) were significantly upregulated in P2, suggesting their involvement in regulating expression of salt-responsive genes for salt tolerance. Functional analysis of DEGs revealed that biosynthesis of secondary metabolites, carbohydrate metabolism and signal transduction were the main pathways in response to salt stress. Weighted gene co-expression network analysis (WGCNA) based on RNA-Seq data showed that membrane transport and ABC transporters were significantly correlated with salt tolerance-related traits. The combined transcriptomics and metabolomics analysis demonstrated that the phenylpropanoid biosynthesis pathway was a major secondary metabolic pathway in the salt response of perennial ryegrass. Especially, the tolerant genotype P2 had greater amounts of upregulated phenylpropanoids, flavonoids and anthocyanins and higher expressions of relevant genes in the pathway than the sensitive genotype P1, indicating a role of phenylpropanoid biosynthesis for perennial ryegrass to adapt to salt stress. The results provided insights into the molecular mechanisms of perennial ryegrass adaptation to salinity and laid a base for genetic improvement of salt tolerance in perennial grass species.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12870-024-05961-1.
Keywords: Perennial ryegrass, Salt tolerance, Transcriptomic profile, Metabolomic profile, Phenylpropanoid biosynthesis
Introduction
Soil salinization is a crucial environmental constraint that hinders the growth and development of plants. It is believed that approximately 6% of global terrestrial land has been subjected to primary salinization [1]. Additionally, approximately 20% of all cultivated land and up to 1/3 of irrigated land are salt-affected [2]. High salt in the soil decreases water and nutrient acquisition and causes osmotic imbalances, oxidative damage and ionic toxicity to plants [3]. Among them, the accumulation of excessive reactive oxygen species (ROS) such as superoxide anions (O2·−), hydrogen peroxide (H2O2), hydroxyl radicals (HO·), ozone, and singlet oxygen (1O2) can lead to oxidative damage to proteins, DNA, and lipids, and severely inhibit the metabolic processes of root cells [4]. Plants, including perennials, have evolved various intrinsic mechanisms and adaptive strategies to cope with long-term salt stress to a certain level [5–7]. These activities may include but are not limited to maintaining osmotic equilibrium, balancing ion concentrations, enhancing secondary metabolism, and modulating hormone levels and antioxidant enzyme activities. In physiological mechanisms, plants maintain ROS homeostasis through the regulation of both enzymatic and nonenzymatic antioxidative systems. Enzymes such as superoxide dismutase (SOD) and catalase (CAT) play crucial roles in scavenging O2·− and H2O2 under salt stress [8]. Additionally, nonenzymatic antioxidants, including glutathione, anthocyanins, and flavonoids, also maintain cellular stability by regulating cellular osmoregulation and clearing excess ROS to enhance plant salt tolerance [9, 10]. Moreover, salt stress significantly increased the expression of genes involved in phenylpropanoid and flavonoid synthesis, increased flavonoids content, and improved the activity of antioxidant enzymes, thus enhancing plant survival from the stress [11, 12]. Previous studies have identified a large number of genes related to salt stress in plant species using transcriptome analysis [13]. In poplar (Populus tomentosa) trees, HSFA5a module regulated flavonoid biosynthesis and controlled ROS accumulation, and thus regulating the root system to adapt to salt stress [14]. Additionally, transcription factors, such as MYB, bZIP, and WRKY, can directly regulate downstream genes in the phenylpropanoid biosynthesis and are involved in salt tolerance [15]. For example, foxtail millet (Setaria italica) SiMYB16 enhanced rice (Oryza sativa) salt tolerance by regulating phenylpropanoid biosynthesis, fatty acid elongation, phenylalanine metabolism, and flavonoid biosynthesis pathways [16]. Therefore, it is crucial to gain a better understanding of metabolic pathways in relation to salt tolerance in plants, especially in the perennial grass systems.
Many secondary metabolic pathways in plants are altered under abiotic stress. The metabolomics analysis showed that amino acid, sugar, plant hormone metabolism, phenylpropanoid and flavonoids biosynthesis contributed to salt tolerance of various plant species [17, 18]. It is known that plant hormones mediate salinity signals to regulate plant growth adaptation and the construction of a defense system. For example, abscisic acid (ABA) is involved in regulation of osmosis, ions, ROS under salt stress [19]. Brassinolide (BR) can increase the activity levels of selected hormones and antioxidant enzymes (SOD and CAT), promote the accumulation of proline and ions (K+, Ca2+, and Mg2+) in perennial ryegrass under salt stress [20]. To explore the key metabolites of salt tolerance and their molecular regulatory mechanisms, the integrated metabolome and transcriptome approaches have been used to investigate salt tolerance mechanisms in some plant species in recent years, including kiwifruit (Actinidia) [21], sorghum (Sorghum bicolor) [22], rice [23] and watermelon (Citrullus lanatus) [24], but such combined analysis has not been extensively used in perennial grass species. Most previous studies on perennial grasses have focused on the analysis of physiological responses, gene expression or pathways identified through a single omics approach [25, 26].
In perennial grass production, alternative water sources such as effluent recycled water may be used for irrigating plants for the purpose of water conservation, especially in an arid or semi-arid area. However, recycled water sources typically have a high soluble salt content, potentially leading to salt stress damage to plants over the time [27]. Perennial ryegrass (Lolium perenne) is a highly valuable cool-season grass species in temperate climates, due to its high forage quality and superior turfgrass appearance. Its widespread geographical distribution subjects to a range of environmental stressors, notably drought and salinity [28, 29]. Previous studies have shown that this species has significant natural variations of salt tolerance [28, 30, 31], making it a suitable material for elucidating physiological and molecular mechanisms of salt tolerance. In this study, the integrated transcriptomic and metabolomics analyses were performed on genotype P1 and P2 of perennial ryegrasses with different salt tolerance, and gene expression and metabolic pathways of roots in response to salt stress were examined. The results would provide a better understanding of salt tolerance mechanisms of perennial grass species.
