Abstract
To optimize patient outcomes, healthcare decisions should be based on the most up-to-date high-quality evidence. Randomized controlled trials (RCTs) are vital for demonstrating the efficacy of interventions; however, information on how an intervention compares to already available treatments and/or fits into treatment algorithms is sometimes limited. Although different therapeutic classes are available for the treatment of chronic obstructive pulmonary disease (COPD), assessing the relative efficacy of these treatments is challenging. Synthesizing evidence from multiple RCTs via meta-analysis can help provide a comprehensive assessment of all available evidence and a “global summary” of findings. Pairwise meta-analysis is a well-established method that can be used if two treatments have previously been examined in head-to-head clinical trials. However, for some comparisons, no head-to-head studies are available, for example the efficacy of single-inhaler triple therapies for the treatment of COPD. In such cases, network meta-analysis (NMA) can be used, to indirectly compare treatments by assessing their effects relative to a common comparator using data from multiple studies. However, incorrect choice or application of methods can hinder interpretation of findings or lead to invalid summary estimates. As such, the use of the GRADE reporting framework is an essential step to assess the certainty of the evidence. With an increasing reliance on NMAs to inform clinical decisions, it is now particularly important that healthcare professionals understand the appropriate usage of different methods of NMA and critically appraise published evidence when informing their clinical decisions. This review provides an overview of NMA as a method for evidence synthesis within the field of COPD pharmacotherapy. We discuss key considerations when conducting an NMA and interpreting NMA outputs, and provide guidance on the most appropriate methodology for the data available and potential implications of the incorrect application of methods. We conclude with a simple illustrative example of NMA methodologies using simulated data, demonstrating that when applied correctly, the outcome of the analysis should be similar regardless of the methodology chosen.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-03056-x.
Keywords: Bayesian, Bucher ITC, Chronic obstructive pulmonary disease, Frequentist, GRADE, Head-to-head comparison, Indirect treatment comparison, Network meta-analysis, Randomized controlled trials, Single-inhaler triple therapy
Plain Language Summary
There are several different treatments available for chronic obstructive pulmonary disease (COPD). Finding out which of these treatments is the most effective is difficult, especially if conflicting results from clinical trials have been reported, or if treatments have never been directly compared to each other. Meta-analysis allows the results from multiple studies to be combined together to give a single summary of findings. This can be useful in cases where previous trials have shown contradictory findings. However, this method can only be used if there is more than one study looking at the same two treatments (e.g., several studies that compared treatment A to treatment B). For treatments that have never been compared in clinical trials, network meta-analysis (NMA) can be used. This method allows several treatments to be compared at the same time using the results from trials comparing different treatments. This method creates ‘indirect evidence’. Indirect evidence refers to cases where two treatments have never been directly compared to each other in a clinical study, but both have been separately compared to a common treatment (e.g., treatment A and treatment C have never been directly compared to each other, but both have been separately compared to treatment B in a clinical study). NMA can be carried out using different methods. However, if the correct method is not chosen, this can lead to inaccurate results. It is becoming more common for NMA findings to be used to help make clinical decisions. Therefore, it is important that healthcare professionals are able to assess the results of published NMAs, including the methods used, to find the most appropriate results to support their clinical decisions. This tutorial provides an overview of different NMA methods, with a focus on the use of these methods within the context of COPD treatments. We also present an example where we use various NMA methods on the same data set to show that different methods should lead to similar results if the methods are used correctly.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12931-024-03056-x.
Introduction
To optimize patient outcomes in chronic obstructive pulmonary disease (COPD), it is important that decisions on funding and reimbursement made by health technology assessment (HTA) bodies and payers are based on a thorough appraisal of the evidence for efficacy of treatments [1, 2]. It is also important that decisions made by healthcare professionals (HCPs) and management recommendations in national and international guidelines are based on the most up-to-date and highest-quality evidence available [3–7].
Randomized controlled trials (RCTs) demonstrate the efficacy of therapies, but often these studies do not compare all available treatments or provide information on how individual treatments fit into treatment algorithms [8]. RCTs usually compare the intervention of interest to an established treatment and/or placebo and are seldom replicated. RCTs examining the same treatments can also sometimes result in contradictory conclusions—this can be due to different trial designs or populations, but also as a result of random variation [9, 10]. Synthesizing evidence from multiple RCTs provides a balanced and comprehensive assessment of all available evidence on a given topic, as well as a “global summary” of findings. This is a fundamental way in which HTA bodies, payers, providers, and those developing clinical management guidelines make informed decisions [1, 3, 4, 11].
Various methods of evidence synthesis can be used in the development of management recommendations and HTA appraisals/reimbursement decisions, and HTAs and payers often have set preferred approaches. For example, the National Institute for Health and Care Excellence (NICE) Technical Support Documents (TSDs) on evidence synthesis make recommendations for preparing, reviewing, and appraising submissions to NICE [12]. However, the TSDs do not attempt to recommend the form that the analysis must take or the methods to be used. Any methods fulfilling the required properties are valid; the appropriateness of the approaches often depends upon the data available [13]. However, methodological problems can hinder interpretation of findings or lead to invalid summary estimates [14–17]. Such problems include inappropriate searching and selection of relevant trials for inclusion in analyses [18, 19], lack of publication bias assessment or evidence appraisal [20, 21], poor reporting of methodology [14], and drawing inappropriate or unsupported conclusions [22, 23].
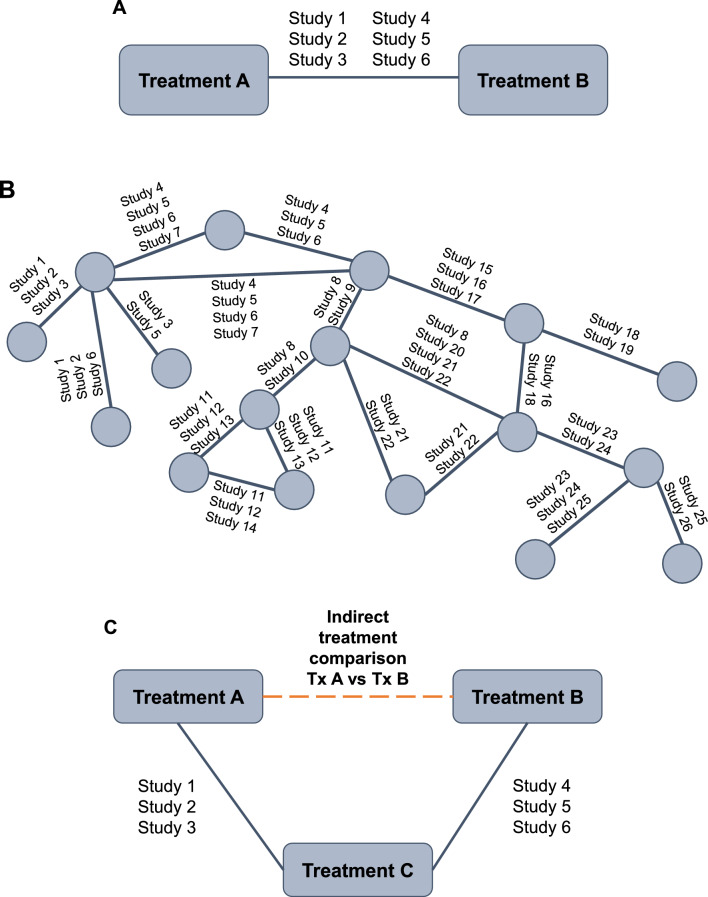
Meta-analysis of RCTs is now widely used to provide a summary measure of effect for an individual treatment as part of evidence synthesis [13]. Pairwise meta-analysis compares the efficacy or safety of two treatments that have been directly compared in head-to-head clinical trials, assuming the population and outcomes are comparable across trials [13, 24, 25] (Fig. 1A). A “network” diagram is constructed, which consists of “nodes” representing interventions and “lines” representing available direct comparisons between interventions (Fig. 1B) [26]. The methodology for performing pairwise meta-analysis is well established and HCPs are familiar with the outputs seen in publications such as Cochrane reviews [27]. All evidence comparing the two treatments is combined and statistical methods are used to calculate a “pooled treatment effect”, which can help inform comparative efficacy and/or safety of interventions. Common measures generated via meta-analysis include odds ratio (OR; odds of an event in the treatment group vs odds of the event in the control group); relative risk (absolute risk in the treatment group vs absolute risk in the control group); and risk difference (difference between the observed risks [proportions of individuals with the outcome of interest] in the treatment group and the control group).
