Abstract
Tissue engineering and in vitro modeling of the airways and lungs in the respiratory system are of substantial research and clinical importance. In vitro airway and lung models aim to improve treatment options for airway and lung repair and advance respiratory pathophysiological research. The construction of biomimetic native airways and lungs with tissue-specific biological, mechanical, and configurable features remains challenging. Bioprinting, an emerging 3D printing technology, is promising for the development of airway, lung, and disease models, allowing the incorporation of cells and biologically active molecules into printed constructs in a precise and reproducible manner to recreate the airways, lung architecture, and in vitro microenvironment. Herein, we present a review of airway and lung bioprinting for applications in tissue engineering and in vitro modeling. The key pathophysiological characteristics of the airway, lung interstitium, and alveoli are described. The bioinks recently used in 3D bioprinting of the airways and lungs are summarized. Furthermore, we propose a bioink categorization based on the structural characteristics of the lungs and airways. Finally, the challenges and opportunities in the research on biofabrication of airways and lungs are discussed.
Keywords: Bioprinting, bioinks for airways and lungs, tissue engineering, lung disease, trachea
Introduction
Airway and lung diseases are major causes of death and economic burden worldwide. Three of the top ten causes of death worldwide are related to the respiratory system, namely chronic obstructive pulmonary disease (COPD), pneumonia, and lung cancer. In addition, respiratory diseases such as asthma, tuberculosis, sleep-disordered breathing, pulmonary arterial hypertension, and occupational lung disease have become heavy burdens on the quality of life and economy worldwide.1–3
The repair and regeneration of the airways and lungs is worldwide problem. Owing to the lack of efficient research methods for airway and lung diseases, many respiratory diseases, such as asthma and acute respiratory distress syndrome (ARDS), require in vivo studies4,5 that euthanize many animals. Therefore, there is a need for a research method that is mass-producible, effectively simulates the in vivo environment, and quickly obtains experimental results. The treatment of long-segment tracheal defects and end-stage lung diseases, including COPD and pulmonary fibrosis, requires airway reconstruction or lung transplantation.6,7 However, donor shortage is a global problem, and there is an urgent need for airway reconstruction and lung regeneration. It is difficult to solve these problems by relying on a single technology; therefore, multidisciplinary and multitechnical approaches have been explored. 8
Emerging techniques for engineering living tissue constructs hold great promise in the aforementioned aspects for treating respiratory diseases.9–12 The development of living tissue constructs that replicate the cellular composition, structural features, and biological functions of the airways and lungs is supported by advances in disciplines such as biomaterials, biotechnology, and manufacturing technology. Tracheal and lung substitutes based on 3D printing trend to transfer from the laboratory to the clinic. The 3D printed extracellular tracheal stent has realized the treatment of tracheomalacia.13,14 Biomimetic alveoli, which use special gel materials and bioprinting technology to prevent collapse, construct biomimetic gas exchange and blood exchange pathway and realize trachea exchange function. 15 As for vitro models, for example, a simplistic alveolar air-liquid model can be formed by seeding cells on a Transwell membrane and cross-linking a mixture of cells and precursor materials with hydrogels.16,17 These living tissue constructs, involving the manipulation and design of living cells to create functional tissues or organs outside of the body, can be used as in vitro models for pathological studies and drug development and replicate the functions of airway and lung tissues more accurately than 2D cell culture.18–20 In surgical procedures for replacing and repairing defective respiratory tissues, these living tissue constructs can be utilized to address challenges such as the shortage of autotransplantation and allotransplantation.21–23 The engineered living constructs are effective for the study and treatment of airway and lung diseases.24,25 However, the pathophysiological processes, functions, and fine structures of the airways and lungs have not been well reproduced. Generating 3D biomimetic airway and lung tissues with specific biological, mechanical, and configurable features is challenging.
Bioprinting is a promising novel method for creating living tissue constructs with intricate features that mimic native tissues. Bioprinting has tremendous potential for tissue engineering applications.26–28 The ability to build individualized designs with patient-derived cells to prevent rejection, and complex 3D biological structures with high resolution are some advantages. Bioprinting is developed from 3D printing techniques and can incorporate the spatial arrangement of cells and materials, offering a novel method for creating 3D multicellular architectures.29,30 Bioinks serve as the “ink” or printable material in bioprinting processes and are designed to incorporate living cells and biomolecules to create three-dimensional structures that mimic natural tissues. For example, a biomimetic artificial trachea can be formulated using an extrusion-based bioprinter with a dual-head printing strategy to simultaneously print stiff ring-shaped scaffolds and soft tissue-like constructs containing bioink and cells. 31 Despite this remarkable research progress based on bioprinting, some critical functions of airways and lungs are yet to be achieved with 3D-printed tissue constructs; for instance, the digital light processing (DLP)-based bioprinting resolution of 15–100 μm is not suitable for the alveolar-like structures size (300 μm).32,33
This article reviews the latest developments in bioprinting techniques for constructing 3D airway and lung tissues to study and treat respiratory diseases. The guidelines for adopting 3D printing techniques for respiratory diseases summarize the key characteristics of the lungs and airways (Figure 1). Cells, bioinks, and 3D printing techniques are surveyed to provide a toolbox for printing lungs and airways (Figure 2(a) to (c)). The applications of 3D printing of the lungs and airways in tissue engineering and in vitro biological modeling are elaborated (Figure 2(d)). Finally, the future outlook for 3D printing of the airways and lungs is discussed, focusing on possible strategies and challenges for accurately reconstructing additional airway and lung functions.
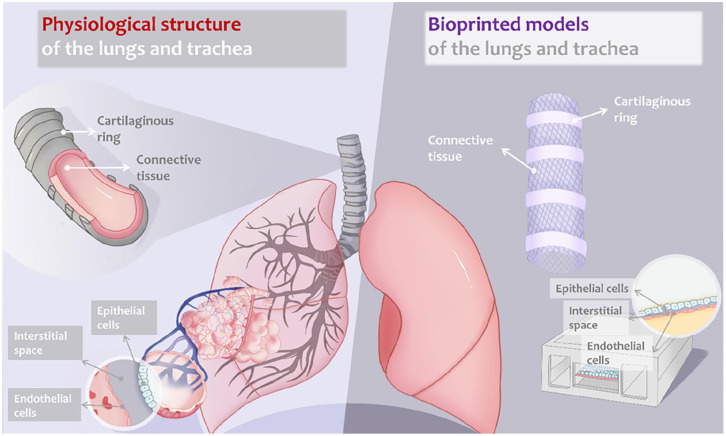
Figure 1.
Schematic structure of human lung and airway: bioprinting of airway and lungs for tissue engineering and in vitro model. The physiological structures of the lungs (featuring the architecture of alveoli) and the trachea (featuring cartilaginous rings and connective tissue) are illustrated on the left. The bioprinted models of the lungs and the trachea are illustrated on the right, with engineered features corresponding to the native tissues.
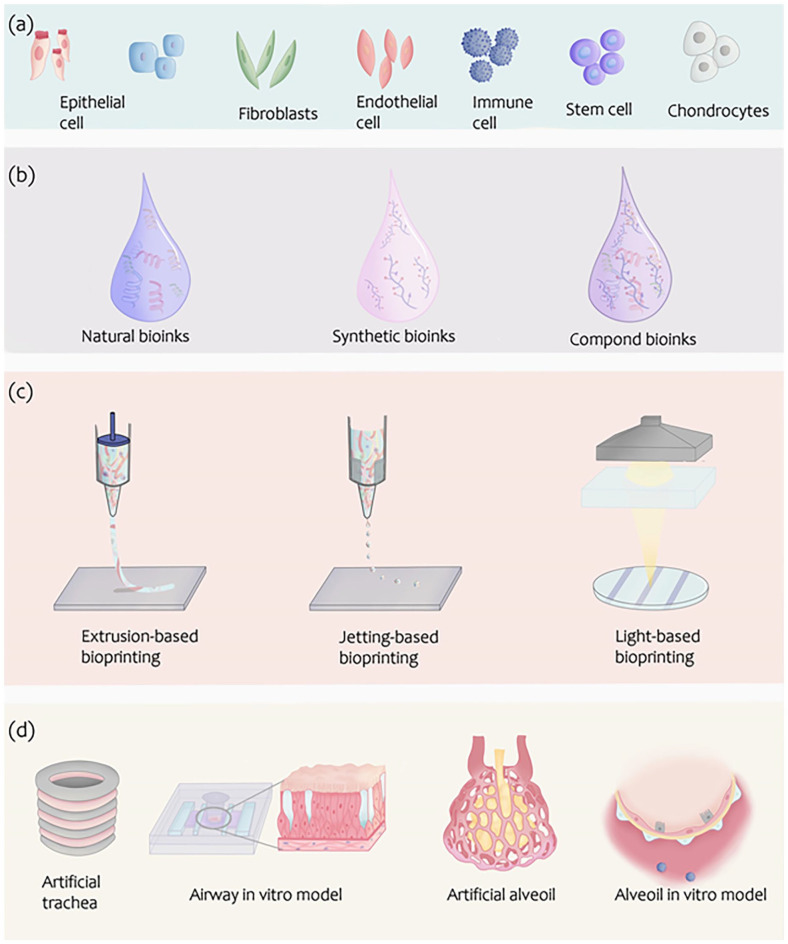
Figure 2.
Bioinks and bioprinting techniques for airway and lung. (a) Various cells for bioinks of airway and lung. (b) Biomaterials for the 3D printing of airway and lung. (c) The representation of bioprinting strategies: Inkjet printers eject small droplets of the bioink sequentially to construct tissues; Laser-assisted printers use a laser to vaporize a region in the donor layer forming a bubble that drives the suspended bioink to fall onto the substrate; Extrusion printers use pneumatics or mechanical pressure to continuously extrude a highly viscous bioink for bioprinting trachea and lung structures. (d) Typical models of airway and lung for modeling and reconstructing by bioprinting.
Physiological features of the airway and lung
Airway
As an important part of the respiratory system, the airway has a special anatomical deconstruction and includes the trachea and bronchi. The trachea begins at the lower border of the cricoid cartilage of the larynx and divides into the left and right bronchi at the upper border of the fifth thoracic vertebra. The left and right main bronchi are connected to the lungs via the secondary and tertiary bronchi, respectively. 34 The main functions of the airway include ventilation, foreign body removal, and respiratory regulation. 35
Various components coordinate the ventilatory function of the airway. Airway cartilage is primarily used to maintain the patency of the airway cavity. The posterior wall and cartilaginous space of the airway are usually closed by smooth muscle and fibrous tissue to prevent gas from escaping into the surrounding tissue. The mucosal epithelium can prevent the attack of air-borne microorganisms and clear foreign bodies from the airway. Moreover, respiratory regulation and immunity are important and are realized by the airway’s submucosal nerve and immune system, respectively.
Although the natural airway has many functional requirements, the ventilatory and foreign body removal functions of the airway are vital. Therefore, the pressing challenges in tissue-engineered airway construction include regenerating the epithelium to clear foreign bodies in the airway, regeneration of cartilage tissue for ventilation, and tissue revascularization to maintain the epithelium and cartilage. 36
Lung
The lung is an important respiratory organ of the human body and comprises the lung parenchyma and interstitium. The pulmonary parenchyma includes alveoli, and the lung interstitium includes connective tissue and microvascular and lymphatic vessels around the alveoli. 37 The lungs mainly perform functions such as gas exchange and immunity in the respiratory system.