Materials and methods
Plant materials and salt treatment
Two perennial ryegrass genotypes, salt sensitive PI265351 (abbreviation P1, from Chile) and salt tolerant PI368892 (abbreviation P2, from Algeria), were used as plant materials. Genotypes P1 and P2 were identified from our previous salt study consisting of globally collected germplasms [32]. The seeds of these genotype were obtained from United States Department of Agriculture’s National Plant Germplasm System (USDA-GRIN). Originated from a single seed, five to six tillers were propagated in pots containing sand in a greenhouse. The grasses were cut weekly to about 5–6 cm, irrigated twice a week with a 50 mL half-strength Hoagland solution (pH 6.5), and grown under average temperature of 22°C/18°C (day/night), a relative humidity of 70% and photosynthetically active radiation of approximately 300 µmol·m− 2·s− 1 for 14 h. Salt treatment was imposed after 10 weeks of seedling growth. For the non-salt control, seedlings of both P1 and P2 plants were irrigated with Hoagland solution, while salt treated plants were watered with the same nutrient solution amended with 200 mM NaCl. To avoid salt shock, the NaCl concentration was increased daily by 25, 50, 100, 150 and 200 mM NaCl. Previous study showed that this NaCl concentration for 10 days were adequate in separating salinity tolerance of genotypes including P1 and P2 [32]. Samples were collected at 10 days under salt stress and saved for transcriptomic and metabolomic analyzes. A randomized complete block design was used for this experiment, with three replicates for genotype P1 and P2 under the salt and control treatment, respectively. Six replicates were included in metabolomic analyzes and three for transcriptome sequencing. All samples were rapidly frozen and stored at -80 °C for further analysis.
Physiological and biochemical responses to salt stress
Physiological parameters and Na+ contents were determined after 10 d of 200 mM NaCl treatment. Plants were cut to 5–6 cm after reaching 200 mM NaCl, and plant height (PH) was determined by the difference at the time of 200 mM NaCl and 10 d after. Fresh leaves were harvested and dry wright (DW) was determined after tissue was dried in an oven at 65 °C for 3 days. Chlorophyll fluorescence (Fv/Fm) was determined by the Pocket PEA plant analyzer produced by Hansatech, UK. For the extraction of antioxidant enzyme, up to 0.2 g leaf samples were collected under NaCl stress and control, with three replicates for each treatment. The activity levels of superoxide dismutase (SOD) and catalase (CAT) were determined using commercially reagent kits (Nanjing Jian Cheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. For Na+ concentration of leaf tissue, the powder sample (0.5 g) was digested with H2SO4 and 30% H2O2 on a heating block at 200 °C for 30 min. The digested solutions were filtered after dilution with deionized water. The diluted extract was then used to determine the Na+ concentration by atomic absorption spectrometry (PerkinElmer Analyst 700; PerkinElmer, Norwalk, CT, USA).
Transcriptome sequencing and data analysis
The total RNA of roots was extracted using the Trizol method, and RNA integrity and concentration were evaluated using an Agilent 2100 Bioanalyzer (Agilent Technologies, USA). The libraries of perennial ryegrass were constructed using the NEBNext Ultra RNA Library Prep Kit (Illumina, NEB, USA). The constructed cDNA was sequenced using an Illumina HiSeq™. Low-quality reads were removed based on the conditions of containing only adaptors, with unknown nucleotides > 5%, or a Q20 score < 20%. Clean reads were mapped to the perennial ryegrass genome (https://ryegrassgenome.ghpc.au.dk/) [33], using HISAT2-2.1.0 with default settings [34]. The featureCounts was used to obtain raw counts [35], and reads per kilobase of exon per million reads (RPKM) were calculated to measure the expression levels of genes. The differentially expressed genes (DEGs) were identified when criteria of a |log2 Fold Change|>1 and an adjusted P-value < 0.05 were met. DEGs related to metabolism were identified on the basis of the Kyoto Encyclopedia of Genes and Genomes (KEGG) (www.kegg.jp).
Identification of antioxidant enzyme encoding genes and transcription factors
To identify the SOD and CAT family genes of perennial ryegrass, the amino acid sequences of 8 AtSODs (AT1G08830, AT2G28190, AT5G18100, AT4G25100, AT5G51100, AT5G23310, AT3G10920, and AT3G56350) and 3 AtCAT genes (AT1G20630, AT4G35090, and AT1G20620) were obtained from the TAIR Arabidopsis genome database (https://www.arabidopsis.org) and used as reference sequences to search the perennial ryegrass protein sequence using the BLASTP method with an e-value set to 1e-5. To identify transcription factors (TFs), the protein sequences of the DEGs of the perennial ryegrass were compared with the Plant Transcription Factor Database (PlantTFDB v 5.0, http://planttfdb.gao-lab.org/).
RT-qPCR analysis of salt-responsive genes
To verify the accuracy of RNA-seq, the candidate gene were selected and verified by qRT-PCR. Briefly, RNA reverse transcription of roots was performed using iScript™ cDNA (Bio-Rad Laboratories Inc.). The gene CDS sequences of perennial ryegrass were obtained from the perennial ryegrass genomics database and primers were designed using Primer BLAST software on the NCBI website (Table S1). Perennial ryegrass elongation factor 1-alpha (EU168438) was used as a housekeeping gene for internal control [36]. The qRT-PCR was completed in triplicate using the SYBR Green Real-time PCR Master Mix Plus (Toyobo, Osaka, Japan) and the ABI PRISM 7500 Real-Time PCR system (Applied Biosystems, Foster City, CA, USA). The relative gene expression level between the control and salinity stress treatment was calculated using the method of 2−ΔΔCt method.
Weighted gene co-expression network analysis (WGCNA)
The WGCNA package in R was used to analyze co-expression networks. After filtering, co-expression modules were constructed with 5699 genes with following parameters: the power of 16, unsigned TOMType, mergeCutHeight of 0.25, and minModuleSize of 20. The co-expression networks were constructed by using Cytoscape_3.3.0 software. The color modules were obtained based on phenotype data, and the correlation matrix between the gene module and the phenotype was generated with the WGCNA package.
Metabolite profiling analysis
The powder sample (0.5 g) of perennial ryegrass roots was transferred to 2.0 mL centrifuge tubes, and 1000 µL extraction solvent containing internal standard (methanol acetonitrile water volume ratio = 2:2:1) was added. The mixture was vortexed for 1 min and subjected to ultrasound for 10 min (in an ice water bath) for metabolite extraction. LC-MS analysis was performed using the Waters Acquity I-Class PLUS ultra-high performance liquid chromatography system coupled with the Waters Xevo G2-XS QTOF high-resolution mass spectrometer. The data analysis of LC-MS was performed by the platform BMKCloud (www.biocloud.net). VIP values obtained from OPLS-DA model and t test for normalized peak areas from different groups were used to screen differential accumulated metabolites (DAMs) with following criteria: screened based on VIP values > 1, fold change (FC) > 2, and P < 0.05. The significant differences among metabolites in KEGG pathway enrichment were determined using the hypergeometric distribution test.
Statistical analysis
Physiological and biochemical measurements were analyzed with three biological replicates. Statistical analyzes and data visualization were carried out using the rstatix and ggplot2 packages within the R software environment, and the significant differences between groups were determined based on the t-test.