Fig. 1.
A Pairwise meta-analysis; B Network meta-analysis; C Indirect treatment comparison. Tx treatment
In our example, a range of molecules in different therapeutic classes and different inhaler devices are available for the treatment of COPD [28] and new therapies based on combinations of these molecules have been developed and approved over recent years. Assessing the relative efficacy and effectiveness of these treatments within and between classes of monotherapy, dual, and triple therapy is challenging but important.
Sometimes, RCTs are not available to inform clinically important comparisons, such as the comparative efficacy of single-inhaler triple therapies for the treatment of COPD, and it is highly unlikely any will be undertaken. To address this problem, meta-analysis methods have been developed, which allow indirect comparisons of treatments by assessing their relative efficacy versus a common comparator using data from multiple studies [24, 29, 30] (Fig. 1C). In effect, this methodology allows researchers and decision makers to ask additional research questions beyond those originally studied. These indirect treatment comparisons (ITCs) are increasingly used by HTA bodies that are interested in the costs and benefits of the entire algorithm of treatments available (e.g., Pharmaceutical Benefits Advisory Committee in Australia, Canadian Agency for Drugs and Technologies in Health, NICE in the United Kingdom) [13, 31–33]. ITCs are able to inform decision makers of the relative effects of different medicines on individual outcomes, and provide a hierarchy of competing treatments, without compromising the rigor of the original RCT.
A pairwise meta-analysis of all relevant RCTs may be judged as being the highest level of evidence (if the analysis is of sufficient design quality) [5, 34]. Network meta-analysis (NMA) allows the simultaneous analysis of direct (head-to-head) and indirect (through a common comparator) data and is less prone to confounding bias than cohort or observational studies [24, 35].
With an increasing use of ITCs to inform clinical decisions, it is important that HCPs are able to critically appraise published analyses. Therefore, the purpose of this tutorial is to fill an important gap in the literature surrounding this topic, by providing an overview of NMA as an evidence synthesis method with a worked example of COPD pharmacotherapy. This tutorial will outline key considerations when planning, conducting, and interpreting NMAs. The tutorial will end with a simple illustrative example of different NMA methodologies using simulated data to illustrate their impact on the conclusions.
The basics of evidence synthesis
Step 1: a systematic literature review
Prior to conducting a meta-analysis, all relevant RCTs in the research area must be identified through a systematic literature review (SLR). This ensures that all relevant studies are systematically identified for inclusion or exclusion in the analysis.
The SLR should follow best practice methodology, including a priori registration with the International Prospective Register of Systematic Reviews (PROSPERO) [36], and should be communicated using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [37]. Cochrane, the gold standard, recommends that the search should be based on a pre-defined search string (specific for each database) and all records identified from the searches must be evaluated for their eligibility for inclusion, usually defined by the research question of interest [38]. Research questions are defined using the population, intervention, comparator, outcome, setting (PICOS) framework (Table 1). Other criteria, such as time horizon and language of studies, should also be pre-specified before the SLR is carried out.
Table 1.
PICOS criteria
| Population | Adults or children, comorbidities permitted |
| Intervention | Drug or drug combination of interest |
| Comparator | Active treatment or placebo |
| Outcome | e.g., change from baseline in forced expiratory volume in 1 s |
| Setting | Study design, duration, location/country |
A non-systematic review introduces a high risk of bias, even before an ITC is completed. For example, exclusion of studies based on their design (e.g., those with placebo or no treatment arms) or restricting the inclusion of trials to those undertaken in a particular location or time period can have a significant effect on the conclusions of the analysis [39]. Theoretically, if the excluded trials are similar to those included, their omission will not have any systematic impact on the estimates, although it will lead to wider confidence intervals. However, if the excluded trials are different from those included, their omission may cause an over- or under-estimation of treatment effect. As such, the research question must be closely related to the inclusion and exclusion criteria, and all of these elements impact the application of study conclusions.
Step 2: data extraction and meta-analysis
Once all relevant studies have been identified, data should be extracted, and a quality assessment/risk of bias completed. A gold standard framework for assessing risk of bias is the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2) [40]. Bias can occur when there are flaws in the design, conduct, analysis, and reporting of randomized trials, causing the study findings to be underestimated or overestimated. This is important for transparency of results; if a large number of included studies are deemed as having a high risk of bias, then overall findings of any combination of these studies should be interpreted with caution [41]. It is recommended that quality assessment and data extraction should be completed independently by at least two reviewers [42].
An overview of the different approaches to network meta-analysis
NMA/multiple treatment comparison (MTC) allows the simultaneous evaluation of direct and indirect evidence across multiple treatments and studies (example in Fig. 1B). Using NMA, the identity of each treatment can be preserved (i.e., different doses and/or co-treatments), with no requirement to combine (pool) different treatment doses or combinations [43]. Other treatments that are not necessarily of interest to the research question can be included in the network of comparisons to provide additional evidence (e.g., to ‘connect’ other treatments into the network that otherwise would not be connected by providing a common comparator) [43]. All treatments in an NMA can be compared, providing they are linked (either directly or indirectly) in the final network. In some cases, the researcher may wish to compare treatments that are not linked either directly or indirectly within the study network. In this case, other methods such as matching-adjusted indirect comparisons (MAIC) can be used to make indirect comparisons [11].
Key assumptions of NMA
Four assumptions are fundamental to NMA: similarity, transitivity, consistency, and homogeneity [4, 11, 26]. An overview of these assumptions is shown in Table 2.
Table 2.
Key assumptions of NMA
| Assumption | |
|---|---|
| Similarity | Are the studies included in the NMA similar enough in terms of research question (PICOS) to be pooled together? |
| Transitivity | Are there any effect modifiers (patient or study characteristics) known or thought to influence the treatment effect? If so, are there no systematic differences in the distribution of effect modifiers between the included trials? |
| Consistency | For mixed comparisons, is there agreement between the direct (head-to-head) and indirect (via a common comparator) evidence within the network? |
| Homogeneity | Are there no imbalances in population, interventions, outcomes, or study design across direct and indirect comparisons within the NMA? |
NMA network meta-analysis; PICOS population, intervention, comparator, outcome, setting
Similarity
Studies included in an NMA must be similar. Similarity includes both clinical and methodological similarity and is based on clinical judgement and knowledge rather than statistical methods. Visualizing relevant patient characteristics (e.g., age, sex, and disease severity) across all trials included in the NMA using a summary table or showing covariate distribution via a scatter plot can help identify dissimilarity between studies (i.e., with outlying data points potentially indicating a violation of the similarity assumption) [4, 44]. Studies brought together in a network must have a similar research question (PICOS) to be pooled together without affecting effect estimations (i.e., the estimated impact of a treatment on the outcome of interest or on the association between variables). If two studies are adequately similar (e.g., mean age ranging between 45 and 60 years), the relative effect of one treatment versus placebo should remain unchanged if tested under the conditions of the other treatment versus placebo.
Transitivity
Transitivity implies that there are no systematic differences between the included comparisons other than the interventions being compared [26]. For example, intervention A must be similar when it appears in A versus B studies and A versus C studies with respect to all patient and study characteristics that may affect the two relative effects. Although clinical and methodological differences between studies are inevitable, prior to conducting NMA, it should be assessed whether such imbalances are considered large enough to potentially violate the transitivity assumption (e.g., the degree of lung function impairment can heavily impact results in COPD—if some studies contain mostly patients with mild lung function impairment and others include patients with more severe lung function impairment, the transitivity assumption may be violated) [26].
Consistency
Networks should be consistent, i.e., there should be agreement between direct (RCT) and indirect (based on a common comparator) evidence within a network [44]. Combining inconsistent evidence is inappropriate and may lead to a biased result—either an under- or overestimation of treatment effect. For closed loops within an NMA (i.e., both direct and indirect comparisons are possible; Fig. 2), inconsistency assessment can be conducted. If both direct and indirect estimations are aligned, the network can be considered consistent.
Fig. 2.
