Abstract
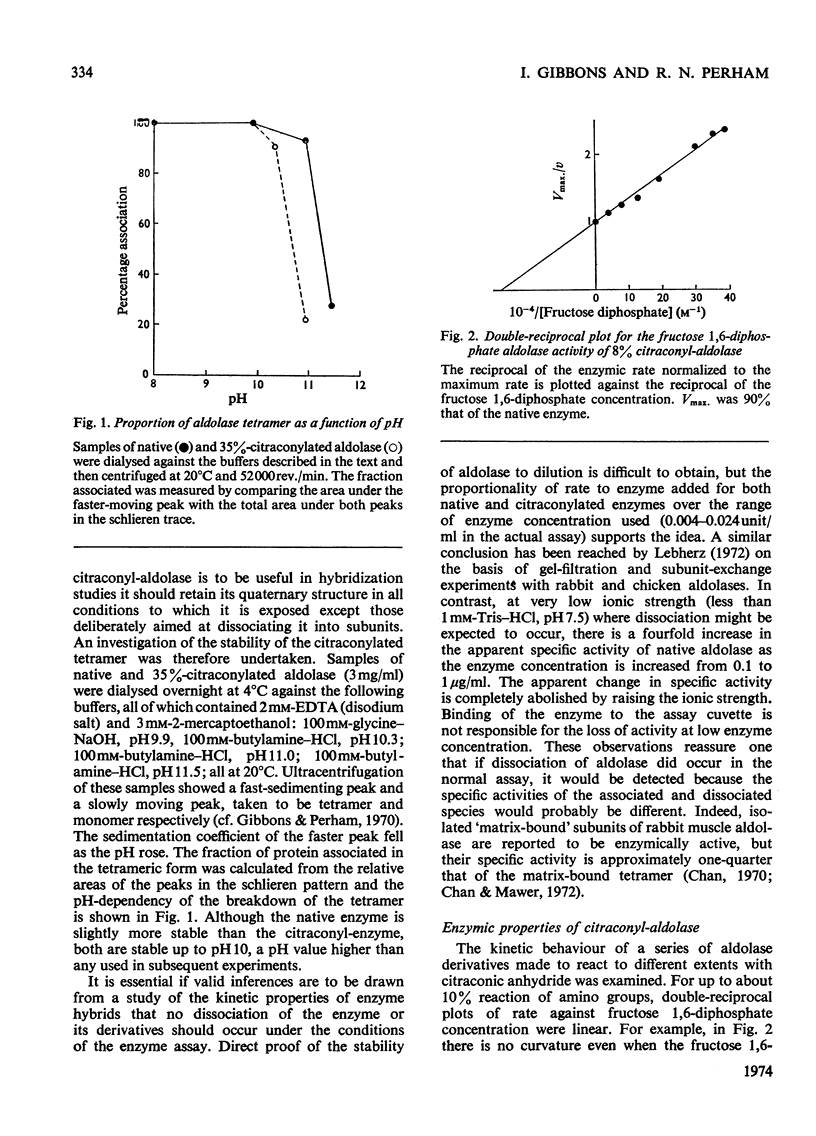
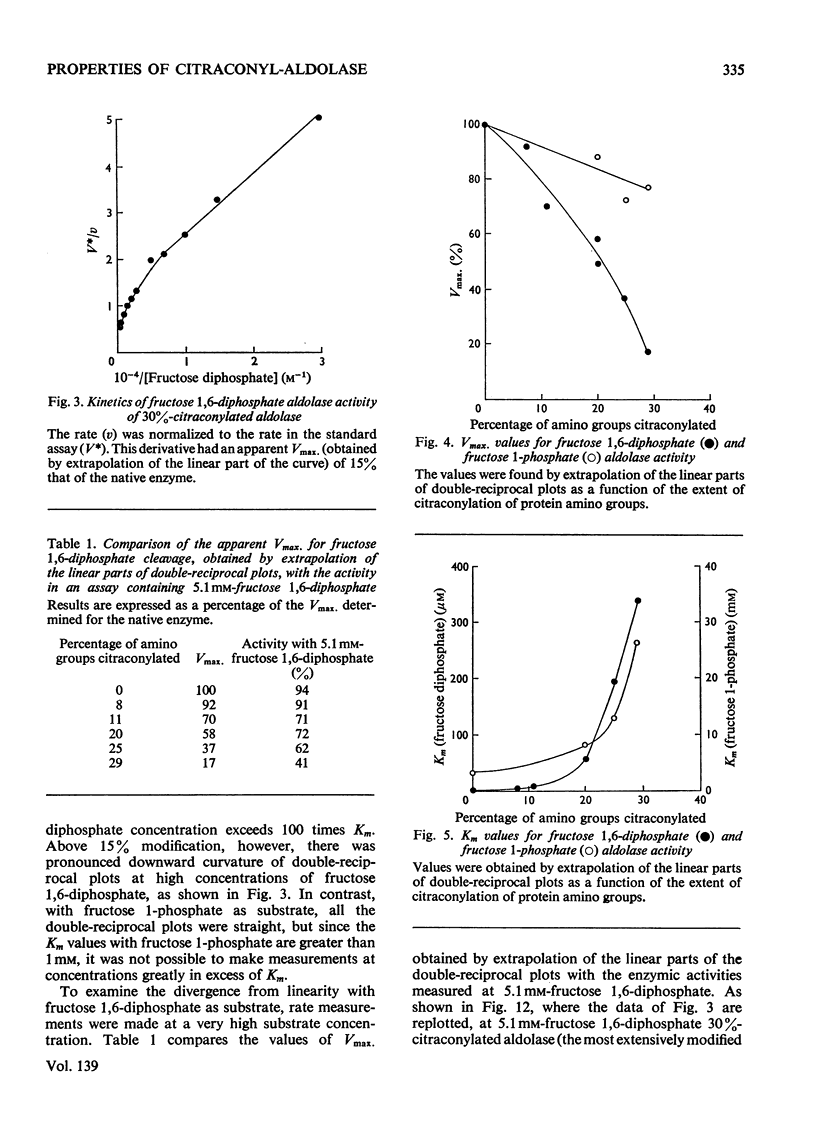
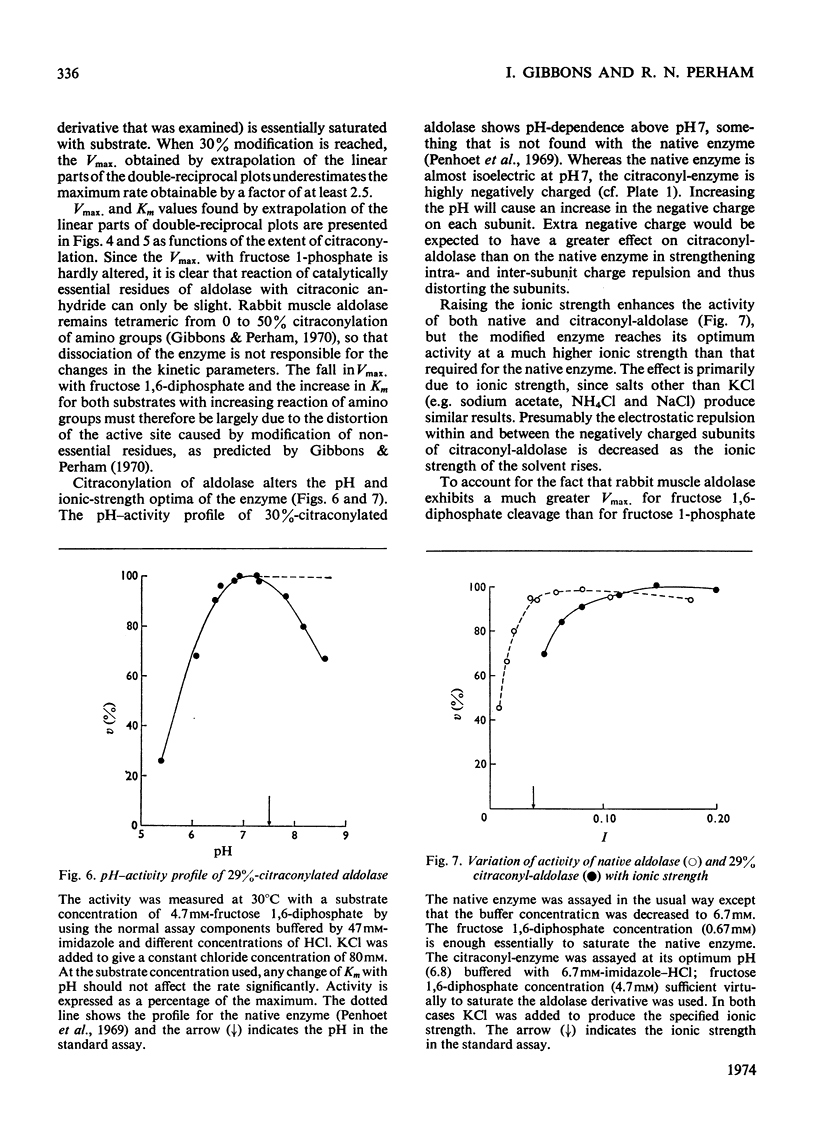
1. The preparation of enzymically active N-citraconyl derivatives of fructose diphosphate aldolase from rabbit muscle is described. Reaction is restricted to amino groups and the derivatives are not very heterogeneous with respect to the number of substituents. 2. Linear double-reciprocal plots of enzyme velocity against substrate concentration are found up to about 15% blocking of amino groups. With more than 15% blocking, there is a marked downward curvature in the double-reciprocal plots at high substrate concentrations. 3. Over the range 0–25% blocking of amino groups the apparent Vmax. for fructose diphosphate falls to 10% that of the native enzyme, and the apparent Km rises from 1 to 400μm. 4. Various pieces of evidence suggest that citraconyl-aldolase is slightly distorted in structure compared with the native enzyme. However, the kinetic properties and tetrameric structure of citraconyl-aldolase can be completely recovered after denaturation in 4m-guanidine hydrochloride. 5. After removal of the citraconyl groups in acid conditions the kinetic and molecular properties of native enzyme are restored. 6. Hybrid forms of aldolase can be constructed containing native and citraconylated subunits and the suitability of these derivatives for the study of subunit interactions in the enzyme is discussed. 7. The kinetic properties of hybridized aldolase containing native and citraconylated subunits are not exactly those predicted from the kinetic properties of the two parental forms. This result is interpreted in terms of conformational changes induced in the native and modified subunits when both are present in a hybrid molecule, evidently as a result of interactions in the tetramer.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J., Gibbons I., Perham R. N. A comparative study of the structure of muscle fructose 1,6-diphosphate aldolases. Eur J Biochem. 1969 Dec;11(3):503–509. doi: 10.1111/j.1432-1033.1969.tb00802.x. [DOI] [PubMed] [Google Scholar]