Results
Salt stress on plant phenotypic and physiological parameters
The leaves of salt sensitive P1 showed noticeable withered and yellow compared to the salt tolerant genotype P2 at 10 d of 200 mM NaCl (Fig. 1a). The responses of physiological and biochemical parameters to salt stress were shown in Fig. 1b. Under 200 mM NaCl, the PH and DW decreased in both genotypes, but to a less extent in the tolerant P2. Furthermore, the genotype P2 showed a minor reduction of Fv/Fm and a sharp increase of leaf CAT and SOD activity than that in genotype P1, indicating that the genotype P2 had a stronger ability to eliminate reactive oxygen species than that in genotype P1. The leaf concentration of sodium ions (Na+) increased by 16.86 mg·g− 1 in P1 and 4.23 mg·g− 1 in P2 after salt treatment. All these results confirmed that the genotype P2 had better salt tolerance than the genotype P1.
Fig. 1.
Growth and physiological responses of perennial ryegrass genotypes P1 and P2 to 10 d of 200 mM NaCl. (a) Phenotype of two perennial ryegrass genotypes under control and salt stress. (b) Plant height, leaf dry weight, chlorophyll fluorescence (Fv/Fm), activities of catalase (CAT) and superoxide dismutase (SOD) and leaf Na+ concentration under control and salt stress. Three independent biological replicates were used to calculate the means. Error bars indicate the standard deviation (SD). Values indicate means ± SD. Student’s t-test was applied to verify the significant differences among treatments
Transcriptomic analysis of perennial ryegrass to salt stress
The transcriptome sequencing of each sample yielded 33,335,218–47,203,859 clean reads. Furthermore, the Quality Score of 30 (Q30) of each sample after filtering was greater than 89.98% (Table S2), indicating high quality of the RNA-seq data. A total of 2222 DEGs were identified in salt sensitive P1 and 3994 DEGs were found in salt tolerant P2 after salt stress, compared to the control (Table S3). In particular, P2 exhibited 1512 upregulated and 2977 downregulated genes, while the P1 had 710 upregulated and 1017 downregulated DEGs. Based on the intersection and divergence of these responses, a Venn diagram was constructed (Fig. 2a), which revealed a shared set of 518 DEGs between the two genotypes, along with 1704 exclusive DEGs in P1 and 3476 unique to P2.
Fig. 2.
Overview of transcriptome responses of salt-sensitive genotype P1 and salt-tolerant genotype P2 of perennial ryegrass to salt stress (200 mM NaCl). (a) Venn diagram showed all common differentially expressed genes (DEGs), up-regulated and down-regulated genes in genotypes P1 and P2 under salt stress compared with their controls, respectively. (b) The KEGG pathway enrichment scatter map based on differentially expressed genes in P1 and P2 under salt stress compared with their controls, respectively
For exploring the biological functions of DEGs, we labeled different numbers of DEGs in two genotypes in the KEGG database. The top 20 enriched metabolic pathways were identified (Fig. 2b). “Metabolism of terpenoids and polyketides”, “carbohydrate metabolism”, “MAPK signaling pathway-plant”, “environmental information processing” and “amino sugar and nucleotide sugar metabolism” were the five most significant pathways in genotype P1. “Phenylpropanoid biosynthesis”, “biosynthesis of secondary metabolites”, “chromosome and associated proteins”, “carbohydrate metabolism” and “glycosyltransferases” were the five most significant pathways in genotype P2. Notably, the phenylpropanoid biosynthesis pathway and biosynthesis of secondary metabolites were more enriched in salt-tolerant genotype P2.
Expression analysis of antioxidant enzyme encoding genes, ion channel genes and transcription factors in DEGs
Antioxidant enzyme activity of CAT and SOD was consistent to their encoding gene expression. We compared the expression patterns of antioxidant enzyme-encoding genes in genotype P1 and P2. The results showed that significant differences were observed in the expression levels of the SOD and CAT genes under NaCl stress between genotype P1 and P2 (Fig. 3). In genotype P2, four SOD genes and five CAT genes were induced by NaCl treatment, and their expression profiles were significantly increased. Among them, the expression levels of the LpSOD1 (V3.Lp_or1_0G6524) and LpSOD2 (V3.Lp_chr6_0G784) were significantly upregulated by 1.5-fold and 1.8-fold, respectively. However, in genotype P1, only two SOD genes showed a significant increase in expression (Fig. 3a). Interestingly, the expression of 5 CAT genes in P2 under NaCl stress was notably upregulated compared to P1 (Fig. 3b). The transcription levels of gene LpCAT1 (V3.Lp-chr6:0G2408) and LpCAT2 (V3.Lp-chr4_0G8600) and in genotype P2 under salt stress significantly increased by 7.2 and 2.2 fold, respectively. Based on KEGG enrichment analysis, ion channel metabolism was significantly enriched in P1. The expression analysis showed that six of these genes were significantly upregulated in P2. Notably, LpCaC(V3.Lp_chr3_0G14764), which encodes a calcium channel protein, exhibited a significant 2.6-fold upregulation in P2 (P < 0.0032) under salt stress conditions, whereas the expression difference in P1 was not significant. The qRT-PCR results showed that the gene LpSOD1 and LpCAT1 were significantly upregulated in genotype P2, but their expressions did not differ in genotype P1 (Figure S2).
Fig. 3.
The expression profile of superoxide dismutase genes (a), catalase genes (b), ion channel genes (c) and TFs (d) identified in genotype P1 and P2 under salt stress
TFs play a crucial role in regulating the biosynthesis of metabolites, thereby enhancing salt tolerance in plants. Among DEGs, we identified a total of 236 genes were as TFs, including 21 families such as ERF, bHLH, MYB, NAC, bZIP, C2H2, GRAS, etc. (Fig. S1a). Of them, the ERF, bHLH, and MYB families contained more than 30 genes of DEGs (Figure S1b). More importantly, a total of 50 genes were significantly upregulated in either genotype P1 or P2 under salt stress conditions (Fig. 3c). Notably, among these genes, 8 DEGs were annotated as bHLH and ERF, 6 as GLK, 5 as NAC, 4 as MYB, and 3 as bZIP. It was worth mentioned that 25 TFs were significantly upregulated in genotype P2, whereas 11 TFs were exclusively and significantly upregulated in genotype P1 (Fig. 3c). LpERF genes (V3.Lpuchr1_0G13836, V3. Lpuchr3_0G5148, V3.Lpuchr6_0G14952), LpGLK (V3.Lpuchr2_0G12658), LpbZIP1 (V3.Lpuchr3_0G20456), LpHSF1 (V3.Lpuchr3_0G12552), and LpMYB1 (V3.Lpuchr3_0G26020) had the highest expression level in genotype P2 under salt stress, with an average RPKM value of 241.64. The qRT-PCR results showed that the gene LpMYB1, LpbZIP1 and three LpERF genes were induced by salt stress in P1 and P2 (Fig. S2). These results indicated that the number and types of upregulated antioxidant enzyme encoding genes and TFs in salt-tolerant P2 were significantly higher than those in salt-sensitive P1.