Alveoli are the main components of gas exchange, which is usually carried out at the air-liquid exchange interface of the alveoli, called the respiratory membrane. It consists of six layers: the liquid layer containing the alveolar surfactant, alveolar epithelial layer, epithelial basement membrane layer, interstitial layer between the alveoli and capillaries, capillary basement membrane layer, and capillary endothelial cells. Some key features, such as the respiratory membrane area and thickness, affect the rate of gas diffusion. In addition, factors such as the difference in gas partial pressure between the air and capillaries, alveolar ventilation, and capillary blood flow affect the gas diffusion efficiency. 38 The respiratory membrane cells mainly comprise type I/type II alveolar epithelial cells, endothelial cells, and fibroblasts (Figure 1). The respiratory membrane also includes white blood cells, such as macrophages, which participate in immune responses. 39
The normal lung interstitial matrix includes collagen fibers, elastin, structural glycoproteins and proteoglycans. 40 Collagen account for 15% of normal lung weight, which is the most prevalent type of protein in the lung interstitial space. Elastin has hydrophobicity regions interspersed by cross-linking domains, which accounts for 20%–30% of lung matrix. Fibronectin, which is a kind of structural glycoprotein, which can be used in cell adhesion, migration, proliferation, and it also can play the roles in cytoskeletal organization and matrix assembly. Proteoglycans include a core protein and glycosaminoglycans. Proteoglycans can be found in the ECM of lungs as the intracellular structures. Some researchers also found that proteoglycans can play an important role in pulmonary immune function. 41
Several lung diseases require extensive research in lung pharmacology and molecular biology. For example, treating pulmonary fibrosis has become a clinical challenge worldwide, and impaired pulmonary interstitial function often causes pulmonary fibrosis, interstitial pneumonia, and other problems.42–44 Research on lung-related diseases requires a model that can simulate lung anatomy, physiology, and pathology in vitro. 45
In addition, patients with end-stage lung disease often require a lung transplant. 46 Shortage of donors, difficulties in maintaining donor lungs, primary graft dysfunction, and chronic graft failure hinder lung transplantation development. Many techniques are used for lung transplantation, such as donation after cardiac death, ex vivo lung perfusion, and extracorporeal membrane oxygenation,47,48 but problems such as high operating costs and difficulty in lung maintenance remain. Therefore, manufacturing artificial lungs with ventilation functions has attracted interest as a research topic.
Cells of the airway and lung
The lungs and airways are composed of a variety of cells, mainly functional cells and stromal cells. The functional cells mainly include epithelial cells and chondrocytes. We summarize the basic cell types and discuss their combinations for constructing airway and lung tissues (Figure 2(a)).
Epithelial cells
Alveolar cells include type I and II cells. Type I alveolar cells are primarily involved in gas exchange, whereas type II alveolar cells secrete surface-active substances. The airways are lined proximally by tall columnar epithelium and distally by cubic epithelium. Airway epithelial cells are essential for regulating airway biology and function as a physical barrier between the airway and the host.36,49 Epithelial cells are conventionally used to repair airway epithelium and the formation of air-liquid interfaces. Park et al. 50 constructed a 3D bioartificial scaffold loaded with rabbit epithelial cells to treat tracheal lesions and for artificial tissue examination. Bae et al. 51 used rabbit epithelial cells to create a biocompatible artificial trachea thought to be structurally similar to a normal trachea.
There are two types of cells in the alveolar epithelium: large, flattened alveolar type I (ATI) cells that cover 95%–98% of the alveolar surface and permit gas exchange and cuboidal alveolar type II (ATII) cells that are the progenitor cells responsible for regenerating ATI and ATII cells during homeostasis and after injury.52,53 The lung epithelium serves as a barrier that protects the body from airborne pathogens and prevents leakage of bodily fluids into the airspaces. In the event of epithelial cell death, such as during infection or after exposure to cigarette smoke, the barrier is compromised. During homeostasis, alveolar epithelial cells have a long but limited lifespan. 52 After the death of an occasional alveolar epithelial cell, barrier function is maintained by adequate levels of ATII cell proliferation and differentiation to replace the lost cells. During lung injury, excessive epithelial cell death results in impaired barrier function. ATI cells are particularly susceptible to injury, but ATII cells can die in cases of severe or certain types of injury.
It has long been known that ATII cells are the principal progenitor responsible for regenerating the injured alveolar epithelium,52,54,55 although it is increasingly recognized that other progenitors can be mobilized under certain circumstances.56–58 ATII cells proliferate to replace lost cells, and once sufficient cell numbers have been restored, some differentiate into ATI cells to restore normal alveolar structure. Since ATI cells are largely responsible for barrier function and gas exchange, the regeneration of ATI cells is absolutely critical to restore normal lung function.
Chondrocytes
Chondrocytes are the only cells in the cartilage that can generate and are responsible for maintaining the cartilage matrix. Up to now, no cartilage was found in the lung, while cartilage can be found in the airways. Chondrocytes constitute the cartilage tissue, with a dense, strongly organized ECM synthesized by these chondrocytes. Chondrocytes can be derived from autologous ear or nasal cartilage and used for chondrogenesis. 59 In tissue engineering, chondrocytes are used to generate neotracheal cartilage and offer mechanical support. Park et al. 60 used a representative extrusion-based 3D printing strategy with human nasal chondrocytes to generate a bionic trachea that exhibited satisfactory cartilage-regeneration competence and satisfactory biocompatibility with a nude mouse model. Huo et al. 61 developed a new strategy for 3D bioprinting that used cartilaginous vascularized fibrous tissue-integrated trachea with photocrosslinkable tissue-specific bioinks to fabricate a trachea characterized by fibrocartilaginous tissue, which may be effective for tracheal construction.
Stromal cells
Stromal cells of lung and airway mainly include fibroblasts, endothelial cells, stem cells and immune cells et al.
During development, lung fibroblasts are derived from lung interstitial progenitor cells and secrete reticular extracellular matrices (ECMs) that allow the lungs to expand and contract repeatedly and rapidly. In addition, the cells secrete a variety of growth factors that regulate cell proliferation and function.62,63
Endothelial cells play an important role in bodily homeostasis, including controlling vascular permeability and regulating vascular tone. Endothelial cells can affect the immunology and pathology of homeostasis in two ways: if dysfunctional, they can lead to diseases; however, they actively mediate immune responses at the site of injury or infection. 64 Endothelial cells play an important role in bodily homeostasis, including controlling vascular permeability and regulating vascular tone. Role of endothelial cells in physiological processes, including vasoconstriction, inflammation, coagulation, metabolic and oxidative/nitrosative stress, and cell viability, growth and differentiation. 65
Stem cells participate in most lung development periods. 66 The various stem cell types include bone marrow mesenchymal, umbilical cord blood-derived, and adipose stem cells. Mesenchymal stem cells (MSCs) can differentiate into various mesenchymal phenotypes during cultivation. MSCs have complex, multicomponent responsiveness and can be induced by growth factors to differentiate into bone, cartilage, muscle, and fat. MSCs can secrete proteins essential for gas exchange, and when the lung is damaged, they quickly form a transient expansion of cells involved in injury and regenerative repair. 67 Stem cells play a role in lung development, they are self-renewing and can differentiate into multiple cell lines in response to bioactive molecules, and then they further differentiate into organs and tissues. Adipose stem cells have been proved to be used for bioengineering lung construction. 68 Hawkins et al. reported that airway basal stem cells derived from human pluripotent stem cells. Airways basal cells possess adult stem cell self-renewal and multidirectional differentiation properties that can be generated in vitro to mimic airway diseases. Nevertheless, basal stem cells are prime candidates for cell-based therapies aimed at reconstructing the airway epithelium. 69
Pulmonary immune cells, including macrophages, dendritic cells, neutrophils, T cells, and B cells, are essential for protecting gas-exchange structures from invasive bacterial pathogens. These immune cells affect pulmonary infection through both innate and adaptive immunity and may interact with epithelial cells to induce a cascade response. 70 Using immune cells to create lungs and airways allows the simulation of different disease states. For example, immune cells are integral in developing diseases, such as pneumonia and airway inflammation.
The combinations of above basic cell types construct airway and lung tissues. The architecture of bioprinted airways and lungs that can perform biological functions requires different cell type combinations.
Bioprinting techniques for airway and lung
Various 3D printing techniques have been developed over the past few decades. They offer distinct advantages in terms of precision, efficiency, and structural complexity for generating living tissue constructs. The development of various 3D bioprinting techniques, such as extrusion-based and light-based bioprinting, has been comprehensively reviewed by Huang et al., 71 highlighting their potential in creating complex tissue constructs for respiratory applications. Here, we summarize the three primary bioprinting techniques for generating airway and lung tissues: extrusion, inkjet, and light-based bioprinting.
Extrusion-based bioprinting techniques
Extrusion-based bioprinting exerts pressure through a syringe structure and needle-like nozzle to deliver bioink in a layer-by-layer fashion. This printing technique is versatile because it can be applied to a wide range of cells, materials, and growth factors; however, there is the potential of damage to cells resulting from the shear stress during the extrusion process. More information on these technologies can be found in recent reviews.72–74
In extrusion-based bioprinting, multiple factors need to be comprehensively considered to achieve good printability and ensure cell viability (Table 1). From the design of bioinks (including rheological properties, viscoelasticity, surface tension, gelation mechanisms, etc.) to the optimization of bioprinting process parameters (such as pressure, speed, temperature, nozzle parameters, and cross-linking strategies), and then to the rational planning of construct design (filament spacing and orientation), each link is interrelated and influences each other. Understanding the relationships among these factors and implementing precise control and optimization contribute to the construction of high-quality lung/airway tissue models and the effective maintenance of cell functions.75,76
Table 1.
Bioprinting techniques for airway and lung.
| Bioprinting techniques | Classification | Printability | Cell viability | Ref |
|---|---|---|---|---|
| Extrusion-based bioprinting | Pneumatic-based | Bioink design (rheological properties, viscoelasticity, surface tension, gelation mechanism, etc.). Bioprinting process parameters pressure, speed, temperature, nozzle parameters, and crosslinking strategies). Construct design (filament spacing and orientation). |
Shear stress. Material cross-linking. Printing parameters (pressure, speed, nozzle size and shape). Characteristics of bio-ink. |
Fu et al., 75 Boularaoui et al. 76 |
| Piston-driven | ||||
| Screw-driven | ||||
| Jetting-based bioprinting | Inkjet-based bioprinting | Highly dependent on the properties of the bioink. Two important parameters (Reynolds number/Weber number—determining the ink viscosity). The influence of pressure parameters on printability. The influence of nozzle parameters on printability (nozzle size and diameter). The influence of temperature on printability. |
Droplets (impact velocity, volume). Properties of bioink (Reynolds (Re) and Weber (We) numbers). Printing parameters (printing frequency, distance from nozzle to substrate). Shear stress. |
Ng and Shkolnikov, 77 Ng et al.78,79 |
| Laser-assisted bioprinting | ||||
| Acoustic-based bioprinting | ||||
| Microvalve-based bioprinting | ||||
| Electrohydrodynamic jet bioprinting | ||||
| Light-based bioprinting techniques | Digital light processing, DLP | Types of photoinitiators. Wavelength and intensity of light. Composition of bio-ink. Structural resolution. |
Bioink composition (whether there are any additional protective components that can protect cells from light-induced damage). | Levato et al., 80 Fang et al., 81 Garciamendez-Mijares et al. 82 |
| Stereolithography, SLA | ||||
| Two-photon polymerization, 2PP |
For instance, bioinks based on the hybridization of gelatin methacryloyl and glycidyl-methacrylated hyaluronic acid were printed through an extrusion mechanism and further cured with UV light. Lee et al. 83 fabricated a photocurable bioink by mixed gelatin methacryloyl (GelMA) and glycidyl-methacrylated HA (GMHA) and bioprinted a rabbit larynx, including the hyoid bone, thyroid cartilage, cricoid cartilage, arytenoid cartilage, and cervical trachea. This strategy has been applied to generate structures for chondrogenesis of tonsil-derived MSCs.
Jetting-based bioprinting technique
Jetting-based bioprinting techniques play a diverse and crucial role in the field of biofabrication. Inkjet bioprinting, being a significant subset, exhibits unique characteristics. Its core mechanism depends on the precise placement of minute droplets, ranging from 1 to 100 picoliters (pL), which is accomplished through thermal or piezoelectric actuation. This precision is a fundamental advantage, facilitating high-resolution tissue engineering and the creation of intricate 3D structures. The process necessitates meticulous selection of cells, biomaterials, and printing parameters to ensure consistent and controlled droplet ejection, which is vital for the stability and quality of the bioprinted constructs. 84 Generally, two inkjet-based printing techniques are employed within inkjet bioprinting: continuous inkjet printing (CIJ) and drop-on-demand printing (DOD). In the CIJ mode, the morphological change of the liquid streams leads to continuous drops, often required for high-speed printing, due to a surface-tension-driven mechanism known as Plateau–Rayleigh instability. In contrast, the DOD mode can be established using piezoelectric or thermal mechanisms to achieve higher droplet precision. For instance, Kang et al. fabricated a 3D alveolar barrier model through multiple inkjet-based printings in the DOD mode, where an 80-μm piezoelectric nozzle generated ink drops at an approximate jet speed of 3 m/s. The printing parameters were carefully adjusted to attain high precision and stability of the cell-laden drops. 85
Beyond inkjet bioprinting, other variants of jetting-based bioprinting techniques exist. 77 Laser-induced forward transfer (LIFT) bioprinting, related to traditional thermal inkjet printing, utilizes a focused laser beam to generate a vapor bubble on an energy-absorbing layer, propelling liquid droplets for deposition. It is suitable for high-viscosity bioinks and high cell concentrations, with a viscosity range of 120–300 mPa·s and cell concentrations >108 cells/mL. Although it offers advantages such as the ability to print high-viscosity materials, high throughput (up to 100 kHz), and no nozzle clogging, it is sensitive to parameters like laser beam characteristics and energy-absorbing layer properties, which can affect print resolution and cell viability. 86
Acoustic bioprinting employs surface acoustic waves generated by a piezoelectric actuator and interdigitated gold rings to eject droplets from an open pool. Despite being used for various cell types, it has drawbacks including high sensitivity to print-head and substrate movement and the limitation of only being able to eject low viscosity droplets. 87 Microvalve bioprinting, consisting of multiple electromechanical print-heads and gas regulators, controls droplet ejection via valve opening time and pressure. Applicable to bioinks with viscosities between 1 and 70 mPa·s and nozzle diameters of 100–250 μm, it enables reliable high-throughput printing (up to 1 kHz) but has limitations such as a limited number of dispense channels, sensitivity to print pressure, and an impact on cell viability. 88 Electrohydrodynamic jet bioprinting applies a high voltage (0.5–30 kV) between the nozzle and substrate to overcome surface tension and eject droplets. It is suitable for high-resolution printing of thin cell-laden constructs using small-diameter nozzles (<100 μm). Despite its high-resolution capabilities and the ability to minimize lateral variations, it faces challenges such as limited ink choices, disordered fiber arrangement, and the inability to fabricate large-scale 3D constructs (up to 5 mm in height).89–91 Overall, these different jetting-based bioprinting techniques contribute to the diverse landscape of biofabrication with their unique characteristics and trade-offs.