- BLOSTEIN R., RUTTER W. J. COMPARATIVE STUDIES OF LIVER AND MUSCLE ALDOLASE. II. IMMUNOCHEMICAL AND CHROMATOGRAPHIC DIFFERENTIATION. J Biol Chem. 1963 Oct;238:3280–3285. [PubMed] [Google Scholar]
- Brenner-Holzach O., Leuthardt F. Hybridisation of aldolase from Drosophila, blocked at the active site, with native C aldolase from calf brain. Eur J Biochem. 1972 Dec 18;31(3):423–426. doi: 10.1111/j.1432-1033.1972.tb02548.x. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., ORGEL L. E. THE THEORY OF INTER-ALLELIC COMPLEMENTATION. J Mol Biol. 1964 Jan;8:161–165. doi: 10.1016/s0022-2836(64)80156-x. [DOI] [PubMed] [Google Scholar]
- Chan W. W. Matrix-bound protein subunits. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1198–1204. doi: 10.1016/0006-291x(70)90213-5. [DOI] [PubMed] [Google Scholar]
- Chan W. W., Mawer H. M. Studies on protein subunits. II. Preparation and properties of active subunits of aldolase bound to a matrix. Arch Biochem Biophys. 1972 Mar;149(1):136–145. doi: 10.1016/0003-9861(72)90307-4. [DOI] [PubMed] [Google Scholar]
- Chan W., Morse D. E., Horecker B. L. Nonidentity of subunits of rabbit muscle aldolase. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1013–1020. doi: 10.1073/pnas.57.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Dixon H. B., Gibbs K., Walshe J. M. Preparation of triethylenetetramine dihydrochloride for the treatment of Wilson's disease. Lancet. 1972 Apr 15;1(7755):853–853. doi: 10.1016/s0140-6736(72)90845-8. [DOI] [PubMed] [Google Scholar]
- Dixon H. B., Perham R. N. Reversible blocking of amino groups with citraconic anhydride. Biochem J. 1968 Sep;109(2):312–314. doi: 10.1042/bj1090312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Forcina B. G., Perham R. N. Amino acid sequence homology between muscle and liver aldolases. FEBS Lett. 1971 Oct 15;18(1):59–63. doi: 