WGCNA on salt stress responses
WGCNA revealed that a total of 5699 genes were integrated into 25 modules (Fig. 4a, b). Among these modules, three were positively correlated with enzyme activity, including module honeydew1, magenta, and darkred. The honeydew1 module contained only a few genes, while 80% of the genes in the darkred module were significantly upregulated in genotype P1. The magenta module had a significantly negative correlation with PH and DW and was positively correlated with SOD activity in leaves (Fig. 4c). The magenta module contained 120 genes, some of which were upregulated under salt stress and expressed at higher levels in the salt-tolerant P2 than in the sensitive P1 (Fig. 4d). This indicates that the key genes of this module play an important role in the response of perennial ryegrass to salt stress. Moreover, KEGG analysis was performed on the genes in the magenta module, and the results showed that these genes were mainly enriched in membrane transport, ABC transporters, and fructose and mannose metabolism (Fig. 4e). Furthermore, the co-expression network showed that there were 8 hub genes were identified according to degree, clustering coefficient, neighborhood Connectivity, stress and betweenness centrality analysis. Specifically, six genes were upregulated by 4.3 folds in response to salt stress in the genotype P2. Notably, V3.Lp_chr1_0G12130, which encodes reticuline oxidase precursor, exhibited a 13-fold downregulation in P2, whereas it decreased by 1.9 folds in P1.
Fig. 4.
Weighted gene co-expression network analysis of genes related to salt stress (200 mM NaCl) in perennial ryegrass. (a) Clustering dendrogram of genes and module division. (b) The number of differentially expressed genes in each module. (c) Correlations between modules and physiological parameters. (d) Gene expression heatmap of magenta module. (e) KEGG enrichment analysis of genes in magenta module. (f) Degree_top50 gene regulatory network analysis of magenta module
Metabonomic analysis on the salt stress response
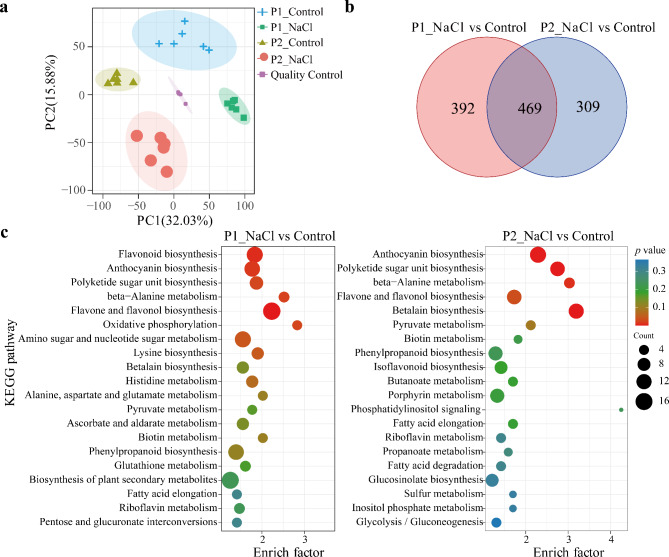
According to non-targeted metabolomic profiling, a total of 3311 metabolomic substances were detected, including 1631 positive and 1680 negative ion modes. PCA well separated the control and salt-treated samples and between genotypes P1 and P2 (Fig. 5a). Specifically, there were 599 and 606 up-regulated and 262 and 172 down-regulated metabolites in P1 and P2 after salt treatment, respectively (Table S4). The intersection analysis revealed a total of 469 salt-responsive metabolites across the P1 and P2. Additionally, there were 392 metabolites in P1 and 309 in P2 that had close associations with salt stress (Fig. 5b). The top 20 enriched metabolic pathways were shown in Fig. 5c. The highly enriched DAMs were involved mainly in “anthocyanin biosynthesis”, “polyketide sugar unit biosynthesis”, “beta-alanine metabolism”, “flavone and flavonol biosynthesis”, “betalain biosynthesis”, “pyruvate metabolism” and “biotin metabolism”.
Fig. 5.
Metabolomics responses of two perennial ryegrass genotypes P1 and P2 to salt stress (200 mM NaCl). (a) PCA clustering under the control and salt stress. (b) Venn diagram showing s all common and specific differentially accumulated metabolites in genotype P1 and P2 under salt stress. (c) The KEGG pathway enrichment scatter map based on differentially accumulated metabolites in P1 and P2 under salt stress
Genes and metabolites involved in the phenylpropanoid and flavonoid biosynthesis pathways
Transcriptome and metabolome KEGG enrichment analysis showed that the biosynthetic pathways of anthocyanins, flavonoids, and flavonols were significantly enriched under salt stress, and these pathways were all key components of the phenylpropanoid pathway. To unravel the phenylpropanoid pathway’s regulatory network under salt stress, we constructed a DEG-DAM regulatory network diagram for genotypes P1 and P2 of perennial ryegrass (Fig. 6), and found that 29 DEGs were significantly correlated with 9 DAMs in the phenylpropanoid pathway. Phenylalanine, a precursor for phenylpropanoid biosynthesis, is regulated by enzymes PAL, C4H, and 4CL. Up-regulation of two PALs, two C4Hs and four 4CLs were observed, but their expression levels were higher in genotype P2 than P1 under salt stress. COMT is involved in the synthesis of ferulic acid, towards the accumulation of sinapic acid. Two COMTs were up-regulated, but to a higher extent in genotype P2 than in P1 after salt stress (Fig. 6). CAD is the key enzyme involved in lignin biosynthesis. Higher expressions of one and two CAD genes were found in genotypes P1 and P2 after salt stress, respectively (Fig. 6). In the synthesis pathway of p-coumaroyl-CoA to eugenol, salt stress significantly induced expression of metabolites in P2, with the amount of increase being higher than that in P1. The results clearly indicated that the salt tolerant genotype P2 could strongly activate phenylpropanoid pathway, thus contributing to salt tolerance.
Fig. 6.