Printability in bioprinting is affected by bioink properties, especially rheological ones characterized by Reynolds (Re) and Weber (We) numbers (Table 2). These dimensionless numbers aid in understanding the relative significance of forces like inertia, surface tension, and viscous forces during printing. In bioprinting, a low Re (<1) implies laminar flow dominated by viscous forces, beneficial for precise droplet formation and placement, while a high Re (>1) indicates turbulent flow, leading to irregular droplet formation and poor print quality. The We number is vital for jet stability and droplet formation. A low We (<1) means surface tension dominates, favorable for jet integrity and preventing droplet breakage, and a high We (>1) shows significant inertial forces, which can cause satellite droplets and loss of print fidelity.77,92
Table 2.
Biomaterials for 3D printing of the airway and lung.
| Types | Advantages | Limitations | Bioinks application of lung and airway | Ref |
|---|---|---|---|---|
| Natural bioinks | ||||
| Collagen | • High binding capacity with integrins and domain receptors • Low immunological response |
• Weak mechanical properties | Neutralized collagen solution loaded with cells: Ingredients: atelocollagen, acetic acid, reconstitution buffer, nutrient buffer, reconstitution buffer, nutrient buffer. Concentration of collagen: 0.3% w/v. |
Kang et al. 85 |
| In the field of trachea reconstruction, collagen can be used to construct scaffolds. Ingredients: monomeric type I and type II atelocollagen, acetic acid, Tris-buffered saline solution. |
Xu et al. 19 | |||
| Alginate | • Biocompatibility • Low immune response |
• Gel strength is weak | Sodium alginate can be used as the middle viscosity layer when manufacturing artificial trachea. Ingredients: calcium chloride solution, alginate hydrogel, 1 × 107 cells/10 mL. |
Bae et al. 51 |
| Alginate can be used to fabricate precise 3D scaffolds for tissue engineering. Ingredients: alginate, CaCl2 solution. |
Khoshnood et al. 93 | |||
| Scaffold fabrication for cartilage regeneration. Ingredients: sodium alginate, CaCl2, NaCl solution. |
Kundu et al. 94 | |||
| Silk fibroin | • Biocompatibility • Well printability • Well mechanical properties |
• Low print resolution • May cause immune responses |
Silk fibroin scaffolds are potential for tracheal substitute and epithelial regeneration. Ingredients: Bombyx mori cocoon silk, Na2CO3, LiBr aqueous solutions, n-butanol. |
Chen et al. 95 |
| Decellularized extracellular matrix | • Biocompatibility • Simulate the microenvironment in vivo |
• Weak Mechanical property • Complex preparation process |
Decellularized extracellular matrix can be used in trachea model. Ingredients: tracheal mucosa part, sodium dodecyl sulfate, phosphate-buffered saline, Triton X-100, peracetic acid, ethanol, acetic acid, pepsin, NaOH. |
Nam et al. 96 |
| Synthetic biomaterials | • High printability • Easy customization |
• Limited cellular interaction capabilities | PSeD/PCL scaffold can be used for bionic cartilage ring construction. Ingredients: PSeD and PCL, tetrahydrofuran. |
Xu et al. 97 |
| Bioprinted tracheal can be fabricated by PCL and human mesenchymal stem cell. Ingredients: PCL, human mesenchymal stem cell. |
Ke et al. 98 | |||
| Compound bioinks | • Can be adjusted according to requirements • Potential for creating complex, functional tissues and organs |
• Complex preparation process | The microfluidic lung chip can be constructed by porous polydimethylsiloxane (PDMS) membrane coated with ECM. Ingredients: PDMS, rat tail collagen type I. |
Guvendiren and Burdick 99 |
Light-based bioprinting techniques
Light-based bioprinting techniques, which are a subset of vat photopolymerization-based bioprinting, play a crucial role in creating three-dimensional (3D) structures. They induce cross-linking, polymerization, or solidification of monomers or precursors at desired locations. 71
Digital light processing (DLP), a common light-based technique, selectively cures a liquid layer by layer with a digitally controlled ultraviolet light source, enabling the achievement of finer and more complex structural features compared to some other bioprinting methods. The achievable resolution in light-based bioprinting is high, typically in micrometers or even sub-micrometer scales, depending on factors like the light source wavelength, optical system precision, and characteristics of the photopolymerizable materials (photoresins). For example, DLP-based bioprinters using a digital micromirror device can achieve resolutions of 10–50 μm (with advanced systems reaching below 10 μm),71,100 Stereolithography (SLA) bioprinters can achieve 5–50 μm, and Two-Photon Polymerization (2PP) can reach sub-micrometer resolutions (100 nm to a few μm), making it suitable for fabricating extremely fine and complex structures for applications such as artificial organ and tissue engineering. 101 Projection stereolithography (PSL), a variation of SLA, uses a digital light projector to solidify liquid photopolymer resin layer by layer. It is known for its speed and precision, allowing for the creation of intricate and highly detailed structures. The resins used, often bio-compatible photopolymer resins, can be tailored for specific applications to support cell adhesion, proliferation, and tissue development.
The ability to achieve such high resolutions in light-based bioprinting makes it a valuable tool for creating intricate tissue constructs and mimicking the microarchitecture of native tissues. This is particularly important for applications where the precise arrangement of cells and materials is critical, such as in the development of artificial organs, tissues, or scaffolds for regenerative medicine and tissue engineering. 102 Projection stereolithography of hydrogels has been developed using food dye additives as biocompatible photoabsorbers, generating vascularized alveolar models with complex topologies. 15
In light-based bioprinting, several key aspects related to printability and cell viability need to be considered. Photo-initiators like Irgacure 2959, Lucirin TPO, and camphorquinone (CQ) are crucial as they start the polymerization process upon light exposure, and their choice affects biocompatibility and the overall printing process. The light wavelength, usually in the 365–405 nm range for curing photopolymers, is specific to the photo-initiator and photopolymer system, and the penetration depth of the light into the bioink is also important, with longer wavelengths penetrating deeper but potentially sacrificing resolution. Light intensity impacts the polymerization rate and printability; higher intensities speed up polymerization but may generate heat harmful to cell viability, so controlling it is essential to balance printing speed and cell survival. Cell viability, a critical factor, can be high if light-based bioprinting is optimized, with factors such as photo-initiator type and concentration, light exposure time and intensity, and protective bioink components affecting it. Printability is influenced by the bioink’s rheological properties like viscosity, elasticity, and thixotropy, which need to be adjusted for accurate extrusion and shape maintenance before polymerization. Additionally, oxygen inhibition in photopolymerization can be mitigated by using oxygen scavengers or modifying the bioink, and the printing environment, including temperature and humidity control, also affects printability and cell viability. Overall, light-based bioprinting offers precision in 3D structure formation but demands careful attention to bioink composition, light parameters, and printing conditions for the best results in tissue engineering and regenerative medicine applications.80,81,103
Emerging bioprinting techniques
In bioprinting strategies, there are an increasing number of new printing methods with advantageous manufacturing efficiency (volumetric printing) and soft structure construction (embedded printing). Volumetric printing can generate three-dimensional objects by solidifying certain volumes of inks, different from the traditional 3D printing approaches based on layer-by-layer strategies. EHDP (Electrohydrodynamic Printing), is a type of additive manufacturing technique that uses electrically driven forces to precisely position nanoparticles, droplets, or fibers at specific locations. This method is known for its high manufacturing accuracy, making it suitable for creating structures with intricate detailing. Embedded printing features the deposition of inks into supporting matrices in predefined paths; therefore, even when the structures formed by the inks are soft, they can be sustained by the matrices. These printing methods could be explored to generate highly complex and soft components of lung and airway with improved accuracy and efficiency.
The current landscape of 3D bioprinting in tissue engineering and the in vitro modeling of the airways and lungs faces several challenges. The intricate architecture of the lung, replete with a diversity of cell types, an elaborate vascular system, and a network of airways, presents a significant hurdle for replication with existing 3D bioprinting technologies. 104 Additionally, the availability of bioinks suitable for lung tissue construction is limited, constraining the ability to print detailed, high-fidelity structures. 105 The prolonged duration of the bioprinting process can also impact cell viability and functionality, potentially reducing the efficacy of the engineered tissues or organs. 60 Post-printing, the maturation of printed constructs into fully functional tissue is a complex task. Furthermore, the critical issue of vascularization remains a major obstacle; without an adequate blood supply, the survival of cells within larger engineered tissues is at risk.106,107 These limitations highlight the need for advancements in bioink development, printing techniques, and post-printing tissue development strategies to improve the viability and functionality of 3D bioprinted airway and lung models.104,108
Bioinks for the airway and lung
Bioinks comprise cells and biomaterials. Below, we survey the different cells and biomaterials involved in airway and lung bioprinting and introduce bioprinting techniques that can generate tissue constructs (Table 2).
Cell-type selection for tracheal and lung model
Cell-type selection is important in tracheal and lung reconstructions. Cell lines and primary cells are both used; for example, the vascularized lung tumor-on-a-chip model consists of primary HUVECs, primary normal human lung fibroblasts, and A549 (human lung adenocarcinoma) cells. In a model of SARS-CoV-2-induced lung injury, the alveolar-capillary barrier was composed of human alveolar epithelial, vascular endothelial, and immune cells. In this model, the epithelial cells used were the immortalized human alveolar epithelial cell line (HPAEpiC), the vascular endothelial cells were the human lung microvasculature cell line (HULEC-5a), and the immune cells were primary isolated peripheral human blood mononuclear cells. The use of primary cells or cell lines has been extensively discussed in previous reviews. In general, primary cells possess characteristics similar to their phenotype in the native environment, which is preferable in lung-on-a-chip models. However, maintaining the functionality of primary cells over extended culture periods is difficult. Cell lines have been widely used because of their facile handling and growth, although they are limited by similar functions in the original lungs and airways.
Biomaterials for 3D printing of the airway and lung
Bioinks are the building blocks for engineered airways and lung tissues and need to recreate specific functional properties and biological activities in bioprinting. The properties of the bioink must match those of the lungs and airways. For instance, bioinks should provide sufficient mechanical strength and flexibility for 3DP structures to mimic the viscoelastic behavior of the lungs, particularly in the alveolar portions. Lung compliance and elasticity must be considered when making bioinks. Bioinks should promote lung and tracheal regeneration, while simultaneously protecting against adverse host reactions after implantation. The development of bioinks suitable for lung and airway printing is a critical aspect of 3D bioprinting. Bioinks must provide not only the structural integrity required for the printed constructs but also the biochemical and biomechanical cues necessary for cell survival, proliferation, and differentiation. 103 Natural biopolymers, such as alginate, collagen, and decellularized extracellular matrix (ECM) proteins, have been widely explored due to their biocompatibility and ability to mimic the native lung microenvironment. However, their printability is often limited, leading to the development of modified natural biopolymers with added reactive functional groups or composite hydrogels combining natural ECM with synthetic components. 103 Bioinks can be categorized as naturally derived, synthetic, or a mixture (Figure 2(b)).