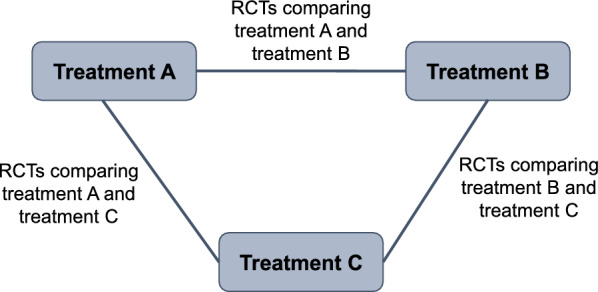
A closed loop with three treatments. RCT randomized controlled trial
Homogeneity
Studies collated in an SLR will inevitably have some level of variability between them, in terms of patient population, interventions/outcomes of interest, or study design/methodological differences. Even when selected using systematic criteria, significant differences in effect modifiers can still be present. Studies may also differ in the way in which the outcomes were measured or defined, the concomitant medications allowed, the length of follow-up, or the timeframe during which the studies were conducted. As in pairwise analyses, homogeneity between studies included in an NMA must be considered. Homogeneity can be assessed in cases where identical treatment comparisons are made and multiple data sources are available. Imbalances in population, interventions, outcomes, and study design across direct and indirect comparisons in an NMA can lead to biased indirect estimations [45, 46].
Overview of NMA methods
There are two common frameworks of NMA: frequentist (including Bucher ITC) and Bayesian. The most frequently used method in the literature is Bayesian NMA, followed by frequentist NMA, and then Bucher ITC. Bucher ITC is based on simple equations whilst frequentist and Bayesian NMA are based on more complex methods (generalized linear models).
Bucher ITC
The method described by Bucher and colleagues in 1997 is an ITC-based approach using simple equations (no statistical model is required) [47]. The indirect comparison of treatment A versus treatment B is estimated by comparing the treatment effects of treatment A and treatment B relative to a common comparator (treatment C; example Fig. 1C) [47]. This allows the comparison of treatments with no head-to-head evidence, whilst preserving the randomization of the original RCTs. This is often the method of choice if evidence is limited (e.g., comparison of just two interventions [48–50]). For example, in the case of three treatments (treatment A, B, and C), the indirect treatment effect of treatment A versus treatment B could be estimated as the treatment effect of A versus a common comparator (treatment C) minus the treatment effect of B versus treatment C [47]. No pooled standard error or standard deviation can be calculated (see Additional file 1, section 1.1). This method may also be the most appropriate if potential effect modifiers vary between studies, and risk introducing bias into the analysis [51]. In larger networks of evidence, indirect comparisons of interventions connected through longer paths can be conducted through multiple steps. However, adding many steps between treatments increases the uncertainty of the estimation.
The advantage of the Bucher ITC is that it is based on simple equations and relatively straightforward to conduct. A key limitation is its unsuitability for performing ITC with more complex networks of treatments with multi-arm studies. The Bucher ITC method is recommended by multiple HTA organizations as a preferred approach for conducting cross-trial ITCs [52]. See Additional file 1, section 1.1 for further information. Recent examples of studies conducted using a Bucher ITC approach in a non-COPD context include Akkoç 2023 [51], Cruz 2023 [50], Merkel 2023 [52], and Pinter 2022 [49].
Frequentist NMA
Frequentist NMA uses the approach most familiar to clinicians, in which measures are thought to have a fixed, unvarying (but unknown) value, without a probability distribution. Frequentist methodologies calculate confidence intervals for the value, or significance tests of hypotheses concerning it. Frequentist NMA is based on generalized linear models and uses weighted least squares regression (LSR; see Additional file 1, section 1.2 for further details).
Frequentist analysis is based solely on observed data. Hypothesis testing is conducted, with the null hypothesis being ‘no statistically significant difference between treatments’. Results are presented as estimated relative effects (mean difference, OR, etc., and a 95% confidence interval [CI; i.e., if the experiment was repeated 100 times, the true value would be covered by the interval 95 times]). P-scores can be calculated to rank treatments and results are interpreted as showing a statistically significant difference or absence thereof. Frequentist analysis is considered more conservative than a standard Bayesian NMA and corresponding 95% intervals are usually narrower.
A frequentist analysis can be implemented relatively straightforwardly using R, Stata, or Python, and there are several packages available, which make the analysis easier. The simplicity of the model can be a deciding factor in choosing frequentist over a Bayesian approach [53]. Advantages of the frequentist method are its suitability for sparse networks of evidence and the fact that the interpretation of classical statistics is more familiar to clinicians. Providing heterogeneity is moderate or low, the estimation bias is considered lower using a frequentist model than with other methods [54]. The main limitation is the inability to incorporate any additional information that may already be known about the parameter of interest (e.g., previously observed evidence from pilot or observational studies obtained through expert clinician opinion) into the analysis. Recent examples of studies conducted using a frequentist approach in a non-COPD context include: Karam [55], Lampl [53], Recchia [56], Shen [57], and Zhang [54].
Bayesian NMA
Bayesian methods are based on the idea that unknown quantities, such as forced expiratory volume in one second (FEV1) differences between treatments, have probability distributions. Bayesian NMA is also based on generalized linear models; however, the Bayesian approach is deemed more flexible than the frequentist approach as it also allows the incorporation of additional information into the model, in the form of prior distributions or ‘priors’. A prior is any external information that is already known or believed about the parameter of interest (for example, additional observational study data on the distribution of change from baseline in FEV1), and it represents the uncertainty about the parameter of interest before the current data are examined. The prior distribution is then updated to produce the posterior distribution by ‘learning’ from the data through an application of Bayes’ theorem [58] (see Additional file 1, section 1.3 for further details). The resulting posterior distribution is the distribution of the parameter of interest.
In contrast to the frequentist approach, during Bayesian analysis, no hypothesis testing takes place. The comparability of treatments can be shown directly but there is no ‘statistical significance’; treatments are deemed comparable, or one treatment is considered favorable/unfavorable over another. Ranking of treatments can be based on the surface under the cumulative ranking (SUCRA), a numeric presentation of the overall ranking with numbers ranging from 0 to 100%. Larger SUCRA numbers represent higher ranked interventions in the network. Results are presented as summaries from the posterior distribution, which can be the mean or median difference (or OR) and their 95% credible interval (CrI; the interval for which there is a 95% probability that the values of the treatment effect will lie within).
Bayesian analysis cannot be conducted without specification of a prior distribution, and these must be selected for basic parameters (e.g., treatment effect) and between-trial variance (in the case of a random effects model—see below section). See Additional file 1, section 1.3 for further information on selection of priors.
The ability to incorporate priors is considered an advantage of Bayesian analyses. The output can also be considered more natural in the context of decision making—i.e., it is possible to rank the orders of treatments. However, Bayesian analysis has a number of disadvantages and weaknesses; it is more computationally challenging than a frequentist approach, and a major criticism is that elicitation of priors can be difficult and subjective. In addition, data sparsity can lead to unrealistically wide CrIs.
Examples of recently published studies conducted using a Bayesian approach in a non-COPD context include: Birkinshaw [59], Chang [60], Panaccione [61], Schettini [62], and Wang [63].
Summary of pairwise comparisons in NMA
Comparative intervention effect estimates can be presented using a square matrix known as a league table [44]. The league table shows relative effectiveness of all pairs of interventions examined along with their 95% CI or CrI. Effect estimates can be graphically represented using forest plots with 95% CIs or CrIs.
Fixed effects versus random effects models
NMAs are usually either based on a fixed effects (FE) or random effects (RE) model (Table 3). An FE model assumes that the relative treatment effect of one treatment compared with another is the same across all trials containing those treatments (i.e., any variation in effect size between studies is due to within-study estimation error). An RE model assumes that the effect size varies between studies (i.e., the studies represent a distribution of effect sizes, and the aim of the analysis is to estimate the mean of the distribution) [64]. A ‘weight’ is assigned to each study within an NMA, which reflects the precision of the individual study estimate and therefore, the relative contribution of each study to the overall pooled result (i.e., more precise studies will contribute more to the overall estimate). In an FE model, the study weight is based solely on within-study variance. In an RE model, study weights consider between-study as well as within-study variance. This means that the relative weights of individual studies will be more alike under an RE model than they are under an FE model [64]. An RE model is more appropriate in many cases as there are differences in study and patient characteristics between the combined studies.