Phenylpropanoid, flavonoid and anthocyanin biosynthesis network diagram in salt sensitive genotype P1 and salt tolerant genotype P2 of perennial ryegrass. The circles and boxes at the bottom show the heatmap of expression of key metabolites and transcripts in different samples. Blue indicates low expression and red high expression. PAL, phenylalanine ammonialyase; C4H, cinnamate 4-hydroxylase; 4CL, 4-coumaroyl CoA ligase; CCR, cinnamoyl-CoA reductase; CAD, cinnamyl-alcoholdehy drogenase; COMT, caffeic acid 3-O-methyltransferase; EGS1, eugenol synthase; CHI, chalcone isomerase; DFR, dihydroflavonol-4-reducatse; F3H, flavanone 3 β-hydroxylase; FLS, flavonol synthase; FG3, flavonol-3-O-glucoside/galactoside glucosyltransferase; F3’H, flavonoid 3’-monooxygenase; BZ1, anthocyanidin 3-O-glucosyltransferase
There were 23 DEGs significantly correlated with 6 DAMs in the flavonoid and anthocyanin pathways. CHI, FLS and F3H are considered the key enzymes that control flavonoids biosynthesis. Higher expression levels of three CHIs, three F3Hs, two FLSs, two F3’H and three FG3 were found in genotype P2 (Fig. 6). Specifically, qRT-PCR results indicated that the expression levels of the LpCAD1 (V3.Lp_chr4_0G11100), LpFLS1 (V3.Lp_chr7_0.1G4668), and LpHSF1 (V3.Lp_chr3_0G12552) genes significantly increased under salt stress (Fig. S2). The metabolomics screening showed that the amount of three DAMs (sophoraflavonoloside, 3-O-β-D-sophorotrioside, 3,7-O-Dimethylquercetin) in the secondary metabolism of flavonoids and three DAMs (delphinidin 3-glucoside, pelargonidin 3-rutinoside 5-glucoside and leucocyanidin) in the anthocyanin biosynthesis pathway were significantly higher in genotype P2 than in genotype P1.
Discussion
The global issues of soil salinization are projected to cause decline of soil quality. The ever-expanding problem of soil salinization poises detrimental effects on plant growth, development, and production [37]. In this study, decreases in plant height, leaf dry weight and Fv/Fm indicated negative effects of salt on plant growth and photosynthetic activity. At cellular level, salt stress increases production of ROS that can cause oxidative damage to the cell. The enhancement of the antioxidant defense systems such as antioxidant compounds and several antioxidant enzymes can contribute to plant salt tolerance [38, 39]. Among the antioxidant enzyme systems, SOD and CAT are important members that remove O2·− and H2O2, thus playing a crucial role in the cellular defense mechanism [40]. In this study, we found that SOD and CAT activities increased significantly after salt stress, but to much higher extent in the salt-tolerant genotype P2, indicating that the salt tolerant genotype had a greater ability to scavenge ROS. Moreover, our results showed that growth changes of plants were consistent with physiological alterations, which provides a good basis for studying molecular mechanisms of salt tolerance in perennial ryegrass.
The regulatory mechanisms of salt responses are complex in plants [41, 42]. Previous works demonstrated that the intensified biosynthesis of specific secondary metabolites under salt stress, such as flavones, anthocyanins, phenolics, and certain phenolic acids, can protect cells from oxidative damage by binding the toxic ions [43]. In this study, through the transcriptomic and metabolomics approaches, we found more number of differentially expressed genes in salt tolerant genotype P2 compared with salt sensitive genotype P1. Meanwhile, through KEGG enrichment analysis of DEGs, we identified several important and enriched pathways, such as “biosynthesis of secondary metabolites”, phenylpropanoid biosynthesis”, “flavonoid biosynthesis”, “carbohydrate metabolism”, and “signal transduction” under salt stress. The results were consistent with previous works in other plant species [12, 18, 44]. Among these pathways, enrichments of “betalain biosynthesis”, “pyruvate metabolism”, “biotin metabolism”, “beta-alanine metabolism”, and “anthocyanin biosynthesis” were specifically noted in P2. Notably, pyruvate, the end product of glycolysis, plays an important role in bolstering energy supplies and facilitating the cellular adjustments necessary for salt tolerance in plants [21], and pyruvate metabolism was also shown in P2. These findings provided a wealth of metabolic pathways to further unravel the salt tolerance mechanisms in perennial ryegrass.
Salt stress enhanced root lignification in different plant species, increasing the mechanical strength of the cell walls [45]. This process limiting the entry of Na+ to the root xylem, subsequently facilitating the salt tolerance of plant roots [46, 47]. Phenylpropanoid metabolism can generate polymer lignin [48]. Phenylpropanoids are secondary metabolites that play an active role in plant growth and response to stress [49]. Beginning with phenylalanine, the phenylpropanoid biosynthesis pathway is a major secondary metabolic pathway in plants, involving the enzymes of PAL, C4H, and 4CL and yielding cinnamic acid, p-hydroxy coumaric acid, and 4-coumaric acid coenzyme A, respectively. This is necessary for the downstream pathways to produce different phenylpropanoid metabolites, including flavonoids, lignans, terpenoids, coumarins, anthocyanins and other specialized metabolites [50]. In this study, DEGs of the tolerant genotype P2 were highly significantly enriched in the phenylpropanoid pathway, and DAMs of genotypes P1 and P2 were also enriched in this pathway (Figs. 2b and 5c and d). Furthermore, the combined transcriptomic and metabolomics analyses showed that P2 had stronger induction of phenylpropanoids and flavonoids pathways under salt stress. We noted that some genes involved in phenylpropanoid biosynthesis and respective metabolites were significantly up-regulated under salt stress, especially in the tolerant genotype P2 (Fig. 6). The results implied that salt tolerance of perennial ryegrass was acquired via enhancing phenylpropanoid synthesis. Increased expression of genes involved in the phenylpropanoid pathway was likewise detected in roots of plant species under salt stress, including Arabidopsis, tomato (Solanum lycopersicum) and barley (Hordeum vulgare) [18, 51, 52].