Natural bioinks for 3D printing of the airway and lung
Bioinks with natural ECM components, such as collagen and fibrin, are naturally derived; alginate, cellulose, and gelatin are derived from various natural sources but not native ECM components.109–113 The intrinsic properties of naturally derived bioinks benefit cellular interaction, adhesion, proliferation, and motility; they are biodegradable and bioabsorbable, have little host response, and participate actively in lung and tracheal regeneration.28,114,115 Natural biomaterial-based bioinks can recreate the viscoelasticity of the airways and lungs, particularly the alveolar area. 104 For example, the elasticity modulus of the airway is 2.57–34.4 kPa, and the elasticity modulus of parenchymal lung is estimated as 0.64–5.13 kPa. Some natural biomaterial moduli, such as collagen (0.5–12 kPa)104,116,117 and alginate (20 kPa) 116 match those of primary tissues. To fabricate a 3D alveolar barrier model, lung fibroblasts (MRC5) were suspended in collagen ink to act as a basement membrane, and type I and type II alveolar cells were printed onto the collagen surface. The results showed high cell viability (>90%) in the bioprinting model. 85 However, naturally derived bioinks are limited by insufficient printability and may not efficiently transition from ink to gel.
Collagen
Collagen is a typical natural macromolecule extracted from the ECM that provides a biomimetic environment; for example, collagen bioinks exhibit high binding capacity with integrins and domain receptors. 118 In the lungs, collagen fibers are present in the conducting airway walls and encompass the alveolar ducts. The gelation of collagen bioinks can be controlled by an alkaline buffer solution that neutralizes the acidic collagen solution and warms it to a physiological temperature. Kang et al. 85 prepared bioinks by suspending fibroblasts in a dilute collagen solution. During printing, the endothelial cell ink, collagen ink, and collagen ink containing fibroblasts were printed layer by layer. Collagen-based inks were printed at 4°C to prevent cross-linking. These bioinks containing collagen were used to construct alveolar tissues with structural and functional characteristics. Xu et al. 19 integrated type II atelocollagen with chondroitin sulfate bioink to mimic the tracheal ECM components, demonstrating that atelocollagen can mitigate immunological responses. The obtained scaffolds reproduced the structural and functional properties of native tracheal tissues during extensive tracheal reconstruction.
Alginate
Alginate biomaterials are natural hydrophilic polysaccharides with negative charges derived from brown algae. Alginate hydrogel can entrap water and allow it to diffuse from inside out.119–121 Alginate-based bioinks are suitable for cartilage cell printing because their modulus is similar to that of cartilage tissue, and their ionic cross-linking properties are suitable for printing. 122 Alginate bioinks cross-link primarily with calcium ions. The majority of the Ca2+ ions originate from the CaCl2 aqueous solution. Gelation occurs immediately when ionic interchain bridges are formed between the polymer chains.123,124 Alginate-based bioprinting is widely used for tracheal tissue engineering. For example, Bae et al. 51 utilized two layers of alginate hydrogels in tracheal scaffolds: one layer contained MSCs or chondrogenic-differentiated MSCs, and the other contained epithelial cells. In vitro experiments revealed that chondrogenically differentiated MSCs result in higher glycosaminoglycan accumulation and higher chondrogenic marker gene expression compared to undifferentiated MSCs. Furthermore, in vivo neocartilage formation was observed in an alginate hydrogel containing -MSCs.
Lower alginate concentrations (1%–2%)
Concentrations in the range of 1%–2% are often chosen for bioprinting lung and airway tissues that require a softer and more compliant matrix, mimicking the elasticity of lung parenchyma and alveoli These concentrations are suitable for creating structures resembling the delicate alveolar sacs and ducts, which require a microenvironment that supports gas exchange and allows for cell spreading and migration. Lower concentrations offer a favorable balance between maintaining cell viability and providing a realistic tissue-like environment. 125
Intermediate alginate concentrations (2%–3%)
Alginate concentrations between 2% and 3% strike a balance between structural stability and printability, making them ideal for constructing branching airway structures and regions of transition within the lung. Bioprinted constructs using these concentrations can capture the mechanical properties of airway epithelium and help maintain the tubular shapes of bronchioles. The resulting constructs may offer a combination of mechanical support and flexibility, crucial for replicating the behavior of airways during breathing and expansion.89,126
Higher alginate concentrations (3%–4%)
Concentrations of 3%–4% are chosen for bioprinting airway sections that require greater mechanical strength and rigidity, such as the trachea or larger bronchi. These concentrations can support the creation of more robust and stable constructs that can better withstand external forces and maintain their structural integrity. Bioprinted tissues with higher alginate concentrations might serve as foundational structures for upper airway or larger bronchial segments.81,94
In addition to concentration, other factors should be considered:
Crosslinking Methods: The choice of crosslinking method (e.g. using calcium ions) and its concentration can significantly impact the mechanical properties and stability of the printed constructs.
Cell Viability and Functionality: The selected alginate concentration should support cell viability and desired functionalities within the bioprinted tissue.
Co-Bioink Formulation: Alginate can be combined with other bioink materials, such as cell-laden hydrogels or growth factors, to create more biomimetic and functional lung and airway constructs.
Silk fibroin
Silk fibroin (SF), derived from the natural fibrous polymer of silk, is recognized for its exceptional mechanical properties, which can be tailored for various tissue engineering applications. The Young’s modulus of SF can vary from 1 to 20 GPa, influenced by processing methods, crosslinking techniques, and fiber alignment. This adjustable stiffness positions SF as a material with moderate to high mechanical strength, suitable for applications requiring robust structural integrity. Native lung tissue is defined by its high compliance and flexibility, essential for the expansion and contraction facilitating respiration. The Young’s modulus for lung tissue is significantly lower, within the range of 0.1 to 1 kPa, reflecting its delicate and elastic nature optimized for physiological functions such as gas exchange. 117 The airway system, comprising cartilage and smooth muscle, demands a slightly higher Young’s modulus to maintain structural integrity and function. 127 Cartilage, which provides structural support, has a Young’s modulus ranging from 0.5 to 10 MPa, while the smooth muscle, responsible for airway constriction and dilation, exhibits a modulus of about 1–5 kPa.127,128 These values are indicative of the balance between flexibility and rigidity required for the airway system’s functionality. When considering SF for the bioprinting of lung or airway models, its significantly higher stiffness compared to native tissues could affect the mechanical behavior and cellular interactions. Nevertheless, SF’s favorable biological attributes, including cell adhesion, proliferation, and low inflammatory response, render it a promising candidate for bioinks that seek to harmonize mechanical properties with biological compatibility. 129 To align SF’s mechanical properties with those of the target lung or airway tissues, researchers have employed various modification techniques. Blending, crosslinking, and electrospinning are among the methods used to enhance SF’s properties, aiming to produce biomimetic models with realistic mechanical behavior and biological functionality for research and therapeutic applications. 130 SF’s utility in tissue engineering is further expanded through modifications that improve its mechanical properties and bioprinting performance. For instance, the addition of methacrylate to SF allows for polymerization under aqueous conditions and light-triggered processes, enhancing its use in bioprinting. Studies indicate that SF promotes chondrocyte proliferation and differentiation by reversing the dedifferentiation process, guiding the cells toward a cartilage lineage and potentially enhancing the synthesis of cartilage-specific extracellular matrix (ECM). 131 Chemical modifications, such as the use of glycidyl methacrylate, have been successfully implemented to create an artificial trachea with human and rabbit chondrocytes, demonstrating the potential of SF in bioprinting complex structures. 132 The glycosaminoglycan content and chondrogenic expression associated with SF bioinks suggest their role in facilitating new cartilage formation (Glycosaminoglycan and chondrogenic expression). In vitro cultivation studies of glycidyl methacrylate-modified silk (silk-GMA) hydrogel have shown cell viability, proliferation, and differentiation toward chondrogenesis, highlighting the suitability of SF bioink for partial defected trachea model requiring chondrogenic differentiation (Up to 4 weeks of in vitro cultivation of silk-GMA hydrogel). Additionally, incorporating glycosaminoglycans (GAGs) into silk fibroin (SF) bioinks is critical for the development of bioprinted trachea, as GAGs are essential for replicating the structural and functional properties of native cartilage. GAGs, such as chondroitin sulfate and hyaluronic acid, enhance the compressive resistance and mechanical resilience of cartilage, which are vital for the trachea’s structural integrity. Moreover, GAGs facilitate chondrogenic differentiation, driving stem cells to become chondrocytes that produce a cartilage-specific extracellular matrix. This bioactive environment not only guides cell behavior and tissue organization but also promotes better integration with host tissues post-transplantation, leveraging GAGs’ known roles in tissue regeneration and wound healing. Thus, GAG-enhanced SF bioinks hold significant promise for advancing tracheal tissue engineering through bioprinting.
Decellularized extracellular matrix
Decellularized extracellular matrices (dECMs) are a highly promising group of bioink materials because they can form an extracellular microenvironment that contains structural features and extracellular cues for cell attachment, growth, differentiation, and function, as well as vascularization. As an example of adapting a decellularized ECM for the 3D printing of tracheal tissues, Nam et al. 96 utilized a decellularized ECM derived from porcine tracheas and blood vessels. Tracheal and blood vessel modules were fabricated and assembled into perfusable vascularized tracheal modules as an in vitro model.
Synthetic biomaterials as bioinks for 3D printing of the airway and lung
Synthetic biomaterials exhibit high flexibility for property tuning and chemical modification. Accordingly, bioinks based on these materials can be adjusted in terms of the mechanical properties, degradation rate, and printability to support 3D bioprinting development. 133 Synthetic biomaterials used for bioprinting include Polycaprolactone (PCL),50,51,134–136 Polyurethane (PU),137,138 poly(sebacoyl Diglyceride) (PSeD), 97 and polylactic acid (PLA). 139 In tracheal tissue fabrication, PCL can be printed into fibrous networks to mechanically support the hollow tissue and form microscale pores for nutrient diffusion. In the study by Ke et al., a hydrogel material based on a hydrogels kit, which includes thiol-modified collagen, thiol-modified hyaluronan, and heparin, was used. The bioprinting method employed was an extrusion-based system facilitated the precise deposition of PCL and MSC-laden hydrogels to construct patient-matched trachea constructs. The resultant tracheal tissues were comparable to native tissues in terms of elastic modulus and yield stress. 130 Xu et al. 97 mixed PSeD with PCL to fabricate ring-shaped porous scaffolds for loading chondrocytes in tracheal tissue regeneration.
The use of synthetic biomaterials as bioinks has the advantages of high printability and easy customization; however, synthetic biomaterials may not fully replicate native tissues due to their lack of inherent bioactivity, which is crucial for cell function and tissue development. Additionally, their limited cellular interaction capabilities can affect cell behavior, as they may not provide the necessary binding sites or ligands present in natural extracellular matrices. Furthermore, synthetics often fall short in replicating the specific mechanical properties of various native tissues, potentially leading to functional and stability issues in the printed constructs.
Compound bioinks for airway and lung 3D printing
Although natural biomaterials can create an ideal milieu for cell adhesion and proliferation by mimicking a native ECM, the adjustable qualities of natural polymers are low.122,140 “Adjustable qualities” in biomaterials refer to the capacity to modify or customize a material’s characteristics, such as mechanical properties, biodegradability, bioactive component incorporation, and structural design, to align with the specific needs of a biomedical application. Consequently, these natural polymers can be blended with synthetic or other natural polymers to produce stable structures with adjustable properties for 3D bioprinting. The mechanical qualities, printability, cross-linking, and other characteristics of synthetic polymers can be adjusted to improve them, despite not supporting cellular adhesion or promotion as well as natural polymers. 99 Many polymers, such as alginate and collagen, have been combined to form different bioinks and their properties can be adjusted according to requirements. Traditional synthetic biodegradable polymers, such as PLA, polyglycolic acid, and polylactic-co-glycolic acid, combined with natural sources of polymers, are popular for generating cartilage with a variety of cell types, including mesenchymal stem cells (MSCs), MSC aggregates (clusters or spheroids of MSCs), and cartilage-resident chondroprogenitor cells. These aggregates generally present an earlier developmental stage and have a greater differentiation capability than spread cells. In tissue engineering, MSC aggregates are considered to have a higher regenerative capacity compared to individual MSCs.141–143
Various gelation techniques have been employed to compound bioinks, which are essential for the 3D printing of complex structures like the airway and lung. These techniques enable the utilization of different bioprinting methods to create constructs that mimic the native tissue architecture and biological functions. gelation techniques used in bioprinting including thermal gelation, chemical crosslinking, photochemical crosslinking, enzymatic gelation, hydrogel swelling and diffusion, electrostatic interactions. Advanced bioprinting may employ a combination of gelation methods to ensure both rapid initial setting and long-term structural integrity. The selection of a gelation technique is tailored to the bioink composition, the specific 3D printing technology (such as extrusion, inkjet, or laser-assisted bioprinting), and the target application in tissue engineering or regenerative medicine. Each method can be optimized to balance the need for rapid gelation with maintaining cell viability and achieving the required mechanical properties for the printed tissue construct. As 3D bioprinting technology evolves, innovative gelation strategies continue to emerge, enhancing the potential for creating complex, functional tissues and organs.
