Abstract
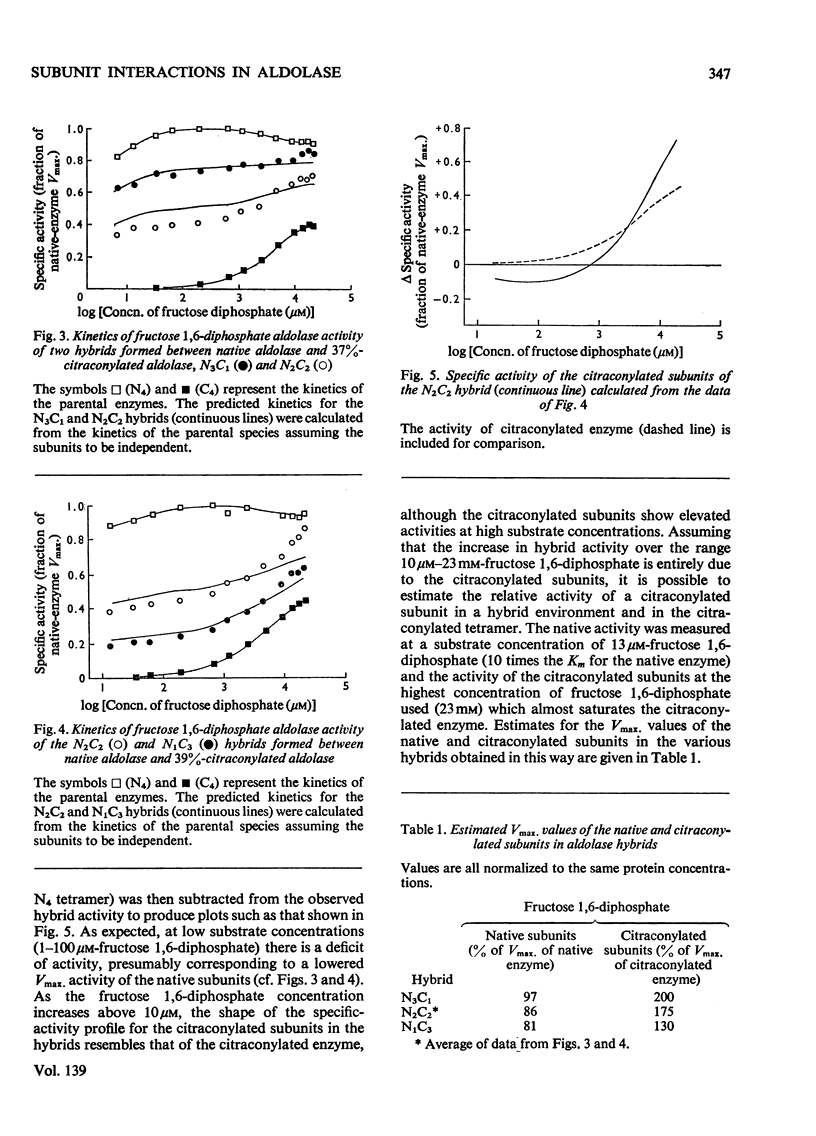
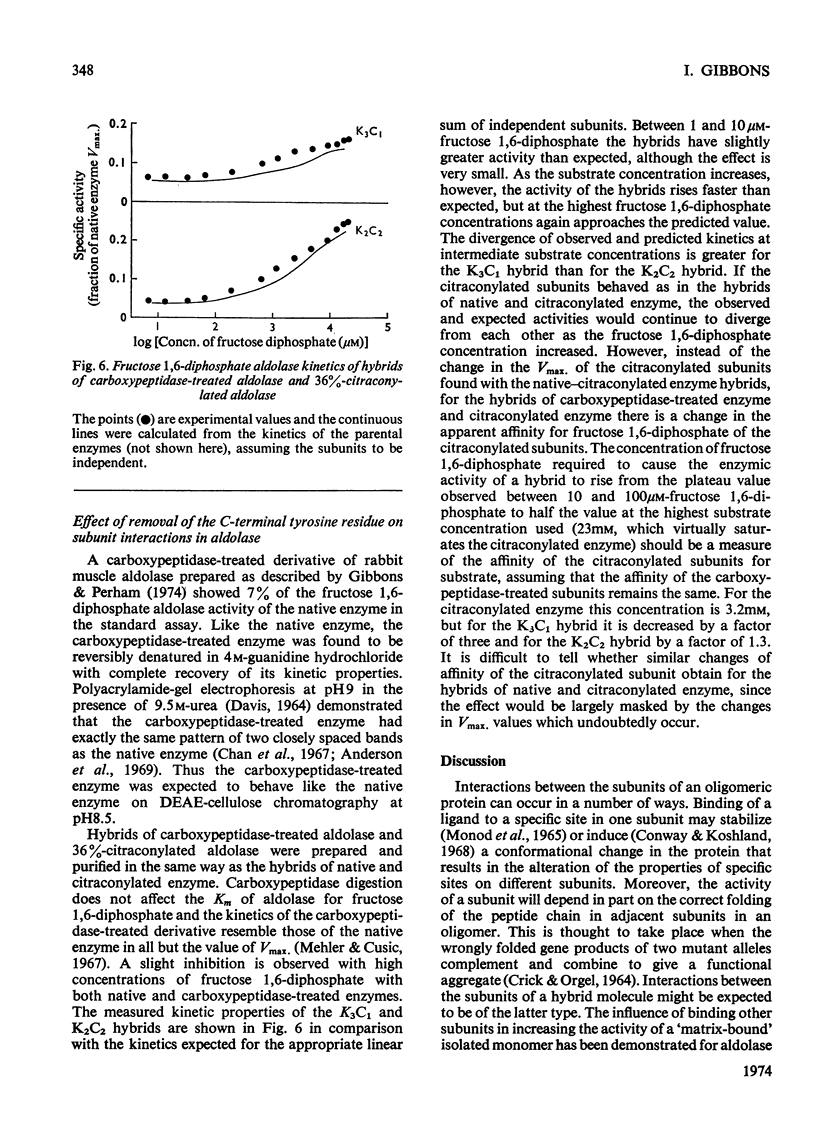
1. The kinetic properties of hybrids of native (or carboxypeptidase-treated) and citraconylated rabbit muscle aldolase are compared with those of equivalent mixtures of the parental enzymes. 2. In the hybrids, the native subunits function slightly less well than in the homotetramer, but the citraconylated subunits have enhanced activity. 3. Subunits of carboxypeptidase-treated aldolase behave essentially as expected in a hybrid environment, but the citraconylated subunits do not show the same enhancement of activity found in the hybrids of native and citraconylated enzyme. The apparent affinity for fructose 1,6-diphosphate of the citraconylated subunits in hybrids of carboxypeptidase-treated and citraconylated aldolase is increased. 4. These results are interpreted in terms of a substrate-induced conformational difference between native and carboxypeptidase-treated aldolase. 5. This conformational change can take place within a single native subunit in the hybrids and does not require a similar conformational change to occur simultaneously in the other three subunits.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson P. J., Gibbons I., Perham R. N. A comparative study of the structure of muscle fructose 1,6-diphosphate aldolases. Eur J Biochem. 1969 Dec;11(3):503–509. doi: 10.1111/j.1432-1033.1969.tb00802.x. [DOI] [PubMed] [Google Scholar]
- Brenner-Holzach O., Leuthardt F. Hybridisation of aldolase from Drosophila, blocked at the active site, with native C aldolase from calf brain. Eur J Biochem. 1972 Dec 18;31(3):423–426. doi: 10.1111/j.1432-1033.1972.tb02548.x. [DOI] [PubMed] [Google Scholar]
- CRICK F. H., ORGEL L. E. THE THEORY OF INTER-ALLELIC COMPLEMENTATION. J Mol Biol. 1964 Jan;8:161–165. doi: 10.1016/s0022-2836(64)80156-x. [DOI] [PubMed] [Google Scholar]
- Chan W. W. Matrix-bound protein subunits. Biochem Biophys Res Commun. 1970 Dec 9;41(5):1198–1204. doi: 10.1016/0006-291x(70)90213-5. [DOI] [PubMed] [Google Scholar]