Table 3.
Fixed effect model versus random effect model
| Fixed effects model | Random effects model | |
|---|---|---|
| Treatment effect | Assumed that the treatment effect of one agent compared with another is the same across all trials containing those treatments | Assumed that the treatment effect size varies between studies |
| Study weights | Based upon within-study variance only | Based upon within-study and between-study variances |
| Use |
If there are a small number of studies with large sample sizes (i.e., sparse evidence base) If there are too few studies to accurately estimate between-study variance |
If substantial heterogeneity in study or patient characteristics between the combined studies is suspected |
Source [64]
Potential approaches to deal with heterogeneity or a sparse evidence base
A Chi-squared test can be used to assess whether observed differences in results across studies are due to chance alone. A low p-value (often < 0.10) indicates evidence of heterogeneity [65]. However, care must be taken when interpreting the results of the Chi-squared test, as it has low power to detect heterogeneity in analyses containing studies with small sample sizes and/or few studies. In analyses with many studies, the test has high power to detect a small amount of heterogeneity that is not necessarily clinically important. Some heterogeneity will inevitably be present in meta-analyses, and the I2 statistic can be used to describe the percentage of variability in effect estimates that is due to heterogeneity rather than chance [65, 66] (see Additional file 1, section 1.4). I2 is derived from the Chi-squared heterogeneity statistic but is independent of the number of studies and the treatment effect metric. An I2 of 0% to 40% suggests heterogeneity might not be important; 30% to 60% suggests moderate heterogeneity; 50% to 90% suggests substantial heterogeneity; and 75% to 100% suggests considerable heterogeneity [65]. If between-study heterogeneity is suspected within a network, use of an RE model should be considered [67]. If there is a large number of studies, meta-regression can also be used to investigate whether particular covariates (i.e., potential effect modifiers such as patient age) explain any of the heterogeneity of treatment effects seen between studies (see Additional file 1, section 1.5) [68]. Multi-level network meta-regression (ML-NMR) is a relatively recent extension of NMA that uses aggregate data along with individual patient data to adjust for differences in effect modifiers between studies [11]. In the case of few studies (with large sample sizes), the use of an FE model is considered more appropriate; RE models are not recommended if there are too few studies to accurately estimate between-study variance [64].
Evaluating confidence in the results of an NMA
GRADE
The Grading of Recommendations, Assessment, Development, and Evaluation (GRADE) framework is recommended for use in NMA to assess the confidence in (or the quality of) the evidence for each main comparison [26, 69–71]. GRADE is used to rate evidence at an outcome level, rather than an individual study level [70, 71]. The certainty of the evidence is categorized as ‘high’, ‘moderate’, ‘low’, or ‘very low’ by outcome and the results are commonly reported using ‘summary of findings’ tables [69, 71].
GRADE assessments are determined via consideration of five domains: (1) risk of bias (i.e., are limitations in individual study designs or implementation large enough to lower confidence in the overall treatment effect); (2) consistency of effect (i.e., was there unexplained heterogeneity or variability of results across studies, which could affect the overall effect estimation); (3) indirectness (i.e., have only indirect comparisons been made or are the patients studied different from those for whom treatment recommendations would apply); (4) imprecision (i.e., do studies include few participants and/or few events); and (5) publication bias (i.e., how likely is it that selective reporting has occurred) [26, 69–71].
Although categorization is subjective, GRADE provides a transparent and reproducible framework for evidence grading; any judgements other than ‘high’ certainty should be justified using explanatory footnotes within the summary table [71].
CINeMA
Confidence in Network Meta-Analysis (CINeMA) is another methodological framework that can be used to evaluate confidence in the results of an NMA [72, 73]. Although broadly based on the GRADE framework, the CINeMA approach has several conceptual differences [72, 73].
CINeMA assessments are determined by consideration of six domains: (1) within-study bias; (2) reporting bias; (3) indirectness; (4) imprecision; (5) heterogeneity; and (6) incoherence. Using the CINeMA framework, judgements are assigned to each domain (no concerns, some concerns, or major concerns). Judgements across the six domains can then be summarized to obtain a level of confidence for each treatment effect—these correspond to the GRADE categorizations: ‘high’, ‘moderate’, ‘low’, or ‘very low’.
The CINeMA framework can be applied to any NMA via use of the freely available web application [73].
Appropriateness of NMA models
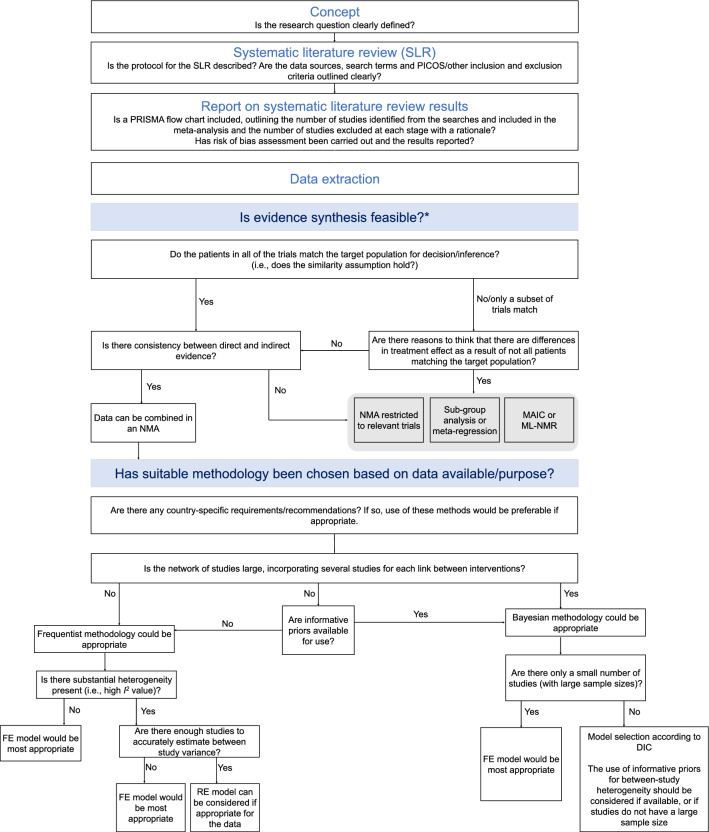
A comparison of frequentist and Bayesian methodologies is shown in Table 4 and a summary of key inputs and outputs for each method is shown in Table 5. Table 6 summarizes the key steps involved in NMA and Fig. 3 outlines a framework for assessing the robustness of a study and the suitability of methods chosen, given the data.
Table 4.
Comparison of frequentist and Bayesian approach
| Frequentist approach | Bayesian approach | |
|---|---|---|
| NMA data input preparation | Input required for all pairwise comparisons per study (k (k − 1)/2), with k representing number of arms, equating to 1 for a 2-arm study, 3 for a 3-arm study, 6 for a 4-arm study etc | Input required for all comparisons to the baseline treatment, corresponding to number of arms − 1 (k − 1): 1 for a 2-arm study, 2 for a 3-arm study, 3 for a 4-arm study etc |
| Prior specification | No priors used – based solely on observed data | Prior distribution must be specified |
| Analysis |
Straightforward implementation using available R package netmeta Statistical hypothesis testing is conducted. Output is presented as the estimates of effects and corresponding 95% CIs and associated p-values for the tests of significance |
More computationally intensive, yet OpenBUGS code readily available at NICE Decision Support Unit No hypothesis testing takes place. Output is presented as the estimates of effect and corresponding 95% CrI, Bayes factor, and posterior probabilities of effect |
| Interpretation |
Results are presented as estimated relative effects (mean difference or odds ratio) and 95% CIs, statistical significance can be determined Ranking of treatments through p-score, the frequentist equivalent to the SUCRA |
Results are presented as summaries of the posterior distribution (of the mean difference or odds ratio) and 95% CrIs, no statistical significance can be determined. Results are interpreted as one treatment to be favorable/unfavorable over another treatment, or two treatments to be comparable Ranking of treatments through SUCRA. Probability of one treatment to be better than another treatment can additionally be estimated (not feasible in a frequentist approach) |
CI confidence interval, CrI credible interval, NICE National Institute for Health and Care Excellence, NMA network meta-analysis, SUCRA surface under the cumulative ranking curve
Table 5.