Flavonoids react to environmental stresses and have excellent ROS scavenging ability. They help plants resist cold, saline-alkali, and other stresses [53]. In the flavonoid biosynthesis pathway, we detected significant upregulation of LpCHI1, LpFLS1, and LpFG3 genes in P2 (Fig. 6). Chalcone isomerase (CHI) is a key enzyme involved in controlling the synthesis of flavonoids. Under salt stress conditions, the expression levels of salt-stress-related genes in soybean (Glycine max) such as GmSOD1, GmAPX1, GmSOS1, and GmNHX1 were significantly upregulated in hairy roots overexpressing GmCHI4. Additionally, the content of isoflavones was notably increased, which had a beneficial impact on the salt tolerance and isoflavone content of soybeans [54]. Overexpression of MsFLS13 in alfalfa (Medicago sativa) promoted plant tolerance to saline-alkali stress by enhancing flavonol accumulation, antioxidant capacity, osmotic balance, and photosynthetic efficiency [55]. In addition, the upregulation of metabolite 3,7-O-dimethylquercetin, sophoraflavonoloside and 3-O - β - D-sophoricoside in P2 was significantly higher than that in P1. Multiple studies have reported that the accumulation of anthocyanins can also enhance plant tolerance to abiotic stresses, including salt stress [56–58]. Collectively, these findings demonstrated that the phenylpropanoid and flavonoid pathways were pivotal contributors to the salt tolerance mechanisms in perennial ryegrass. Especially, the enhanced accumulation of flavonoid in the genotype P2 suggested that the change of this specific metabolite could be a promising indicator of enhanced salt tolerance in perennial turf and forage grass species.
Conclusion
The molecular response mechanisms of plants to salt stress are complex. In this study, we analyzed the metabolomic and transcriptomic responses of genotype P1 (salt-sensitive) and P2 (salt-tolerant) of perennial ryegrass under salt stress conditions. Functional enrichment analysis of DEGs and DAMs demonstrated that biosynthesis of secondary metabolites, carbohydrate metabolism and signal transduction were the main pathways to respond to salt stress. Notably, the tolerant genotype P2 had greater amounts of upregulated phenylpropanoids, flavonoids and anthocyanins and higher expressions of relevant genes in this pathway than the sensitive genotype P1. Our results contributed to a better understanding of the complex regulatory mechanisms of grass plants in response to salinity and also provided important information for molecular breeding to produce salt-tolerant perennial grass varieties. Future work can be conducted to validate the key metabolites and regulatory genes of phenylpropanoid pathway involved in salt tolerance, to explore the possibility in using these biomarkers to improve the efficiency of early selection of salt tolerant varieties.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Supplementary Table S1. Sequences of primers used for qRT-PCR. Table S2. Quality of the transcriptome sequencing data. Table S3. Number of differential expression genes in different comparisons. Table S4. Number of differential accumulated metabolites in different comparisons.
Supplementary Fig. S1. Clustering heat map (a) and number (b) of transcription factors (TFs) identified in genotype P1 and P2 under salt stress. Fig. S2. qRT-PCR verification of DEGs. The relative gene expression levels after 10 days of treatment with 200 mM NaCl. Error bars indicate mean ± SD based on three biological replicates.
Acknowledgements
Not applicable.
Abbreviations
- Na+
Sodium Ions
- PH
Plant Height
- DW
Dry Wright
- Fv/Fm
Chlorophyll fluorescence
- SOD
Superoxide Dismutase
- CAT
Catalase
- Q20/30
Quality Score of 30/20
- DEGs
Differently Expressed Genes
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- WGCNA
Weighted gene co-expression network analysis
- RPKM
Reads per Kilobase of Exon per Million Reads
- FC
Fold Change
- DAMs
Differential Accumulated Metabolites
Author contributions
YHC: Writing–original draft, Visualization, Methodology, Investigation, Conceptualization. ZLL: Methodology, Formal analysis. YHL: Methodology, Formal analysis. YJ: Writing – review & editing, Methodology, Conceptualization. JLZ: Writing – review & editing, Methodology, Funding acquisition, Formal analysis, Conceptualization. All authors read and approved the manuscript.
Funding
This work was supported by the China Central Government-Guided Local Science and Technology Development Project (23ZYQA291), the Science & Technology Project from China Huaneng Group Co. LTD. (HNKJ21-H76) and the Key Science & Technology Project of Gansu Province (22ZD6NA008).
Data availability
The datasets and materials supporting the research findings of this article are presented within the manuscript and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yiwei Jiang, Email: yjiang@bjfu.edu.cn.
Jin-Lin Zhang, Email: jlzhang@lzu.edu.cn.
References
- 1.Yang Y, Guo Y. Elucidating the molecular mechanisms mediating plant salt-stress responses. New Phytol. 2018;217(2):523–39. [DOI] [PubMed] [Google Scholar]
- 2.Hopmans JW, Qureshi A, Kisekka I, Munns R, Grattan S, Rengasamy P, Ben-Gal A, Assouline S, Javaux M, Minhas P. Critical knowledge gaps and research priorities in global soil salinity. Adv Agron. 2021;169:1–191. [Google Scholar]
- 3.Zhang Y, Li D, Zhou R, Wang X, Dossa K, Wang L, Zhang Y, Yu J, Gong H, Zhang X. Transcriptome and metabolome analyses of two contrasting sesame genotypes reveal the crucial biological pathways involved in rapid adaptive response to salt stress. BMC Plant Biol. 2019;19:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miller G, Suzuki N, Ciftci-Yilmaz S, Mittler R. Reactive oxygen species homeostasis and signalling during drought and salinity stresses. Plant Cell Environ. 2010;33(4):453–67. [DOI] [PubMed] [Google Scholar]
- 5.Gong Z. Plant abiotic stress: New insights into the factors that activate and modulate plant responses. J Integr Plant Biol. 2021;63(3):429. [DOI] [PubMed] [Google Scholar]
- 6.Ismail AM, Horie T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu Rev Plant Biol. 2017;68(1):405–34. [DOI] [PubMed] [Google Scholar]
- 7.Afzal M, Hindawi SES, Alghamdi SS, Migdadi HH, Khan MA, Hasnain MU, Arslan M, Habib ur Rahman M, Sohaib M. Potential breeding strategies for improving salt tolerance in crop plants. J Plant Growth Regul. 2023;42(6):3365–87. [Google Scholar]
- 8.van Zelm E, Zhang Y, Testerink C. Salt tolerance mechanisms of plants. Annu Rev Plant Biol. 2020;71:403–33. [DOI] [PubMed] [Google Scholar]
- 9.Naing AH, Kim CK. Abiotic stress-induced anthocyanins in plants: their role in tolerance to abiotic stresses. Physiol Plant. 2021;172(3):1711–23. [DOI] [PubMed] [Google Scholar]
- 10.Arif Y, Singh P, Siddiqui H, Bajguz A, Hayat S. Salinity induced physiological and biochemical changes in plants: an omic approach towards salt stress tolerance. Plant Physiol Biochem. 2020;156:64–77. [DOI] [PubMed] [Google Scholar]
- 11.Zhang L, Zhang Z, Fang S, Liu Y, Shang X. Integrative analysis of metabolome and transcriptome reveals molecular regulatory mechanism of flavonoid biosynthesis in Cyclocarya paliurus under salt stress. Ind Crop Prod. 2021;170:113823. [Google Scholar]
- 12.Zhu Y, Wang Q, Wang Y, Xu Y, Li J, Zhao S, Wang D, Ma Z, Yan F, Liu Y. Combined transcriptomic and metabolomic analysis reveals the role of phenylpropanoid biosynthesis pathway in the salt tolerance process of Sophora alopecuroides. Int J Mol Sci. 2021;22(5):2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu Z, Chen X, Lu X, Zhao B, Yang Y, Liu J. Integrative analysis of transcriptome and metabolome reveal mechanism of tolerance to salt stress in oat (Avena sativa L). Plant Physiol Biochem. 2021;160:315–28. [DOI] [PubMed] [Google Scholar]
- 14.Song Q, He F, Kong L, Yang J, Wang X, Zhao Z, Zhang Y, Xu C, Fan C, Luo K. The IAA17.1/HSFA5a module enhances salt tolerance in populus tomentosa by regulating flavonol biosynthesis and ROS levels in lateral roots. New Phytol. 2024;241(2):592–606. [DOI] [PubMed] [Google Scholar]
- 15.Fernando VD. Major transcription factor families involved in salinity stress tolerance in plants. In: Edited by Wani SH, Ed., Transcription factors for abiotic stress tolerance in plants. Cambridge, MA, USA: Academic; 2020: pp. 99–109.