Emerging bioinks for 3D bioprinting
New biomaterials, such as self-healing polymers, injectable rapid adhesive hydrogels, and on-demand antibacterial biomaterials, have proven promising for biomedical applications. Their printability and compatibility with lungs and airway tissues deserve further verification as they may suit the specific requirements in tissue regeneration and biological model constructure of lungs and airways. For example, when self-healing polymers are printed to repair lungs and airways, they can mimic the regenerative capabilities of living tissues to support cell growth and function and even to restore tissue when subjected to damage. Besides, when used as part of tissue-engineered scaffolds, adhesive hydrogels can not only promote the attachment to cells and tissues but also potentially eliminate the need for suturing in implantation. Self-healing polymers: The respiratory system is continuously subjected to physical stress and repeated mechanical deformation during the breathing process. Within these circumstances, self-healing polymers could offer significant advantages for lung and airway bioprinting applications. In particular, these biomaterials could help maintain the longevity of bioprinted lung constructs or tracheal replacements by repairing any microscale damage that may occur during physiological processes.
Injectable rapid adhesive hydrogels: While hydrogels offer excellent potential for cellular encapsulation and the formation of scaffold materials in lung and airway bioprinting, achieving proper adhesion and integration within the host tissue is a challenge. 144 Injectable rapid adhesive hydrogels could address this issue by creating strong bonds between the bioprinted construct and native tissue, regardless of the tissue’s mechanical properties. This would be particularly crucial for bioprinted bronchi, bronchioles, and alveoli, where constructs would need to withstand repetitive mechanical strain during respiration.
On-demand antibacterial biomaterials: The risk of infection is a major concern for patients undergoing lung or airway grafting procedures. Bioprinted constructs can be particularly susceptible to bacterial invasion due to their high surface area and the presence of biomaterials, which may serve as conducive environments for bacteria. On-demand antibacterial biomaterials have the potential to prevent or treat infections in lung and airway bioprinting applications, thereby lowering the risk associated with implant failure or the need for additional surgeries. For instance, bioprinted bronchial or tracheal replacements can be designed with these biomaterials, releasing antibacterial agents upon detection of an infection to minimize infection-related complications and promote long-term graft success.
Despite the promising applications, each biomaterial requires extensive testing and development to ensure its biocompatibility, mechanical properties, and functional characteristics are optimized for lung and airway bioprinting. The performance of these biomaterials under physiological conditions, as well as their long-term safety and effectiveness, will ultimately be key to their successful implementation in clinical settings
Applications of 3D bioprinting in tissue engineering and in vitro modeling of the airways and lungs
Bioprinted airway tissues
Bioprinted trachea for tissue engineering
Geometric tracheal features can be generated with 3D printing resolution. Biological structures and functions such as cartilage and epithelium can also be realized by bioprinting. For cartilage tissue, bioprinting offers precise chondrocyte placement for controlled cartilage construct development, with adjustable ECM composition and mechanical properties to replicate native tissue. 60 Cartilage tissue regeneration in bioprinted tracheal constructs has been widely proven. Park et al. 50 fabricated a multilayered artificial trachea with autologous chondrocytes and applied it to lesions measuring >6 cm. The bioprinting of tracheal tissues of clinically relevant sizes takes a long time, which may compromise cell viability and functionality; the research team also developed a two-step bioprinting method to create a trachea-mimicking cellular construct with cartilage formation in a nude mouse model.60,130 Their key strategy was to utilize extrusion-based printing to generate a porous bellows framework, and selective printing of cellular components (e.g. cartilage rings and epithelium lining) was performed on the framework. Compared with other bioprinting techniques that typically involve additional sacrificial components, this two-step strategy significantly reduces the printing time. The printed tracheal scaffold and neo-cartilage both add mechanical strength and can adapt to the mechanical requirements of the native trachea. In addition to cartilage regeneration, biological properties of the smooth muscle tissue and epithelium, and vascularization similar to those of the native trachea are important. Extrusion-based bioprinting was used to generate separate cartilage and smooth muscle regions using polycaprolactone and human MSC-laden hydrogels. The obtained structures exhibited mechanical properties comparable to those of native tracheal tissues, and the formation of cartilage and smooth muscle was demonstrated through in vitro culture. 31 Extrusion-based scaffold printing has been widely adopted to generate tracheal tissues, whereas other approaches have also been explored. Huo et al. developed a functional cartilage-vascularized fibrous tissue using 3D bioprinting with photo-crosslinkable tissue-specific bioinks. For epithelial tissues, bioprinting enables the creation of multilayered structures with barrier functions, specialized features like cilia and microvilli, mucin production for protection, and the formation of tight junctions for tissue integrity and substance regulation. 26 Epithelium-like tissue and regenerated cartilage rings help to recover tracheal physiological functions for segmental tracheal defects in a rabbit model. 61 Although scaffold-based tracheal tissue engineering can provide mechanical support and structural guidance for tissue generation, there are issues such as the limited biocompatibility of the scaffold materials and inflexibility of the scaffold design. To offer a different approach for tracheal tissue engineering, Machino et al. bioprinted a cartilaginous and fibrous tracheal construct with tissue-specific bioinks embedding human chondrocytes, MSCs, fibroblasts, and umbilical vein endothelial cells. These bioprinted scaffold-free trachea-like tubes replace the epithelium and capillaries lost during surgical resection of rat tracheas. 145
Bioprinted in vitro airway models
In vitro airway models are vital to study airway physiology and disease mechanisms and development, such as rhinosinusitis, asthma, and COPD (chronic obstructive lung disease). 146 A personalized and specific airway disease model can be achieved using 3D bioprinting. Bioprinting facilitates the placement of various cell types at desired locations and mimics airway cells and microenvironmental arrangements. Park et al. created an in vitro airway model featuring printed airway epithelium and a vascular network that exhibited respiratory symptoms such as asthmatic inflammation and allergen-induced asthma exacerbation, with a significant increase in mucus production observed in a model simulating inflammatory conditions through the addition of IL-13. 147 Airway models can be developed further with printing technology and bioinks. Nam et al. fabricated the tracheal modules by using the tracheal mucosa-derived dECM and microporous membrane, and utilized the prepared vascular-tissue-derived dECM to fabricate the blood vessel modules. After assembling tracheal and blood vessel modules together to manufacture the tracheal model, which imitated the interface between the tracheal epithelium and blood vessels. These modules were assembled to form a tracheal model with perfusable blood vessels, which could be used to study respiratory diseases. 96 Physiologically biomimetic in vitro airway model is more potential for clinical trial. An inkjet-based bioprinting airway model, facilitated precise structural arrangement of cells to reflect cell-cell and cell-ECM interactions, composed of epithelium, ECM and endothelium, including tight junctions. 148
Bioprinted lung tissues
Bioprinted lungs for tissue engineering
Different strategies have been used to reconstruct the physiological characteristics of lungs. Huang et al. have specifically addressed the challenge of donor shortage in organ transplantation by exploring 3D bioprinted lungs for transplantation. They have investigated the bioprinting of functional lung tissues that can potentially be used for the treatment of end-stage lung diseases, where traditional transplant options are limited. The advancements in their research present a unique opportunity to study diseases like chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, and even the implications of COVID-19 on lung function, within an in vitro setting that closely resembles the human lung microenvironment. 71 Huang et al. employed extrusion-based 3D bioprinting to create silk- and cellulose-based hydrogel scaffolds to investigate the influence of fiber alignment on LESC behavior. The aligned cellulose-based nanofibers acted as biomimetic structures of the lung ECM, contributing to LESC orientation. This lung-like tissue structure with anisotropic ECM is promising for lung tissue regeneration. 149 To study the effects of physical cues on myofibroblasts in a 3D microenvironment, Matera et al. 150 replicated the key features (dimensionality, matrix stiffness, and fiber density) of pulmonary fibrosis. A study of lung regeneration guided by in vivo 3D biomimetic porous collagen scaffolds delivered hepatocyte growth factors to improve the microenvironment. 151 All the 3D biomimetic lung structures mentioned above can be fabricated by bioprinting, although some use another method.
Bioprinted alveoli structural models
The respiratory mucosa and alveoli reside at the air-liquid interface (ALI). 3D bioprinting has been applied to generate ALI culture models and can precisely control the composition and surface structure on which the cells grow to mimic the basement membrane of the epithelium. Kang et al. used inkjet printing to achieve a single-cell-level arrangement with high-resolution control of alveolar cells and established an accurate arrangement of the three-layer structure of four cells: pulmonary microvascular endothelial cells (HUC-5A) were printed on the bottom layer, pulmonary fibroblasts (MRC5) were printed on the middle layer, and alveolar epithelial cells of types I and II were arranged in an orderly manner on the top layer, forming a respiratory membrane structure. The bionic respiratory membrane structure thickness was only 10 μm. 85 A recent study described a comprehensive organ-/disease-specific model that recapitulated the key attributes of pulmonary fibrosis and conditions during inhalation therapy. 152 To address the challenge of generating 3D complex tissue structures, Grigoryan et al. 15 reported on light-based printing using food dyes as biocompatible and potent photoabsorbers. Hydrogel structures with 3D complex topological features were generated using this technique, which could mimic the oxygenation and flow of red blood cells during tidal ventilation and airway distension. 15 Das et al. 153 constructed an in vivo lung-cancer-on-a-chip model, including fluidic channels, air channels and a porous membrane, which can provide an air-liquid interface used in inhalation and exhalation cycles. Similar to the biochemical composition of native lung tissues is important for lung related models. A 3D bioprinted vascularized lung cancer organoid models, including patient-derived lung cancer organoids, lung fibroblasts, and perfusable vessels, which are constructed by 3D bioprinting technology. 154 (Table 3)
Table 3.
3D bioprinting promotes airways and lungs for applications in tissue engineering and in vitro modeling.
| Model | Bioinks composition | Concentration | Cell sources | Cell density | Bioprinting technology | Reference |
|---|---|---|---|---|---|---|
| Airway in vitro modeling | Tracheal mucosa-derived dECM (tmdECM) hydrogel | 1% (w/v) | 1. Human dermal microvascular endothelial cells 2. Human lung fibroblasts |
5 × 106 cells/ml | An in-house 3D cell cell-printing system by a one one-step process. | Park et al. 147 |
| Trachea in vitro modeling | Upper module: tmdECM Inklower module: VdECM |
Upper module: 3 ml of 1% tmdECM Inklower module: Not mention |
Upper module: primary bronchial/tracheal epithelial cells Lower module: HUVECs |
Upper module: fifth to sixth passage Lower module: 107/ml |
Upper module: a multiplehead 3D printer and a stainlesssteel print head (HN series, Musashi Engineering, Japan) were used. Lower module: The multiplehead 3D printer, G-code generator program, and custom coaxial needles were used to print the perfusable blood vessel. |
Nam et al. 96 |
| Aveolar in vitro model | In a Transwell permeable support Endothelial cell ink, collagen ink, fibroblast containing collagen ink |
0.3% | Human lung cellline NCI-H1703 as flat squamous AT I, NCI-H441 as cuboidal AT II, endothelial HULEC-5a, and MRC5 as fibroblasts. | NCI-H1703 cells: 8 × 104/ml NCI-H441 cells: 1.6 × 105/ml HULEC-5a cells 3 × 105/ml |
The bio-ink was ejected in the form of droplets using the drop-on-demand (DoD) inkjet bioprinting system. | Kang et al. 85 |
| Trachea tissue engineering | Multicellular spheroids | / | Normal human dermal fibroblasts, Normal human articular chondrocytes, Human umbilical vein endothelial cells, Human mesenchymal stem cells. | 2 × 105 cells/ml |
Inject printing | Machino et al. 145 |
| Trachea tissue engineering | 1. Gelatin methacryloyl (GelMA) and methacrylate-modified chondroitin sulfate (CSMA) and methacryloyl-modified acellular cartilage matrix (ACMMA) 2. Methacrylate-modified hyaluronic acid (HAMA)+8-arm-polyethylene glycol-succinic acid ester (8-PEG NHS)+methacryloyl-modified acellular derm matrix (ADMMA) |
1. 10% w/v + 2% w/v + 1% w/v 2. 2% w/v + 5% w/v + 1% w/v |
Rabbit auricular chondrocytes Rabbit auricular skin dermis fibroblasts |
1 × 108 cells/ml 5 × 107 cells/ml |
Extrusion type printing Photocrosslinking |
Huo et al. 61 |
| Trachea tissue engineering | 1. 2-Hydroxy-4’-(2-hydroxyethoxy)-2-methylpropiophenone 2. Hyaluronic acid (HA) 3. Gelatin |
1. 0.1% w/v 2. 1.5 mg/ml 3. 30 mg/ml |
Human mesenchymal stem cell(hMSCs) | 1.5 × 107 cells/ml | Extrusion-based bioprinting system Crosslinking |
Ke et al. 98 |
Future perspectives
In the field of 3D bioprinting, the construction of complex tissues such as airways and lungs is extremely challenging. There are numerous existing theoretical and technical bottlenecks that limit its broader application and development. However, it also holds great potential and is expected to open up new paths for tissue engineering and respiratory disease research.