Summary of key inputs and outputs
| Frequentist approach | Bayesian approach | |
|---|---|---|
| Is the network of evidence sparse? (<5 studies) | Works well |
Does not work well for standard non-informative priors but works well for informative priors Non-informative priors could result in unrealistically wide credible intervals |
| Is prior specification justified? | No priors used—based solely on observed data | Non-informative if enough data are available, informative in the case of sparse data, choice of suitable distributions and additional information such as expert clinician opinion |
| Are there few large studies of high quality? | Consider FE model | Consider FE model |
| Are there country-specific regulations? | Required by German G-BA and Australian PBAC | Preferred by NICE in the UK |
| Interpretation | Statistical significance or the absence thereof |
One treatment favorable/unfavorable over another treatment, or two treatments comparable Often falsely interpreted as significant or not significant; common misconception |
FE fixed effects, G-BA Federal Joint Committee, NICE National Institute for Health and Care Excellence, PBAC Pharmaceutical Benefits Advisory Committee
Table 6.
Summary of steps involved in NMA
| Step | Further information/considerations | Additional resources |
|---|---|---|
| Systematic literature review |
Prospective registration with PROSPERO Well-defined research question using the PICOS framework Searches carried out using a pre-defined search string (specific to each database) Systematic inclusion/exclusion of studies per the research question |
PROSPERO: [36] Cochrane handbook: [38] |
| Data extraction and network generation |
Quality/risk of bias assessment Treatment network defined |
RoB 2 tool: [40] Cochrane handbook: [26] |
| Assessment of NMA assumptions |
Similarity: similarity in PICOS criteria of all included studies Transitivity: no systematic differences in the distribution of effect modifiers between included studies Consistency: agreement between direct and indirect evidence within the network Homogeneity: no imbalances in PICOS across direct and indirect comparisons within the network |
Cochrane handbook: [26] |
| Conducting an NMA |
Appropriate statistical model used for the available data and/or any specific country requirements Justified use of FE vs RE methods Appropriate presentation of results For frequentist analysis: estimates of effects and corresponding 95% CIs and associated p-values For Bayesian analysis: estimates of effects and corresponding 95% CrIs |
Cochrane handbook: [26] Bucher 1997: [47] Netmeta: [74] NICE DSU: [12] |
| Interpretation of NMA findings |
Appropriate and careful interpretation of findings For frequentist analysis: ranking of treatments through p-scores. Can be interpreted as statistical significance or absence thereof For Bayesian analysis: ranking of treatments through SUCRA. No significance testing Use of the GRADE framework to assess the confidence in the evidence |
Cochrane handbook: [26] NMA worked example for clinicians: [75] |
| Reporting of NMA findings | Communicated following the PRISMA guidelines for NMA | PRISMA: [37] |
CI confidence interval, CrI credible interval, FE fixed effects, GRADE Grading of Recommendations, Assessment, Development and Evaluation, NICE National Institute for Health and Care Excellence, NMA network meta-analysis, PICOS population, intervention, comparator, outcome(s) and setting, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, PROSPERO International Prospective Register of Systematic Reviews, RE random effects, RoB risk of bias, SUCRA surface under the cumulative ranking curve
Fig. 3.
Decision framework—evidence synthesis eligibility and method selection. *Cope S, Zhang J, Saletan S, Smiechowksi B, et al. A process for assessing the feasibility of a network meta-analysis: a case study of everolimus in combination with hormonal therapy versus chemotherapy for advanced breast cancer. BMC Medicine 2014,12:93. DIC deviance information criterion, FE fixed effects, MAIC matching-adjusted indirect comparisons, ML-NMR multi-level network meta-regression, NMA network meta-analysis, PICOS population, intervention, comparator, outcomes, setting, PRISMA Preferred Reporting Items for Systematic Reviews and Meta-Analyses, RE random effects, SLR systematic literature review
Illustrative example of different NMA methods in COPD
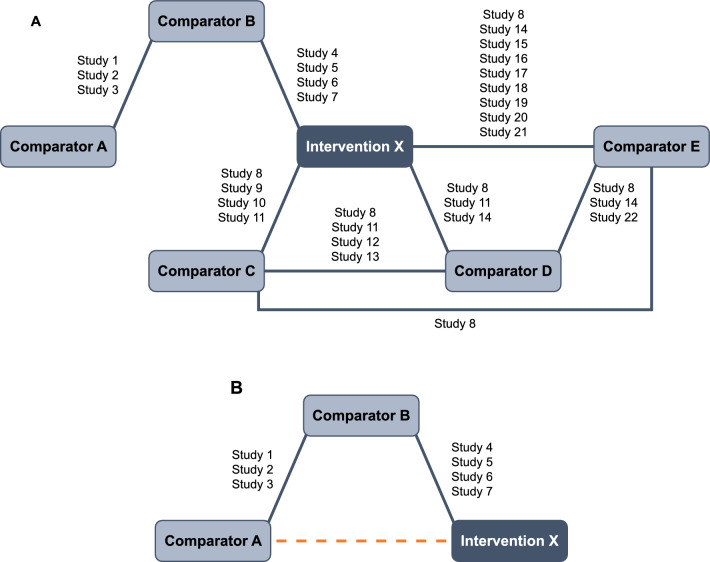
We have used a simulated data set to show the results from the three different statistical frameworks (frequentist NMA, Bayesian NMA, and Bucher ITC). The example compares the efficacy of fictitious “intervention X” with five comparators (interventions A–E) on change in FEV1 from baseline. The setting was defined as a large evidence base (i.e., more than three interventions in total and more than one study informing most links in the network; Fig. 4A). Data were simulated using a normal random number generator in R, with the same mean for pairwise comparisons on the same interventions, and standard deviations (SDs) ranging from 7 to 20 to incorporate a realistic amount of heterogeneity in the data. The mean was estimated from real FEV1 data, and then varied for the different pairwise comparisons, ranging from 40 to 65. The amount of heterogeneity in the simulated data was set at a realistic, moderate level (I2 = 55%). Further details regarding data simulation and the final data set are shown in Additional file 1 (section 1.6 and Tables S1–S3).
Fig. 4.