- 16.Yu Y, Guo DD, Min DH, Cao T, Ning L, Jiang QY, Sun XJ, Zhang H, Tang WS, Gao SQ, et al. Foxtail millet MYB-like transcription factor SiMYB16 confers salt tolerance in transgenic rice by regulating phenylpropane pathway. Plant Physiol Biochem. 2023;195:310–21. [DOI] [PubMed] [Google Scholar]
- 17.Cao B-l, Li N, Xu K. Crosstalk of phenylpropanoid biosynthesis with hormone signaling in Chinese cabbage is key to counteracting salt stress. Environ Exp Bot. 2020;179:104209. [Google Scholar]
- 18.Jia C, Guo B, Wang B, Li X, Yang T, Li N, Wang J, Yu Q. Integrated metabolomic and transcriptomic analysis reveals the role of phenylpropanoid biosynthesis pathway in tomato roots during salt stress. Front Plant Sci. 2022;13:1023696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu Z, Duan X, Luo L, Dai S, Ding Z, Xia G. How plant hormones mediate salt stress responses. Trends Plant Sci. 2020;25(11):1117–30. [DOI] [PubMed] [Google Scholar]
- 20.Wu W, Zhang Q, Ervin EH, Yang Z, Zhang X. Physiological mechanism of enhancing salt stress tolerance of perennial ryegrass by 24-epibrassinolide. Front plant Sci. 2017;8:1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Abid M, Gu S, Zhang Y-J, Sun S, Li Z, Bai D-F, Sun L, Qi X-J, Zhong Y-P, Fang J-B. Comparative transcriptome and metabolome analysis reveal key regulatory defense networks and genes involved in enhanced salt tolerance of Actinidia (kiwifruit). Hortic Res. 2022;9:uhac189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang F, Lu F, Wang Y, Zhang Z, Wang J, Zhang K, Wu H, Zou J, Duan Y, Ke F, et al. Combined transcriptomic and physiological metabolomic analyses elucidate key biological pathways in the response of two sorghum genotypes to salinity stress. Front Plant Sci. 2022;13:880373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Huang L, Du F, Wang J, Zhao X, Li Z, Wang W, Xu J, Fu B. Comparative transcriptome and metabolome profiling reveal molecular mechanisms underlying OsDRAP1-mediated salt tolerance in rice. Sci Rep. 2021;11(1):5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Zhang W, Elango D, Liu H, Jin D, Wang X, Wu Y. Metabolome and transcriptome analysis reveals molecular mechanisms of watermelon under salt stress. Environ Exp Bot. 2023;206:105200. [Google Scholar]
- 25.Xu H-S, Guo S-M, Zhu L, Xing J-C. Growth, physiological and transcriptomic analysis of the perennial ryegrass Lolium perenne in response to saline stress. R Soc Open Sci. 2020;7(7):200637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng X, Yu D, Yan J, Zhang N, Lin J, Wang J. Physiological and proteomic analyses reveal adaptive mechanisms of ryegrass (annual vs. perennial) seedlings to salt stress. Agronomy. 2019;9(12):843. [Google Scholar]
- 27.Qian YL, Mecham B. Long-term effects of recycled wastewater irrigation on soil chemical properties on golf course fairways. Agron J. 2005;97(3):717–21. [Google Scholar]
- 28.Tang J, Yu X, Luo N, Xiao F, Camberato JJ, Jiang Y. Natural variation of salinity response, population structure and candidate genes associated with salinity tolerance in perennial ryegrass accessions. Plant Cell Environ. 2013;36(11):2021–33. [DOI] [PubMed] [Google Scholar]
- 29.Miao C, Zhang Y, Bai X, Qin T. Insights into the response of perennial ryegrass to abiotic stress: underlying survival strategies and adaptation mechanisms. Life. 2022;12(6):860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang J, Camberato JJ, Yu X, Luo N, Bian S, Jiang Y. Growth response, carbohydrate and ion accumulation of diverse perennial ryegrass accessions to increasing salinity. Sci Hortic-Amsterdam. 2013;154:73–81. [Google Scholar]
- 31.Song X, Wang S-m, Jiang Y. Genotypic variations in plant growth and nutritional elements of perennial ryegrass accessions under salinity stress. J Am Soc Hort Sci. 2017;142(6):476–83. [Google Scholar]
- 32.Cao Y-H, Zhao X-W, Nie G, Wang Z-Y, Song X, Zhang M-X, Hu J-P, Zhao Q, Jiang Y, Zhang J-L. The salt-tolerance of perennial ryegrass is linked with root exudate profiles and microflora recruitment. Sci Total Environ. 2024;916:170205. [DOI] [PubMed] [Google Scholar]
- 33.Nagy I, Veeckman E, Liu C, Bel MV, Vandepoele K, Jensen CS, Ruttink T, Asp T. Chromosome-scale assembly and annotation of the perennial ryegrass genome. BMC Genomics. 2022;23(1):505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim D, Langmead B, Salzberg SL. HISAT: a fast spliced aligner with low memory requirements. Nat Methods. 2015;12(4):357–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao Y, Smyth GK, Shi W. featureCounts: an efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics. 2014;30(7):923–30. [DOI] [PubMed] [Google Scholar]
- 36.Liu M, Song X, Jiang Y. Growth, ionic response, and gene expression of shoots and roots of perennial ryegrass under salinity stress. Acta Physio Plant. 2018;40:1–8. [Google Scholar]
- 37.Chele KH, Tinte MM, Piater LA, Dubery IA, Tugizimana F. Soil salinity, a serious environmental issue and plant responses: a metabolomics perspective. Metabolites. 2021;11(11):724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seckin B, Turkan I, Sekmen AH, Ozfidan C. The role of antioxidant defense systems at differential salt tolerance of Hordeum marinum Huds. (sea barleygrass) and Hordeum vulgare L. (cultivated barley). Environ Exp Bot. 2010;69(1):76–85. [Google Scholar]