Theoretical Bottlenecks: ①Challenges in Alveolar Structure and Function: Alveoli in the lungs are responsible for gas exchange. Their thin-walled structures are precise and complex, requiring the precise implantation of alveolar epithelial cells and capillary endothelial cells, as well as taking into account functions like surfactant production. Replicating them is extremely difficult. ②Dilemmas in Airway Branching and Cartilage Support: Airways are in the form of branching tubes, lined with epithelial cells and supported by cartilage externally. It is quite challenging to accurately reproduce the branching morphology, match the appropriate cell types, and incorporate cartilage to achieve mechanical support in bioprinting. ③Obstacles in Immune Response and Graft Integration: Bioprinted lung and airway tissues need to be compatible with the recipient’s immune system to avoid rejection. Reconciling immunogenicity issues is an urgent theoretical barrier to overcome.
Technical Bottlenecks: ①Bottlenecks in High-Resolution Bioprinting: Current bioprinting technologies have deficiencies in resolution and precision and struggle to print multiple cell types simultaneously, making it difficult to finely reproduce the structures of airways and alveoli. ②Difficulties in Mimicking Mechanical Properties: Lung tissues experience variable forces during respiration. Bioprinted products need to mimic their mechanical characteristics such as elasticity and compliance, which is yet to be achieved by existing biomaterials. ③Challenges in Vascularization and Perfusion: Efficient vascularization within bioprinted lung tissues is crucial for oxygen and nutrient supply. Printing blood vessels within complex structures and connecting them to the host vascular network is technically highly challenging. ④Problems in Cell Sourcing and Differentiation: It is not easy to obtain sufficient quantities of specific cells such as alveolar epithelial cells, endothelial cells, and smooth muscle cells and maintain their functions for bioprinting.⑤Barriers in Scaling-up and Clinical Translation: Scaling up the bioprinting process to produce large-sized lung and airway constructs that meet clinical needs, while ensuring reproducibility and quality control, poses significant technical challenges.
To overcome these bottlenecks, efforts from multiple fields are indispensable. Interdisciplinary collaboration is the foundation. Doctors, biologists, and engineers work together to deeply analyze the physiological functions of airways and lungs, providing a scientific basis for the design of tissue-engineered scaffolds and in vitro biological models. Materials scientists develop new multi-material composite bioinks, mix functional materials and cells in specific proportions to create bionic structures and enhance the physiological functions of tissues.
Artificial intelligence (AI) and big data may be the keys to breaking the deadlock. 155 On the one hand, AI deeply analyzes vast amounts of biomedical data to quickly gain insights into the physiological laws of airways and lungs, facilitating the rational design and precise manufacturing of bioprinted tissues. On the other hand, with the help of machine learning algorithms, it optimizes printing paths and parameters, improves printing resolution, achieves precise positioning of single cells and the construction of micro/nanoscale extracellular matrices, which is beneficial for alveolar reconstruction. In conclusion, integrating AI and big data and strengthening interdisciplinary cooperation, and continuously working on improving physiological understanding, printing precision, bionic effects, vascularization, and tissue integration are expected to break the existing constraints, unlock more possibilities for 3D bioprinting of airways and lungs, and accelerate the entry into a new stage of clinical application.
Footnotes
Author contributions: (I) Conception and design: Jinbo Zhao, Yanning Zhang, Yujian Liu, Chen Shu; (II) Administrative support: Jinbo Zhao, Nan Ma; (III) Conduct and analyze the search on related literature: Yanning Zhang, Yujian Liu, Chen Shu, Yang Shen, Mengchao Li; (IV) Manuscript writing: All authors; (V) Final approval of manuscript: All authors.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Natural Science Foundation of China [Grant number 82070101]; the Natural Science Foundation of Hubei Province [Grant number 2024AFB541]; Shaanxi Youth Science and Technology Rising Star Fund [Grant number 2018KJXX-051]; Xi’an General Medical Research projects [24YXYJ0178]; The Science and Technology Innovation Fund [Grant number 2019QYTS004]; the Certificate of China Postdoctoral Science Foundation Grant [Grant number 2024M754284]; Youth Innovation Science Fund Project of Tangdu Hospital (2023CTQN009), and the Top Talent Fund of Tangdu Hospital [Grant number 2019].
ORCID iD: Jinbo Zhao  https://orcid.org/0000-0002-7806-6072
https://orcid.org/0000-0002-7806-6072
References
- 1. Labaki WW, Rosenberg SR. Chronic obstructive pulmonary disease. Ann Intern Med 2020; 173: ITC17–ITC32. [DOI] [PubMed] [Google Scholar]
- 2. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet 2017; 389: 1931–1940. [DOI] [PubMed] [Google Scholar]
- 3. Levine SM, Marciniuk DD. Global impact of respiratory disease: what can we do, together, to make a difference? Chest 2022; 161: 1153–1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sauler M, Bazan IS, Lee PJ. Cell death in the lung: the apoptosis-necroptosis axis. Annu Rev Physiol 2019; 81: 375–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chaudhuri D, Sasaki K, Karkar A, et al. Corticosteroids in COVID-19 and non-COVID-19 ARDS: a systematic review and meta-analysis. Intensive Care Med 2021; 47: 521–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Christenson SA, Smith BM, Bafadhel M, et al. Chronic obstructive pulmonary disease. Lancet 2022; 399: 2227–2242. [DOI] [PubMed] [Google Scholar]
- 7. Lareau SC, Fahy B, Meek P, et al. Chronic Obstructive Pulmonary Disease (COPD). Am J Respir Crit Care Med 2019; 199: P1–P2. [DOI] [PubMed] [Google Scholar]
- 8. Baiguera S, D’Innocenzo B, Macchiarini P. Current status of regenerative replacement of the airway. Expert Rev Respir Med 2011; 5: 487–494. [DOI] [PubMed] [Google Scholar]
- 9. Galliger Z, Vogt CD, Panoskaltsis-Mortari A. 3D bioprinting for lungs and hollow organs. Transl Res 2019; 211: 19–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mejías JC, Nelson MR, Liseth O, et al. A 96-well format microvascularized human lung-on-a-chip platform for microphysiological modeling of fibrotic diseases. Lab Chip 2020; 20: 3601–3611. [DOI] [PubMed] [Google Scholar]
- 11. Huh D, Matthews BD, Mammoto A, et al. Reconstituting organ-level lung functions on a chip. Science 2010; 328: 1662–1668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. da Silva da, Costa FA, Soares MR, Malagutti-Ferreira MJ, et al. Three-dimensional cell cultures as a research platform in lung diseases and COVID-19. Tissue Eng Regen Med 2021; 18: 735–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang L, Wang L, He J, et al. Tracheal suspension by using 3-dimensional printed personalized scaffold in a patient with tracheomalacia. J Thorac Dis 2016; 8: 3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wang L, Liu W, He J, et al. Treatment of bronchomalacia using three-dimensional printed polycaprolactone scaffold in a pediatric patient. J Thorac Cardiovasc Surg 2019; 157: e287–e290. [DOI] [PubMed] [Google Scholar]
- 15. Grigoryan B, Paulsen SJ, Corbett DC, et al. Multivascular networks and functional intravascular topologies within biocompatible hydrogels. Science 2019; 364: 458–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vera D, García-Díaz M, Torras N, et al. Engineering tissue barrier models on hydrogel microfluidic platforms. ACS Appl Mater Interfaces 2021; 13: 13920–13933. [DOI] [PubMed] [Google Scholar]
- 17. Costa A, de Souza Carvalho-Wodarz C, Seabra V, et al. Triple co-culture of human alveolar epithelium, endothelium and macrophages for studying the interaction of nanocarriers with the air-blood barrier. Acta Biomater 2019; 91: 235–247. [DOI] [PubMed] [Google Scholar]
- 18. Sellgren KL, Butala EJ, Gilmour BP, et al. A biomimetic multicellular model of the airways using primary human cells. Lab Chip 2014; 14: 3349–3358. [DOI] [PubMed] [Google Scholar]
- 19. Xu Y, Dai J, Zhu X, et al. Biomimetic trachea engineering via a modular ring strategy based on bone-marrow stem cells and atelocollagen for use in extensive tracheal reconstruction. Adv Mater 2022; 34: e2106755. [DOI] [PubMed] [Google Scholar]
- 20. Paek J, Park SE, Lu Q, et al. Microphysiological engineering of self-assembled and perfusable microvascular beds for the production of vascularized three-dimensional human microtissues. ACS Nano 2019; 13: 7627–7643. [DOI] [PubMed] [Google Scholar]
- 21. Tang H, Sun WY, Chen Y, et al. Future directions for research on tissue-engineered trachea. Biodes Manuf 2022; 5: 627–632. [Google Scholar]
- 22. Udelsman B, Mathisen DJ, Ott HC. A reassessment of tracheal substitutes-a systematic review. Ann Cardiothorac Surg 2018; 7: 175–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Law JX, Liau LL, Aminuddin BS, et al. Tissue-engineered trachea: a review. Int J Pediatr Otorhinolaryngol 2016; 91: 55–63. [DOI] [PubMed] [Google Scholar]
- 24. Zhang M, Wang P, Luo R, et al. Biomimetic human disease model of SARS-CoV-2-induced lung injury and immune responses on organ chip system. Adv Sci (Weinh) 2021; 8: 2002928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Varone A, Nguyen JK, Leng L, et al. A novel organ-chip system emulates three-dimensional architecture of the human epithelia and the mechanical forces acting on it. Biomaterials 2021; 275: 120957. [DOI] [PubMed] [Google Scholar]
- 26. Zimmerling A, Zhou Y, Chen X. Bioprinted constructs for respiratory tissue engineering. Bioprinting 2021; 24: e00177. [Google Scholar]
- 27. Murphy SV, De Coppi P, Atala A. Opportunities and challenges of translational 3D bioprinting. Nat Biomed Eng 2020; 4: 370–380. [DOI] [PubMed] [Google Scholar]
- 28. Matai I, Kaur G, Seyedsalehi A, et al. Progress in 3D bioprinting technology for tissue/organ regenerative engineering. Biomaterials 2020; 226: 119536. [DOI] [PubMed] [Google Scholar]
- 29. Zhang YS, Haghiashtiani G, Hübscher T, et al. 3D extrusion bioprinting. Nat Rev Methods Primers 2021; 1: 75. [Google Scholar]
- 30. Levato R, Jungst T, Scheuring RG, et al. From shape to function: the next step in bioprinting. Adv Mater 2020; 32: e1906423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kaye R, Goldstein T, Grande DA, et al. A 3-dimensional bioprinted tracheal segment implant pilot study: rabbit tracheal resection with graft implantation. Int J Pediatr Otorhinolaryngol 2019; 117: 175–178. [DOI] [PubMed] [Google Scholar]
- 32. Reimelt AM, Vasilescu DM, Beare R, et al. Analysis of the alveolar shape in 3-D. Am J Physiol Lung Cell Mol Physiol 2023; 324: L358–L372. [DOI] [PubMed] [Google Scholar]
- 33. Li H, Dai J, Wang Z, et al. Digital light processing (DLP)-based (bio) printing strategies for tissue modeling and regeneration. Aggregate 2023; 4: e270. [Google Scholar]
- 34. Brand-Saberi BEM, Schäfer T. Trachea: anatomy and physiology. Thorac Surg Clin 2014; 24: 1–5. [DOI] [PubMed] [Google Scholar]
- 35. Yesil-Celiktas O, Hassan S, Miri AK, et al. Mimicking human pathophysiology in organ-on-chip devices. Adv Biosyst 2018; 2(10): 1800109. [Google Scholar]