Illustrative example: A Network of evidence and B Bucher ITC network. ITC indirect treatment comparison
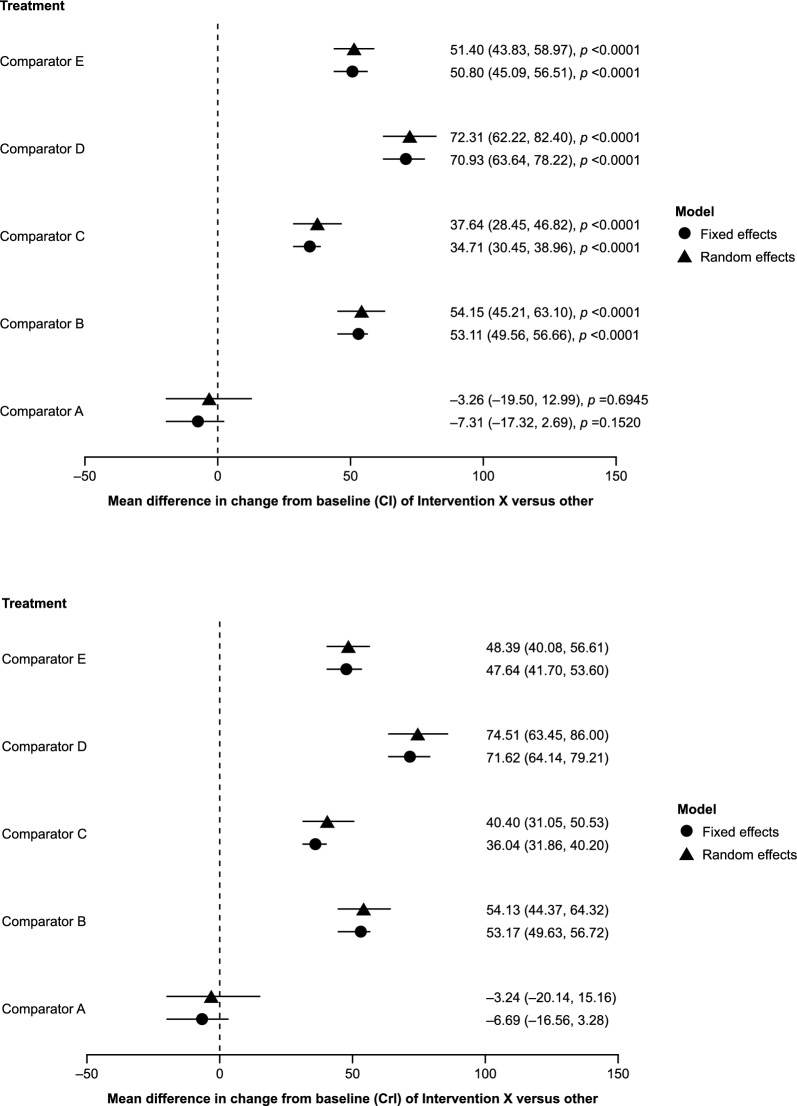
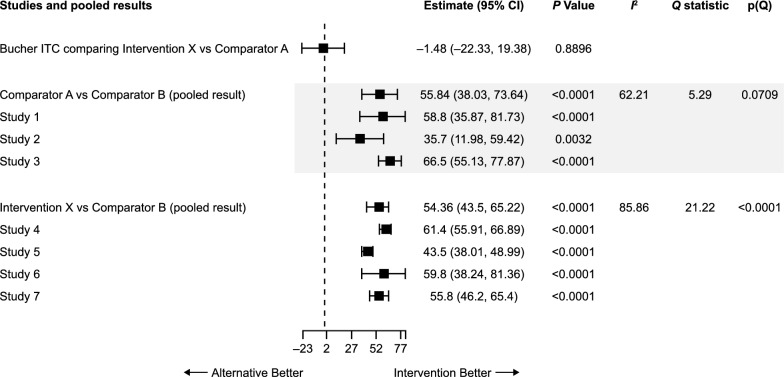
Results of the frequentist and Bayesian analyses are shown in Fig. 5. The FE and RE models were compared to account for between-study heterogeneity. Although some differences in point estimates were seen, the overall results of the analyses were similar using both frequentist and Bayesian frameworks as well as both FE and RE models. The ranking of interventions using each method is shown in Table 7. Despite minor numerical differences, the overall ranking of treatments was consistent across all analyses. Pairwise results from each analysis and the “probability of intervention X being better than the comparator” for the Bayesian analyses are shown in Additional file 1 (Tables S4 and S5, respectively). The section of the network used for the Bucher ITC is shown in Fig. 4B. The result of the indirect comparison of intervention X versus comparator A is shown in Fig. 6 and Additional file 1, Table S6. Results were consistent with frequentist and Bayesian approaches.
Fig. 5.
Illustrative example: Mean change from baseline FEV1: A frequentist and B Bayesian comparison. Abbreviations: CI confidence interval, CrI credible interval, FEV1 forced expiratory volume in 1 s
Table 7.
Illustrative example: ranking of treatments
| Frequentist FE model | Frequentist RE model | ||
|---|---|---|---|
| Intervention | p-score | Intervention | p-score |
| Comparator A | 0.984798 | Comparator A | 0.930552 |
| Intervention X | 0.815202 | Intervention X | 0.869446 |
| Comparator C | 0.600000 | Comparator C | 0.597584 |
| Comparator E | 0.349816 | Comparator E | 0.336729 |
| Comparator B | 0.250183 | Comparator B | 0.264845 |
| Comparator D | 1.71E − 06 | Comparator D | 0.000843 |
| Bayesian FE model | Bayesian RE model | ||
|---|---|---|---|
| Intervention | SUCRA | Intervention | SUCRA |
| Comparator A | 0.981027 | Comparator A | 0.92899 |
| Intervention X | 0.818973 | Intervention X | 0.870971 |
| Comparator C | 0.599903 | Comparator C | 0.577122 |
| Comparator E | 0.388257 | Comparator E | 0.382042 |
| Comparator B | 0.211838 | Comparator B | 0.23979 |
| Comparator D | 1.9E − 06 | Comparator D | 0.001084 |
FE fixed effects, RE random effects, SUCRA surface under the cumulative ranking curve
Fig. 6.
Illustrative example: Bucher ITC—intervention X versus Comparator A. CI confidence interval, ITC indirect treatment comparison
In summary, the illustrative example demonstrates that Bucher ITC, frequentist NMA, and Bayesian NMA, using both FE and RE models, give results that are similar and are in alignment. Although there were some numerical differences in point estimates, and the width of the intervals differed slightly across analyses, all conclusions drawn were identical. If the evidence base is large, and non-informative priors are used in the Bayesian model, the results obtained using frequentist and Bayesian methods are comparable. This example should be considered within the limitation of using simulated data; for example, it is not possible to present a GRADE summary of findings for this analysis.
Conclusions
A range of molecules in different therapeutic classes are available for the treatment of COPD; assessing the relative effectiveness of these molecules within and between classes can be challenging. There are various ways of synthesizing the available efficacy data of different interventions when head-to-head studies do not exist. Frequentist (including Bucher ITC) and Bayesian are two commonly used NMA frameworks. Network sparsity, authority requirements, and general preference may influence the choice of statistical model, and authors should be able to justify the method selected. HCPs can assess the appropriateness of the model and the assumptions that underpin it using the information in this tutorial. However, providing the methods are applied correctly, the outcome should be consistent regardless of which method is chosen.
Supplementary Information
Additional file 1: Fig. S1. Supplementary appendix, sections 1.1–1.6. [NMA methods – additional information and details of illustrative example data simulation.] Table S1. [Illustrative example: frequentist data.] Table S2. [Illustrative example: Bayesian data.] Table S3. [Illustrative example: Bucher ITC data.] Table S4. [Illustrative example: pairwise results.] Table S5. [Illustrative example: probability of being better than comparator – Bayesian analysis.] Table S6. [Illustrative example: indirect comparison of intervention X versus comparator A] Figure S1. [LSR model.]
Acknowledgments
Editorial support (in the form of writing assistance, including preparation of the draft manuscript under the direction and guidance of the authors, collating and incorporating authors’ comments for each draft, assembling tables and figures, grammatical editing, and referencing) was provided by Rebecca Cunningham of Luna, OPEN Health Communications, and was funded by GSK, in accordance with Good Publication Practice (GPP) guidelines (www.ismpp.org/gpp-2022).
Abbreviations
- CI
Confidence interval
- CINeMA
Confidence in Network Meta-Analysis
- COPD
Chronic obstructive pulmonary disease
- CrI
Credible interval
- DIC
Deviance information criterion
- FE
Fixed effects
- FEV1
Forced expiratory volume in 1 s
- GRADE
Grading of Recommendations, Assessment, Development, and Evaluation
- HCP
Healthcare professional
- HTA
Health technology assessment
- ITC
Indirect treatment comparison
- LSR
Least squares regression
- MAIC
Matching-adjusted indirect comparisons
- ML-NMR
Multi-level network meta-regression
- MTC
Multiple treatment comparison
- NICE
National Institute for Health and Care Excellence
- NMA
Network meta-analysis
- OR
Odds ratio
- PBAC
Australian Pharmaceutical Benefits Advisory Committee
- PICOS
Population, intervention(s), comparator(s), outcome(s), setting
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta-Analyses
- PROSPERO
International Prospective Register of Systematic Reviews
- RCT
Randomized controlled trial
- RE
Random effects
- SD
Standard deviation
- SLR
Systematic literature review
- SUCRA
Surface under the cumulative ranking
- TSD
Technical Support Document
- Tx
Treatment
Author contributions
KH, ASI, MM, SGN, NG, CC, LT, CFV, and DMGH were involved in the drafting, revising, and critical review of the manuscript. KH, ASI, and MM were involved in the simulation and analysis of the data for the illustrative example. All authors gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by GSK (study number 206272). GSK-affiliated authors were involved in study conception and design, data analysis, data interpretation, and the decision to submit the article for publication. The sponsor funded the article processing charges and open access fee.