- 39.Waseem M, Rong X, Li Z. Dissecting the role of a basic Helix-Loop-Helix transcription factor, SlbHLH22, under salt and drought stresses in transgenic Solanum lycopersicum L. Front Plant Sci. 2019;10:734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garcia-Caparros P, De Filippis L, Gul A, Hasanuzzaman M, Ozturk M, Altay V, Lao MT. Oxidative stress and antioxidant metabolism under adverse environmental conditions: a review. Bot Rev. 2021;87:421–66. [Google Scholar]
- 41.Ma L, Liu X, Lv W, Yang Y. Molecular mechanisms of plant responses to salt stress. Front Plant Sci. 2022;13:934877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu J-K. Abiotic stress signaling and responses in plants. Cell. 2016;167(2):313–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gengmao Z, Yu H, Xing S, Shihui L, Quanmei S, Changhai W. Salinity stress increases secondary metabolites and enzyme activity in safflower. Ind Crop Prod. 2015;64:175–81. [Google Scholar]
- 44.Zhang X, Han C, Liang Y, Yang Y, Liu Y, Cao Y. Combined full-length transcriptomic and metabolomic analysis reveals the regulatory mechanisms of adaptation to salt stress in asparagus. Front Plant Sci. 2022;13:1050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Oliveira DM, Mota TR, Salatta FV, Sinzker RC, Končitíková R, Kopečný D, Simister R, Silva M, Goeminne G, Morreel K. Cell wall remodeling under salt stress: insights into changes in polysaccharides, feruloylation, lignification, and phenolic metabolism in maize. Plant Cell Environ. 2020;43(9):2172–91. [DOI] [PubMed] [Google Scholar]
- 46.Zou Y, Zhang Y, Testerink C. Root dynamic growth strategies in response to salinity. Plant Cell Environ. 2022;45(3):695–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sexauer M, Shen D, Schön M, Andersen TG, Markmann K. Visualizing polymeric components that define distinct root barriers across plant lineages. Development. 2021;148(23):dev199820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogt T. Phenylpropanoid biosynthesis. Mol Plant. 2010;3(1):2–20. [DOI] [PubMed] [Google Scholar]
- 49.Sharma A, Shahzad B, Rehman A, Bhardwaj R, Landi M, Zheng B. Response of phenylpropanoid pathway and the role of polyphenols in plants under abiotic stress. Molecules. 2019;24(13):2452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yao T, Feng K, Xie M, Barros J, Tschaplinski TJ, Tuskan GA, Muchero W, Chen J-G. Phylogenetic occurrence of the phenylpropanoid pathway and lignin biosynthesis in plants. Front Plant Sci. 2021;12:704697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chun HJ, Baek D, Cho HM, Lee SH, Jin BJ, Yun D-J, Hong Y-S, Kim MC. Lignin biosynthesis genes play critical roles in the adaptation of Arabidopsis plants to high-salt stress. Plant Signal Behav. 2019;14(8):1625697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ho WWH, Hill CB, Doblin MS, Shelden MC, van de Meene A, Rupasinghe T, Bacic A, Roessner U. Integrative multi-omics analyses of barley rootzones under salinity stress reveal two distinctive salt tolerance mechanisms. Plant Commun. 2020;1(3):100031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shomali A, Das S, Arif N, Sarraf M, Zahra N, Yadav V, Aliniaeifard S, Chauhan DK, Hasanuzzaman M. Diverse physiological roles of flavonoids in plant environmental stress responses and tolerance. Plants. 2022;11(22):3158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Wang Y, Li J, Zhu Y, Wang L, Li Z, Liu Y, Yan F, Wang Q. Overexpression of chalcone isomerase-like genes, GmCHI4A and GmCHI4B, enhances salt tolerance of cotyledon hairy roots and composite plant in Soybean (Glycine max (L.) Merr). Agronomy. 2024;14(4):731. [Google Scholar]
- 55.Zhang L, Sun Y, Ji J, Zhao W, Guo W, Li J, Bai Y, Wang D, Yan Z, Guo C. Flavonol synthase gene MsFLS13 regulates saline-alkali stress tolerance in alfalfa. Crop J. 2023;11(4):1218–29. [Google Scholar]
- 56.Kim J, Lee WJ, Vu TT, Jeong CY, Hong S-W, Lee H. High accumulation of anthocyanins via the ectopic expression of AtDFR confers significant salt stress tolerance in Brassica napus L. Plant Cell Rep. 2017;36:1215–24. [DOI] [PubMed] [Google Scholar]
- 57.Dabravolski SA, Isayenkov SV. The role of anthocyanins in plant tolerance to drought and salt stresses. Plants. 2023;12(13):2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Truong HA, Lee WJ, Jeong CY, Trịnh CS, Lee S, Kang C-S, Cheong Y-K, Hong S-W, Lee H. Enhanced anthocyanin accumulation confers increased growth performance in plants under low nitrate and high salt stress conditions owing to active modulation of nitrate metabolism. J Plant Physiol. 2018;231:41–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1. Sequences of primers used for qRT-PCR. Table S2. Quality of the transcriptome sequencing data. Table S3. Number of differential expression genes in different comparisons. Table S4. Number of differential accumulated metabolites in different comparisons.
Supplementary Fig. S1. Clustering heat map (a) and number (b) of transcription factors (TFs) identified in genotype P1 and P2 under salt stress. Fig. S2. qRT-PCR verification of DEGs. The relative gene expression levels after 10 days of treatment with 200 mM NaCl. Error bars indicate mean ± SD based on three biological replicates.
Data Availability Statement
The datasets and materials supporting the research findings of this article are presented within the manuscript and its supplementary information files.