- Chan W., Morse D. E., Horecker B. L. Nonidentity of subunits of rabbit muscle aldolase. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1013–1020. doi: 10.1073/pnas.57.4.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway A., Koshland D. E., Jr Negative cooperativity in enzyme action. The binding of diphosphopyridine nucleotide to glyceraldehyde 3-phosphate dehydrogenase. Biochemistry. 1968 Nov;7(11):4011–4023. doi: 10.1021/bi00851a031. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- DRECHSLER E. R., BOYER P. D., KOWALSKY A. G. The catalytic activity of carboxypeptidase-degraded aldolase. J Biol Chem. 1959 Oct;234:2627–2634. [PubMed] [Google Scholar]
- Feldmann K., Zeisel H., Helmreich E. Interactions between native and chemically modified subunits of matrix-bound glycogen phosphorylase. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2278–2282. doi: 10.1073/pnas.69.8.2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. Kinetic and molecular properties of citraconyl-aldolase. The reversible denaturation and hybridization of the native and modified enzymes. Biochem J. 1974 May;139(2):331–342. doi: 10.1042/bj1390331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons I., Perham R. N. The reaction of aldolase with 2-methylmaleic anhydride. Biochem J. 1970 Mar;116(5):843–849. doi: 10.1042/bj1160843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Mehler A. H., Cusic M. E., Jr Aldolase reaction with sugar diphosphates. Science. 1967 Mar 3;155(3766):1101–1103. doi: 10.1126/science.155.3766.1101. [DOI] [PubMed] [Google Scholar]
- Meighen E. A., Schachman H. K. Hybridization of native and chemically modified enzymes. I. Development of a general method and its application to the study of the subunit structure of aldolase. Biochemistry. 1970 Mar 3;9(5):1163–1176. doi: 10.1021/bi00807a017. [DOI] [PubMed] [Google Scholar]
- Penhoet E., Kochman M., Valentine R., Rutter W. J. The subunit structure of mammalian fructose diphosphate aldolase. Biochemistry. 1967 Sep;6(9):2940–2949. doi: 10.1021/bi00861a039. [DOI] [PubMed] [Google Scholar]
- SHAW C. R. THE USE OF GENETIC VARIATION IN THE ANALYSIS OF ISOZYME STRUCTURE. Brookhaven Symp Biol. 1964 Dec;17:117–130. [PubMed] [Google Scholar]