Availability of data and materials
No datasets were generated or analyzed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
KH and MM are employees of ICON PLC. ASI, SGN, and CC are employees of and/or hold financial equities in GSK. ASI is also a part-time, unpaid professor at McMaster University. NG is part-funded by ICON PLC. LT has worked as a paid consultant or served as a board member for the following companies: Bausch Health, GSK, Baxter, Teva Pharmaceuticals, and Theralase Inc. He also works as Vice-President Research for St Joseph's Healthcare Hamilton. CFV has given presentations at symposia and/or served on scientific advisory boards sponsored by Aerogen, AstraZeneca, Boehringer Ingelheim, Chiesi, CSL Behring, Grifols, GSK, Insmed, MedUpdate, Menarini, Novartis, Nuvaira, Roche, and Sanofi. DMGH reports personal fees from Aerogen, AstraZeneca, Boehringer Ingelheim, Berlin-Chemie, Chiesi, CSL Behring, GSK, Inogen, Menarini, Novartis, Pfizer, and Sanofi.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Joore M, Grimm S, Boonen A, de Wit M, Guillemin F, Fautrel B. Health technology assessment: a framework. RMD Open. 2020;6: e001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Health technology assessment. https://www.who.int/health-topics/health-technology-assessment#tab=tab_1.
- 3.Deliv C, Devane D, Putnam E, Healy P, Hall A, Rosenbaum S, Toomey E. Development of a video-based evidence synthesis knowledge translation resource: drawing on a user-centred design approach. Digit Health. 2023;9:20552076231170696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thano AN. Evidence synthesis: from meta-analysis to network meta-analysis with an application in patients with COPD. Athens University of Economics and Business, Department of Statistics; 2017.
- 5.Sackett DL, Rosenberg WM, Gray JA, Haynes RB, Richardson WS. Evidence based medicine: what it is and what it isn’t. BMJ. 1996;312:71–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connor L, Dean J, McNett M, Tydings DM, Shrout A, Gorsuch PF, Hole A, Moore L, Brown R, Melnyk BM, Gallagher-Ford L. Evidence-based practice improves patient outcomes and healthcare system return on investment: findings from a scoping review. Worldviews Evid Based Nurs. 2023;20:6–15. [DOI] [PubMed] [Google Scholar]
- 7.Djulbegovic B, Trikalinos TA, Roback J, Chen R, Guyatt G. Impact of quality of evidence on the strength of recommendations: an empirical study. BMC Health Serv Res. 2009;9:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Granholm A, Alhazzani W, Derde LPG, Angus DC, Zampieri FG, Hammond NE, Sweeney RM, Myatra SN, Azoulay E, Rowan K, et al. Randomised clinical trials in critical care: past, present and future. Intensive Care Med. 2022;48:164–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paraskevas KI, de Borst GJ, Veith FJ. Why randomized controlled trials do not always reflect reality. J Vasc Surg. 2019;70:607–614.e603. [DOI] [PubMed] [Google Scholar]
- 10.Lu M, Liu S, Yuan Y. Why there are so many contradicted or exaggerated findings in highly-cited clinical research? Contemp Clin Trials. 2022;118: 106782. [DOI] [PubMed] [Google Scholar]
- 11.Evidence synthesis: a solution to sparse evidence, heterogeneous studies, and disconnected networks [Whitepaper] https://www.iconplc.com/insights/value-based-healthcare/evidence-synthesis/.
- 12.NICE Decision Support Unit Technical support documents. https://www.sheffield.ac.uk/nice-dsu/tsds.
- 13.Dias S, Welton NJ, Sutton AJ, Ades AE. Evidence synthesis for decision making 1: introduction. Med Decis Making. 2013;33:597–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehmbi H, Retter S, Shah UJ, Nguyen D, Martin J, Uppal V. Epidemiological, methodological, and statistical characteristics of network meta-analysis in anaesthesia: a systematic review. Br J Anaesth. 2023;130:272–86. [DOI] [PubMed] [Google Scholar]
- 15.Song F, Loke YK, Walsh T, Glenny A-M, Eastwood AJ, Altman DG. Methodological problems in the use of indirect comparisons for evaluating healthcare interventions: survey of published systematic reviews. BMJ. 2009;338: b1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Song F, Clark A, Bachmann MO, Maas J. Simulation evaluation of statistical properties of methods for indirect and mixed treatment comparisons. BMC Med Res Methodol. 2012;12:138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Wely M. The good, the bad and the ugly: meta-analyses. Hum Reprod. 2014;29:1622–6. [DOI] [PubMed] [Google Scholar]
- 18.Wu H, Jin R, Yang S, Park BJ, Li H. Long-term and short-term outcomes of robot- versus video-assisted anatomic lung resection in lung cancer: a systematic review and meta-analysis. Eur J Cardiothorac Surg. 2021;59:732–40. [DOI] [PubMed] [Google Scholar]
- 19.Huang Y, Li H, Zhang J. The inclusion criteria was flawed in the systematic review and meta-analysis by Wu et al. Eur J Cardiothorac Surg. 2022;62: ezac107. [DOI] [PubMed] [Google Scholar]
- 20.Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, Leucht S, Ruhe HG, Turner EH, Higgins JPT, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391:1357–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boesen K, Paludan-Müller AS, Munkholm K. Network meta-analysis of antidepressants. Lancet. 2018;392:1011. [DOI] [PubMed] [Google Scholar]
- 22.Bartoszko JJ, Siemieniuk RAC, Kum E, Qasim A, Zeraatkar D, Martinez JPD, Azab M, Ibrahim S, Izcovich A, Soto GB, et al. Prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373: n949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schilling WHK, Callery J, Chandna A, Cruz C, Hamers RL, Watson JA, White NJ. Rapid response to: prophylaxis against covid-19: living systematic review and network meta-analysis. BMJ. 2021;373: n949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caldwell DM, Ades AE, Higgins JPT. Simultaneous comparison of multiple treatments: combining direct and indirect evidence. BMJ. 2005;331:897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hasselblad V. Meta-analysis of multitreatment studies. Med Decis Making. 1998;18:37–43. [DOI] [PubMed] [Google Scholar]
- 26.Chapter 11: Undertaking network meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane; 2023. https://training.cochrane.org/handbook/current/chapter-11#_Ref390868433.
- 27.https://www.cochranelibrary.com/
- 28.Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease 2024 report. https://goldcopd.org/2024-gold-report/.
- 29.Egger M, Smith GD. Meta-analysis. Potentials and promise. BMJ. 1997;315:1371–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lumley T. Network meta-analysis for indirect treatment comparisons. Stat Med. 2002;21:2313–24. [DOI] [PubMed] [Google Scholar]
- 31.Kanters S, Ford N, Druyts E, Thorlund K, Mills EJ, Bansback N. Use of network meta-analysis in clinical guidelines. Bull World Health Organ. 2016;94:782–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Indirect evidence: indirect treatment comparisons in meta-analysis. Ottawa: Canadian Agency for Drugs and Technologies in Health; 2009. https://www.cadth.ca/sites/default/files/pdf/H0462_itc_tr_e.pdf.
- 33.Guidelines for preparing submissions to the Pharmaceutical Benefits Advisory Committee, version 5.0. https://pbac.pbs.gov.au/content/information/files/pbac-guidelines-version-5.pdf.
- 34.Burns PB, Rohrich RJ, Chung KC. The levels of evidence and their role in evidence-based medicine. Plast Reconstr Surg. 2011;128:305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ades AE, Welton NJ, Dias S, Phillippo DM, Caldwell DM. Twenty years of network meta-analysis: continuing controversies and recent developments. Res Synth Methods. [DOI] [PubMed]
- 36.International Prospective Register of Systematic Reviews (PROSPERO). https://www.crd.york.ac.uk/PROSPERO/.
- 37.Preferred reporting items for systematic reviews and meta-analyses. https://www.prisma-statement.org/. [DOI] [PubMed]
- 38.Cochrane Handbook for Systematic Reviews of Interventions. Version 6.4. https://training.cochrane.org/handbook/current.