- 36. Zhang H, Fu W, Xu Z. Re-epithelialization: a key element in tracheal tissue engineering. Regen Med 2015; 10: 1005–1023. [DOI] [PubMed] [Google Scholar]
- 37. Webb WR. Thin-section CT of the secondary pulmonary lobule: anatomy and the image—the 2004 Fleischner lecture. Radiology 2006; 239: 322–338. [DOI] [PubMed] [Google Scholar]
- 38. Weibel ER. Lung morphometry: the link between structure and function. Cell Tissue Res 2017; 367: 413–426. [DOI] [PubMed] [Google Scholar]
- 39. Joshi N, Walter JM, Misharin AV. Alveolar macrophages. Cell Immunol 2018; 330: 86–90. [DOI] [PubMed] [Google Scholar]
- 40. Crouch E. Pathobiology of pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 1990; 259: L159–L184. [DOI] [PubMed] [Google Scholar]
- 41. Gill S, Wight TN, Frevert CW. Proteoglycans: key regulators of pulmonary inflammation and the innate immune response to lung infection. Anat Rec 2010; 293: 968–981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bernard GR, Artigas A, Brigham KL, et al. The American-European consensus conference on ARDS. Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Am J Respir Crit Care Med 1994; 149: 818–824. [DOI] [PubMed] [Google Scholar]
- 43. Belloli EA, Beckford R, Hadley R, et al. Idiopathic non-specific interstitial pneumonia. Respirology 2016; 21: 259–268. [DOI] [PubMed] [Google Scholar]
- 44. Thannickal VJ, Toews GB, White ES, et al. Mechanisms of pulmonary fibrosis. Annu Rev Med 2004; 55: 395–417. [DOI] [PubMed] [Google Scholar]
- 45. Sabetkish S, Kajbafzadeh AM. Lung extracellular matrix as a platform for lung organ bioengineering: design and development of tissue engineered lung. Adv Exp Med Biol 2021; 1345: 35–46. [DOI] [PubMed] [Google Scholar]
- 46. Rucker AJ, Nellis JR, Klapper JA, et al. Lung retransplantation in the modern era. J Thorac Dis 2021; 13: 6587–6593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal life support organization registry international report 2016. ASAIO J 2017; 63: 60–67. [DOI] [PubMed] [Google Scholar]
- 48. Warnecke G, Van Raemdonck D, Smith MA, et al. Normothermic ex-vivo preservation with the portable Organ Care System Lung device for bilateral lung transplantation (INSPIRE): a randomised, open-label, non-inferiority, phase 3 study. Lancet Respir Med 2018; 6: 357–367. [DOI] [PubMed] [Google Scholar]
- 49. Proud D, Leigh R. Epithelial cells and airway diseases. Immunol Rev 2011; 242: 186–204. [DOI] [PubMed] [Google Scholar]
- 50. Park JH, Yoon JK, Lee JB, et al. Experimental tracheal replacement using 3-dimensional bioprinted artificial trachea with autologous epithelial cells and chondrocytes. Sci Rep 2019; 9: 2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bae SW, Lee KW, Park JH, et al. 3D bioprinted artificial trachea with epithelial cells and chondrogenic-differentiated bone marrow-derived mesenchymal stem cells. Int J Mol Sci 2018; 19(6): 1624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Barkauskas CE, Cronce MJ, Rackley CR, et al. Type 2 alveolar cells are stem cells in adult lung. J Clin Invest 2013; 123: 3025–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Dobbs L, Johnson M, Vanderbilt J, et al. The great big alveolar TI cell: evolving concepts and paradigms. Cell Physiol Biochem 2009; 25: 55–62. [DOI] [PubMed] [Google Scholar]
- 54. Evans MJ, Cabral LJ, Stephens RJ, et al. Transformation of alveolar Type 2 cells to Type 1 cells following exposure to NO2. Exp Mol Pathol 1975; 22: 142–150. [DOI] [PubMed] [Google Scholar]
- 55. Evans MJ, Dekker NP, Cabral-Anderson LJ, et al. Quantitation of damage to the alveolar epithelium by means of type 2 cell proliferation. Am Rev Respir Dis 1978; 118: 787–790. [DOI] [PubMed] [Google Scholar]
- 56. Kathiriya JJ, Brumwell AN, Jackson JR, et al. Distinct airway epithelial stem cells hide among club cells but mobilize to promote alveolar regeneration. Cell Stem Cell 2020; 26: 346–358. e344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Vaughan AE, Brumwell AN, Xi Y, et al. Lineage-negative progenitors mobilize to regenerate lung epithelium after major injury. Nature 2015; 517: 621–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rock JR, Barkauskas CE, Cronce MJ, et al. Multiple stromal populations contribute to pulmonary fibrosis without evidence for epithelial to mesenchymal transition. Proc Natl Acad Sci U S A 2011; 108(52): E1475–E1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hirose T, Fukuma Y, Takeshita A, et al. The role of lymphotoxin-α in rheumatoid arthritis. Inflamm Res 2018; 67: 495–501. [DOI] [PubMed] [Google Scholar]
- 60. Park JH, Ahn M, Park SH, et al. 3D bioprinting of a trachea-mimetic cellular construct of a clinically relevant size. Biomaterials 2021; 279: 121246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Huo Y, Xu Y, Wu X, et al. Functional trachea reconstruction using 3D-bioprinted native-like tissue architecture based on designable tissue-specific bioinks. Adv Sci (Weinh) 2022; 9: e2202181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Correa-Gallegos D, Jiang D, Rinkevich Y. Fibroblasts as confederates of the immune system. Immunol Rev 2021; 302: 147–162. [DOI] [PubMed] [Google Scholar]
- 63. Plikus MV, Wang X, Sinha S, et al. Fibroblasts: origins, definitions, and functions in health and disease. Cell 2021; 184: 3852–3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sturtzel C. Endothelial cells. Adv Exp Med Biol 2017; 1003: 71–91. [DOI] [PubMed] [Google Scholar]
- 65. Evans CE, Cober ND, Dai Z, et al. Endothelial cells in the pathogenesis of pulmonary arterial hypertension. Eur Respir J 2021; 58(3): 2003957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Burri PH. Fetal and postnatal development of the lung. Annu Rev Physiol 1984; 46: 617–628. [DOI] [PubMed] [Google Scholar]
- 67. Caplan AI. MSCs: the sentinel and safe-guards of injury. J Cell Physiol 2016; 231: 1413–1416. [DOI] [PubMed] [Google Scholar]
- 68. Farré R, Otero J, Almendros I, et al. Bioengineered lungs: a challenge and an opportunity. Arch Bronconeumol 2018; 54: 31–38. [DOI] [PubMed] [Google Scholar]
- 69. Hawkins FJ, Suzuki S, Beermann ML, et al. Derivation of airway basal stem cells from human pluripotent stem cells. Cell Stem Cell 2021; 28: 79–95. e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Muruganandah V, Kupz A. Immune responses to bacterial lung infections and their implications for vaccination. Int Immunol 2022; 34: 231–248. [DOI] [PubMed] [Google Scholar]
- 71. Huang G, Zhao Y, Chen D, et al. Applications, advancements, and challenges of 3D bioprinting in organ transplantation. Biomater Sci 2024; 12: 1425–1448. [DOI] [PubMed] [Google Scholar]
- 72. Yang J, He H, Li D, et al. Advanced strategies in the application of gelatin-based bioink for extrusion bioprinting. Biodes Manuf 2023; 6: 586–608. [Google Scholar]
- 73. Herzog J, Franke L, Lai Y, et al. 3D bioprinting of microorganisms: principles and applications. Bioprocess Biosyst Eng 2024; 47: 443–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Xu H-Q, Liu J-C, Zhang Z-Y, et al. A review on cell damage, viability, and functionality during 3D bioprinting. Mil Med Res 2022; 9: 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Fu Z, Naghieh S, Xu C, et al. Printability in extrusion bioprinting. Biofabrication 2021; 13: 033001. [DOI] [PubMed] [Google Scholar]
- 76. Boularaoui S, Al Hussein G, Khan KA, et al. An overview of extrusion-based bioprinting with a focus on induced shear stress and its effect on cell viability. Bioprinting 2020; 20: e00093. [Google Scholar]
- 77. Ng WL, Shkolnikov V. Jetting-based bioprinting: process, dispense physics, and applications. Biodes Manuf 2024; 7: 771–799. [Google Scholar]
- 78. Ng WL, Huang X, Shkolnikov V, et al. Controlling droplet impact velocity and droplet volume: key factors to achieving high cell viability in sub-nanoliter droplet-based bioprinting. Int J Bioprint 2022; 8: 424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Ng WL, Huang X, Shkolnikov V, et al. Polyvinylpyrrolidone-based bioink: influence of bioink properties on printing performance and cell proliferation during inkjet-based bioprinting. Biodes Manuf 2023; 6: 676–690. [Google Scholar]
- 80. Levato R, Dudaryeva O, Garciamendez-Mijares CE, et al. Light-based vat-polymerization bioprinting. Nat Rev Methods Primers 2023; 3: 47. [Google Scholar]
- 81. Fang W, Yu Z, Gao G, et al. Light-based 3D bioprinting technology applied to repair and regeneration of different tissues: a rational proposal for biomedical applications. Mater Today Bio 2024; 27: 101135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Garciamendez-Mijares CE, Aguilar FJ, Hernandez P, et al. Design considerations for digital light processing bioprinters. Appl Phys Rev 2024; 11(3): 031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lee JS, Park HS, Jung H, et al. 3D-printable photocurable bioink for cartilage regeneration of tonsil-derived mesenchymal stem cells. Addit Manuf 2020; 33: 101136. [Google Scholar]
- 84. Mota C, Camarero-Espinosa S, Baker MB, et al. Bioprinting: from tissue and organ development to in vitro models. Chem Rev 2020; 120: 10547–10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Kang D, Park JA, Kim W, et al. All-inkjet-printed 3D alveolar barrier model with physiologically relevant microarchitecture. Adv Sci (Weinh) 2021; 8: 2004990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Serra P, Piqué A. Laser-induced forward transfer: fundamentals and applications. Adv Mater Technol 2019; 4: 1800099. [Google Scholar]
- 87. Demirci U, Montesano G. Single cell epitaxy by acoustic picolitre droplets. Lab Chip 2007; 7: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 88. Ng WL, Lee JM, Yeong WY, et al. Microvalve-based bioprinting–process, bio-inks and applications. Biomater Sci 2017; 5: 632–647. [DOI] [PubMed] [Google Scholar]
- 89. Qiu Z, Zhu H, Wang Y, et al. Functionalized alginate-based bioinks for microscale electrohydrodynamic bioprinting of living tissue constructs with improved cellular spreading and alignment. Biodes Manuf 2023; 6: 136–149. [Google Scholar]
- 90. Onses MS, Sutanto E, Ferreira PM, et al. Mechanisms, capabilities, and applications of high-resolution electrohydrodynamic jet printing. Small 2015; 11: 4237–4266. [DOI] [PubMed] [Google Scholar]
- 91. An S, Lee MW, Kim NY, et al. Effect of viscosity, electrical conductivity, and surface tension on direct-current-pulsed drop-on-demand electrohydrodynamic printing frequency. Appl Phys Lett 2014; 105(21): 214102. [Google Scholar]
- 92. Furbank RJ, Morris JF. An experimental study of particle effects on drop formation. Phys Fluids 2004; 16: 1777–1790. [Google Scholar]
- 93. Khoshnood N, Zamanian A, Abbasi M. The potential impact of polyethylenimine on biological behavior of 3D-printed alginate scaffolds. Int J Biol Macromol 2021; 178: 19–28. [DOI] [PubMed] [Google Scholar]
- 94. Kundu J, Shim J-H, Jang J, et al. An additive manufacturing-based PCL–alginate–chondrocyte bioprinted scaffold for cartilage tissue engineering. J Tissue Eng Regen Med 2015; 9: 1286–1297. [DOI] [PubMed] [Google Scholar]
- 95. Chen Z, Zhong N, Wen J, et al. Porous three-dimensional silk fibroin scaffolds for tracheal epithelial regeneration in vitro and in vivo. ACS Biomater Sci Eng 2018; 4: 2977–2985. [DOI] [PubMed] [Google Scholar]
- 96. Nam H, Choi YM, Cho S, et al. Modular assembly of bioprinted perfusable blood vessel and tracheal epithelium for studying inflammatory respiratory diseases. Biofabrication 2022; 15(1): 014101. [DOI] [PubMed] [Google Scholar]