10.1016/0014-5793(71)80406-4. [DOI] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I. Subunit interactions in hybrids of native, carboxypeptidase-treated and citraconylated rabbit muscle aldolase. Biochem J. 1974 May;139(2):343–350. doi: 10.1042/bj1390343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habeeb A. F. Determination of free amino groups in proteins by trinitrobenzenesulfonic acid. Anal Biochem. 1966 Mar;14(3):328–336. doi: 10.1016/0003-2697(66)90275-2. [DOI] [PubMed] [Google Scholar]
- Halford S. E., Bennett N. G., Trentham D. R., Gutfeund H. A substate-induced conformation change in the reaction of alkaline phosphatase from Escherichia coli. Biochem J. 1969 Sep;114(2):243–251. doi: 10.1042/bj1140243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford S. E. Escherichia coli alkaline phosphatase. Relaxation spectra of ligand binding. Biochem J. 1972 Feb;126(3):727–738. doi: 10.1042/bj1260727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada K., Wolfe R. G. Malic dehydrogenase. VII. The catalytic mechanism and possible role of identical protein subunits. J Biol Chem. 1968 Aug 10;243(15):4131–4137. [PubMed] [Google Scholar]
- Ikai A., Tanford C. Kinetic evidence for incorrectly folded intermediate states in the refolding of denatured proteins. Nature. 1971 Mar 12;230(5289):100–102. doi: 10.1038/230100a0. [DOI] [PubMed] [Google Scholar]
- King L., Perham R. N. Reaction of tobacco mosaic virus with maleic anhydride and some possible applications to x-ray diffraction analysis. Biochemistry. 1971 Mar 16;10(6):981–987. doi: 10.1021/bi00782a008. [DOI] [PubMed] [Google Scholar]
- Lazdunski M., Petitclerc C., Chappelet D., Lazdunski C. Flip-flop mechanisms in enzymology. A model: the alkaline phosphatase of Escherichia coli. Eur J Biochem. 1971 May 11;20(1):124–139. doi: 10.1111/j.1432-1033.1971.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Lebherz H. G. Stability of quaternary structure of mammalian and avian fructose diphosphate aldolases. Biochemistry. 1972 Jun 6;11(12):2243–2250. doi: 10.1021/bi00762a006. [DOI] [PubMed] [Google Scholar]