- 97. Xu Y, Guo Y, Li Y, et al. Biomimetic trachea regeneration using a modular ring strategy based on poly(sebacoyl diglyceride)/polycaprolactone for segmental trachea defect repair. Adv Funct Mater 2020; 30: 2004276. [Google Scholar]
- 98. Ke D, Yi H, Est-Witte S, et al. Bioprinted trachea constructs with patient-matched design, mechanical and biological properties. Biofabrication 2019; 12: 015022. [DOI] [PubMed] [Google Scholar]
- 99. Guvendiren M, Burdick JA. Engineering synthetic hydrogel microenvironments to instruct stem cells. Curr Opin Biotechnol 2013; 24: 841–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sharma R, Perez MR, da Silva VA, et al. 3D bioprinting complex models of cancer. Biomater Sci 2023; 11: 3414–3430. [DOI] [PubMed] [Google Scholar]
- 101. Frankowski J, Kurzątkowska M, Sobczak M, et al. Utilization of 3D bioprinting technology in creating human tissue and organoid models for preclinical drug research-state-of-the-art. Int J Pharm 2023; 644: 123313. [DOI] [PubMed] [Google Scholar]
- 102. Daly AC, Lim KS. High resolution lithography 3D bioprinting. Trends Biotechnol 2023; 41: 262–263. [DOI] [PubMed] [Google Scholar]
- 103. Dabaghi M, Carpio MB, Moran-Mirabal JM, et al. 3D (bio)printing of lungs: past, present, and future. Eur Respir J 2023; 61(1): 2200417. [DOI] [PubMed] [Google Scholar]
- 104. Mahfouzi SH, Safiabadi Tali SH, Amoabediny G. 3D bioprinting for lung and tracheal tissue engineering: criteria, advances, challenges, and future directions. Bioprinting 2021; 21: e00124. [Google Scholar]
- 105. Barreiro Carpio M, Dabaghi M, Ungureanu J, et al. 3D bioprinting strategies, challenges, and opportunities to model the lung tissue microenvironment and its function. Front Bioeng Biotechnol 2021; 9: 773511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Lovett M, Lee K, Edwards A, et al. Vascularization strategies for tissue engineering. Tissue Eng Part B Rev 2009; 15: 353–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Shen Z, Xia T, Zhao J, et al. Current status and future trends of reconstructing a vascularized tissue-engineered trachea. Connect Tissue Res 2023; 64: 428–444. [DOI] [PubMed] [Google Scholar]
- 108. Comber EM, Palchesko RN, Ng WH, et al. De novo lung biofabrication: clinical need, construction methods, and design strategy. Transl Res 2019; 211: 1–18. [DOI] [PubMed] [Google Scholar]
- 109. Rahmani Dabbagh S, Rezapour Sarabi M, Birtek MT, et al. 3D bioprinted organ-on-chips. Aggregate 2023; 4: e197. [Google Scholar]
- 110. Choi Y-J, Park H, Ha D-H, et al. 3D bioprinting of in vitro models using hydrogel-based bioinks. Polymers (Basel) 2021; 13: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Donderwinkel I, van Hest JCM, Cameron NR. Bio-inks for 3D bioprinting: recent advances and future prospects. Polym Chem 2017; 8: 4451–4471. [Google Scholar]
- 112. Yu J, Park SA, Kim WD, et al. Current advances in 3D bioprinting technology and its applications for tissue engineering. Polymers (Basel) 2020; 12: 2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Liu F, Chen Q, Liu C, et al. Natural polymers for organ 3D bioprinting. Polymers (Basel) 2018; 10: 1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Gungor-Ozkerim PS, Inci I, Zhang YS, et al. Bioinks for 3D bioprinting: an overview. Biomater Sci 2018; 6: 915–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Carrow JK, Kerativitayanan P, Jaiswal MK, et al. Polymers for bioprinting. In: Atala A, Yoo JJ. (eds) Essentials of 3D biofabrication and translation. Boston, MA: Academic Press, 2015, pp.229–248. [Google Scholar]
- 116. Soofi SS, Last JA, Liliensiek SJ, et al. The elastic modulus of Matrigel™ as determined by atomic force microscopy. J Struct Biol 2009; 167: 216–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Raub CB, Putnam AJ, Tromberg BJ, et al. Predicting bulk mechanical properties of cellularized collagen gels using multiphoton microscopy. Acta Biomater 2010; 6: 4657–4665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Lin K, Zhang D, Macedo MH, et al. Advanced collagen-based biomaterials for regenerative biomedicine. Adv Funct Mater 2019; 29: 1804943. [Google Scholar]
- 119. Axpe E, Oyen ML. Applications of alginate-based bioinks in 3D bioprinting. Int J Mol Sci 2016; 17(12): 1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Piras CC, Smith DK. Multicomponent polysaccharide alginate-based bioinks. J Mater Chem B 2020; 8: 8171–8188. [DOI] [PubMed] [Google Scholar]
- 121. Rastogi P, Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication 2019; 11: 042001. [DOI] [PubMed] [Google Scholar]
- 122. Gopinathan J, Noh I. Recent trends in bioinks for 3D printing. Biomater Res 2018; 22: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Kuo CK, Ma PX. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: part 1. Structure, gelation rate and mechanical properties. Biomaterials 2001; 22: 511–521. [DOI] [PubMed] [Google Scholar]
- 124. Kaklamani G, Cheneler D, Grover LM, et al. Mechanical properties of alginate hydrogels manufactured using external gelation. J Mech Behav Biomed Mater 2014; 36: 135–142. [DOI] [PubMed] [Google Scholar]
- 125. Jiao T, Lian Q, Lian W, et al. Properties of collagen/sodium alginate hydrogels for bioprinting of skin models. J Bionic Eng 2023; 20: 105–118. [Google Scholar]
- 126. Li Z, Huang S, Liu Y, et al. Tuning alginate-gelatin bioink properties by varying solvent and their impact on stem cell behavior. Sci Rep 2018; 8: 8020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Singh G, Chanda A. Mechanical properties of whole-body soft human tissues: a review. Biomed Mater 2021; 16: 062004. [DOI] [PubMed] [Google Scholar]
- 128. Polio SR, Kundu AN, Dougan CE, et al. Cross-platform mechanical characterization of lung tissue. PLoS One 2018; 13: e0204765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Karamchand L, Makeiff D, Gao Y, et al. Biomaterial inks and bioinks for fabricating 3D biomimetic lung tissue: a delicate balancing act between biocompatibility and mechanical printability. Bioprinting 2023; 29: e00255. [Google Scholar]
- 130. Feng X, Hu Y, Cao L, et al. Artificial trachea design, construction, and application: materials, cells, and growth factors. Appl Mater Today 2023; 35: 101968. [Google Scholar]
- 131. Cheng G, Davoudi Z, Xing X, et al. Advanced silk fibroin biomaterials for cartilage regeneration. ACS Biomater Sci Eng 2018; 4: 2704–2715. [DOI] [PubMed] [Google Scholar]
- 132. Hong H, Seo YB, Kim DY, et al. Digital light processing 3D printed silk fibroin hydrogel for cartilage tissue engineering. Biomaterials 2020; 232: 119679. [DOI] [PubMed] [Google Scholar]
- 133. Tan B, Gan S, Wang X, et al. Applications of 3D bioprinting in tissue engineering: advantages, deficiencies, improvements, and future perspectives. J Mater Chem B 2021; 9: 5385–5413. [DOI] [PubMed] [Google Scholar]
- 134. Park JH, Park JY, Nam IC, et al. Human turbinate mesenchymal stromal cell sheets with bellows graft for rapid tracheal epithelial regeneration. Acta Biomater 2015; 25: 56–64. [DOI] [PubMed] [Google Scholar]
- 135. Rehmani SS, Al-Ayoubi AM, Ayub A, et al. Three-dimensional-printed bioengineered tracheal grafts: preclinical results and potential for human use. Ann Thorac Surg 2017; 104: 998–1004. [DOI] [PubMed] [Google Scholar]
- 136. Chang JW, Park SA, Park JK, et al. Tissue-engineered tracheal reconstruction using three-dimensionally printed artificial tracheal graft: preliminary report. Artif Organs 2014; 38: E95–E105. [DOI] [PubMed] [Google Scholar]
- 137. Jung SY, Lee SJ, Kim HY, et al. 3D printed polyurethane prosthesis for partial tracheal reconstruction: a pilot animal study. Biofabrication 2016; 8: 045015. [DOI] [PubMed] [Google Scholar]
- 138. Best CA, Pepper VK, Ohst D, et al. Designing a tissue-engineered tracheal scaffold for preclinical evaluation. Int J Pediatr Otorhinolaryngol 2018; 104: 155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Goldstein TA, Smith BD, Zeltsman D, et al. Introducing a 3-dimensionally printed, tissue-engineered graft for airway reconstruction: a pilot study. Otolaryngol Head Neck Surg 2015; 153: 1001–1006. [DOI] [PubMed] [Google Scholar]
- 140. Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater 2011; 23: H41–H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Levato R, Webb WR, Otto IA, et al. The bio in the ink: cartilage regeneration with bioprintable hydrogels and articular cartilage-derived progenitor cells. Acta Biomater 2017; 61: 41–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Möller T, Amoroso M, Hägg D, et al. In vivo chondrogenesis in 3D bioprinted human cell-laden hydrogel constructs. Plast Reconstr Surg Glob Open 2017; 5: e1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Hung KC, Tseng CS, Dai LG, et al. Water-based polyurethane 3D printed scaffolds with controlled release function for customized cartilage tissue engineering. Biomaterials 2016; 83: 156–168. [DOI] [PubMed] [Google Scholar]
- 144. Ouyang C, Yu H, Wang L, et al. Tough adhesion enhancing strategies for injectable hydrogel adhesives in biomedical applications. Adv Colloid Interface Sci 2023; 319: 102982. [DOI] [PubMed] [Google Scholar]
- 145. Machino R, Matsumoto K, Taniguchi D, et al. Replacement of rat tracheas by layered, trachea-like, scaffold-free structures of human cells using a bio-3D printing system. Adv Healthc Mater 2019; 8: e1800983. [DOI] [PubMed] [Google Scholar]
- 146. Boschetto P, Quintavalle S, Miotto D, et al. Chronic obstructive pulmonary disease (COPD) and occupational exposures. J Occup Med Toxicol 2006; 1: 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Park JY, Ryu H, Lee B, et al. Development of a functional airway-on-a-chip by 3D cell printing. Biofabrication 2018; 11: 015002. [DOI] [PubMed] [Google Scholar]
- 148. Lee Y, Lee MK, Lee H-R, et al. 3D-printed airway model as a platform for SARS-CoV-2 infection and antiviral drug testing. Biomaterials 2024; 311: 122689. [DOI] [PubMed] [Google Scholar]
- 149. Huang L, Yuan W, Hong Y, et al. 3D printed hydrogels with oxidized cellulose nanofibers and silk fibroin for the proliferation of lung epithelial stem cells. Cellulose (Lond) 2021; 28: 241–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Matera DL, DiLillo KM, Smith MR, et al. Microengineered 3D pulmonary interstitial mimetics highlight a critical role for matrix degradation in myofibroblast differentiation. Sci Adv 2020; 6(37): eabb5069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wang L, Zhao Y, Yang F, et al. Biomimetic collagen biomaterial induces in situ lung regeneration by forming functional alveolar. Biomaterials 2020; 236: 119825. [DOI] [PubMed] [Google Scholar]
- 152. Sengupta A, Dorn A, Jamshidi M, et al. A multiplex inhalation platform to model in situ like aerosol delivery in a breathing lung-on-chip. Front Pharmacol 2023; 14: 1114739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Das P, Najafikhoshnoo S, Tavares-Negrete JA, et al. An in-vivo-mimicking 3D lung cancer-on-a-chip model to study the effect of external stimulus on the progress and inhibition of cancer metastasis. Bioprinting 2022; 28: e00243. [Google Scholar]
- 154. Choi Y-m, Lee H, Ann M, et al. 3D bioprinted vascularized lung cancer organoid models with underlying disease capable of more precise drug evaluation. Biofabrication 2023; 15: 034104. [DOI] [PubMed] [Google Scholar]
- 155. Zhang Z, Zhou X, Fang Y, et al. AI-driven 3D bioprinting for regenerative medicine: from bench to bedside. Bioact Mater 2025; 45: 201–230. [DOI] [PMC free article] [PubMed] [Google Scholar]