- Mehler A. H., Cusic M. E., Jr Aldolase reaction with sugar diphosphates. Science. 1967 Mar 3;155(3766):1101–1103. doi: 10.1126/science.155.3766.1101. [DOI] [PubMed] [Google Scholar]
- Meighen E. A., Schachman H. K. Hybridization of native and chemically modified enzymes. I. Development of a general method and its application to the study of the subunit structure of aldolase. Biochemistry. 1970 Mar 3;9(5):1163–1176. doi: 10.1021/bi00807a017. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Chan W., Horecker B. L. The subunit structure and carboxy-terminal sequence of rabbit muscle aldolase. Proc Natl Acad Sci U S A. 1967 Aug;58(2):628–634. doi: 10.1073/pnas.58.2.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penhoet E. E., Kochman M., Rutter W. J. Molecular and catalytic properties of aldolase C. Biochemistry. 1969 Nov;8(11):4396–4402. doi: 10.1021/bi00839a026. [DOI] [PubMed] [Google Scholar]
- Penhoet E. E., Rutter W. J. Catalytic and immunochemical properties of homomeric and heteromeric combinations of aldolase subunits. J Biol Chem. 1971 Jan 25;246(2):318–323. [PubMed] [Google Scholar]
- Perham R. N. A diagonal paper-electrophoretic technique for studying amino acid sequences around the cysteine and cystine residues of proteins. Biochem J. 1967 Dec;105(3):1203–1207. doi: 10.1042/bj1051203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perham R. N., Richards F. M. Reactivity and structural role of protein amino groups in tobacco mosaic virus. J Mol Biol. 1968 May 14;33(3):795–807. doi: 10.1016/0022-2836(68)90320-3. [DOI] [PubMed] [Google Scholar]
- Turner B. M., Fisher R. A., Harris H. An association between the kinetic and electrophoretic properties of human purine-nucleoside-phosphorylase isozymes. Eur J Biochem. 1971 Dec;24(2):288–295. doi: 10.1111/j.1432-1033.1971.tb19684.x. [DOI] [PubMed] [Google Scholar]