- 39.Zhang J, Yuan Y, Chu H. The impact of excluding trials from network meta-analyses – an empirical study. PLoS ONE. 2016;11:e0165889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.RoB 2: a revised Cochrane risk-of-bias tool for randomized trials. https://methods.cochrane.org/bias/resources/rob-2-revised-cochrane-risk-bias-tool-randomized-trials. [DOI] [PubMed]
- 41.Chapter 8: Assessing risk of bias in a randomized trial. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane; 2023. https://training.cochrane.org/handbook/current/chapter-08]
- 42.Chapter 5: Collecting data. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). https://training.cochrane.org/handbook/current/chapter-05.
- 43.Caldwell DM. An overview of conducting systematic reviews with network meta-analysis. Syst Rev. 2014;3:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ahn E, Kang H. Concepts and emerging issues of network meta-analysis. Korean J Anesthesiol. 2021;74:371–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jansen JP, Crawford B, Bergman G, Stam W. Bayesian meta-analysis of multiple treatment comparisons: an introduction to mixed treatment comparisons. Value Health. 2008;11:956–64. [DOI] [PubMed] [Google Scholar]
- 46.Jansen JP, Schmid CH, Salanti G. Directed acyclic graphs can help understand bias in indirect and mixed treatment comparisons. J Clin Epidemiol. 2012;65:798–807. [DOI] [PubMed] [Google Scholar]
- 47.Bucher HC, Guyatt GH, Griffith LE, Walter SD. The results of direct and indirect treatment comparisons in meta-analysis of randomized controlled trials. J Clin Epidemiol. 1997;50:683–91. [DOI] [PubMed] [Google Scholar]
- 48.Martin AA, Parks D. An indirect comparison of HbA1c treatment effect with albiglutide and exenatide 2.0 mg QW using the Bucher method. Diabetes Metab Syndr Obes. 2016;9:163–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pinter A, Gold LS, Reich A, Green LJ, Praestegaard M, Selmer J, Armstrong AW, Danø A, Dhawan S, Galván J, et al. A novel, fixed-dose calcipotriol and betamethasone dipropionate cream for the topical treatment of plaque psoriasis: direct and indirect evidence from phase 3 trials discussed at the 30th EADV Congress 2021. J Eur Acad Dermatol Venereol. 2023;37:14–9. [DOI] [PubMed] [Google Scholar]
- 50.Cruz F, Danchenko N, Fahrbach K, Freitag A, Tarpey J, Whalen J. Efficacy of abobotulinumtoxinA versus onabotulinumtoxinA for the treatment of refractory neurogenic detrusor overactivity: a systematic review and indirect treatment comparison. J Med Econ. 2023;26:200–7. [DOI] [PubMed] [Google Scholar]
- 51.Akkoç N, Arteaga CH, Auteri SE, Betts M, Fahrbach K, Kim M, Kiri S, Neupane B, Gaffney K, Mease PJ. Comparative efficacy of biologic disease-modifying anti-rheumatic drugs for non-radiographic axial spondyloarthritis: a systematic literature review and Bucher indirect comparisons. Rheumatol Ther. 2023;10:307–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Merkel M, Danese D, Chen C, Wang J, Wu A, Yang H, Lin H. Indirect treatment comparison (ITC) of the efficacy of vutrisiran and tafamidis for hereditary transthyretin-mediated amyloidosis with polyneuropathy. Expert Opin Pharmacother. 2023;24:1205–14. [DOI] [PubMed] [Google Scholar]
- 53.Lampl C, MaassenVanDenBrink A, Deligianni CI, Gil-Gouveia R, Jassal T, Sanchez-Del-Rio M, Reuter U, Uluduz D, Versijpt J, Zeraatkar D, Sacco S. The comparative effectiveness of migraine preventive drugs: a systematic review and network meta-analysis. J Headache Pain. 2023;24:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang KD, Wang LY, Zhang ZH, Zhang DX, Lin XW, Meng T, Qi F. Effect of exercise interventions on health-related quality of life in patients with fibromyalgia syndrome: a systematic review and network meta-analysis. J Pain Res. 2022;15:3639–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karam G, Agarwal A, Sadeghirad B, Jalink M, Hitchcock CL, Ge L, Kiflen R, Ahmed W, Zea AM, Milenkovic J, et al. Comparison of seven popular structured dietary programmes and risk of mortality and major cardiovascular events in patients at increased cardiovascular risk: systematic review and network meta-analysis. BMJ. 2023;380: e072003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Recchia F, Leung CK, Chin EC, Fong DY, Montero D, Cheng CP, Yau SY, Siu PM. Comparative effectiveness of exercise, antidepressants and their combination in treating non-severe depression: a systematic review and network meta-analysis of randomised controlled trials. Br J Sports Med. 2022;56:1375–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Shen Y, Shi Q, Nong K, Li S, Yue J, Huang J, Dong B, Beauchamp M, Hao Q. Exercise for sarcopenia in older people: A systematic review and network meta-analysis. J Cachexia Sarcopenia Muscle. 2023;14:1199–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gelman A, Carlin JB, Stern HS, Dunson DB, Vehtari A, Rubin DB. Bayesian data analysis. 3rd ed. New York: Chapman and Hall/CRC; 2013. [Google Scholar]
- 59.Birkinshaw H, Friedrich CM, Cole P, Eccleston C, Serfaty M, Stewart G, White S, Moore RA, Phillippo D, Pincus T. Antidepressants for pain management in adults with chronic pain: a network meta-analysis. Cochrane Database Syst Rev. 2023;5: CD014682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chang E, Josan AS, Purohit R, Patel CK, Xue K. A network meta-analysis of retreatment rates following bevacizumab, ranibizumab, aflibercept, and laser for retinopathy of prematurity. Ophthalmology. 2022;129:1389–401. [DOI] [PubMed] [Google Scholar]
- 61.Panaccione R, Collins EB, Melmed GY, Vermeire S, Danese S, Higgins PDR, Kwon CS, Zhou W, Ilo D, Sharma D, et al. Efficacy and safety of advanced therapies for moderately to severely active ulcerative colitis at induction and maintenance: an indirect treatment comparison using Bayesian network meta-analysis. Crohns Colitis. 2023;5: otad009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schettini F, Venturini S, Giuliano M, Lambertini M, Pinato DJ, Onesti CE, De Placido P, Harbeck N, Lüftner D, Denys H, et al. Multiple Bayesian network meta-analyses to establish therapeutic algorithms for metastatic triple negative breast cancer. Cancer Treat Rev. 2022;111: 102468. [DOI] [PubMed] [Google Scholar]
- 63.Wang X, Wen D, He Q, Yang J, You C, Tao C, Ma L. Effect of corticosteroids in patients with COVID-19: a Bayesian network meta-analysis. Int J Infect Dis. 2022;125:84–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Borenstein M, Hedges LV, Higgins JP, Rothstein HR. A basic introduction to fixed-effect and random-effects models for meta-analysis. Res Synth Methods. 2010;1:97–111. [DOI] [PubMed] [Google Scholar]
- 65.Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch V, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane; 2023. https://training.cochrane.org/handbook/current/chapter-10#section-10-10-1.
- 66.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–58. [DOI] [PubMed] [Google Scholar]
- 67.Turner RM, Domínguez-Islas CP, Jackson D, Rhodes KM, White IR. Incorporating external evidence on between-trial heterogeneity in network meta-analysis. Stat Med. 2019;38:1321–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dias S, Sutton AJ, Welton NJ, Ades AE. NICE Decision Support Unit Technical Support Documents. In Heterogeneity: subgroups, meta-regression, bias and bias-adjustment. London: National Institute for Health and Care Excellence (NICE). 2012 [PubMed]
- 69.Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, Schünemann HJ. GRADE Working Group: GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336:924–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.What is GRADE? https://bestpractice.bmj.com/info/toolkit/learn-ebm/what-is-grade/.
- 71.Chapter 14: Completing ‘Summary of findings’ tables and grading the certainty of the evidence. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions version 6.4 (updated August 2023). Cochrane, 2023 [https://training.cochrane.org/handbook/current/chapter-14#_Ref527924277]
- 72.Salanti G, Del Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta-analysis. PLoS ONE. 2014;9: e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nikolakopoulou A, Higgins JPT, Papakonstantinou T, Chaimani A, Del Giovane C, Egger M, Salanti G. CINeMA: an approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17: e1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schwarzer G, Carpenter JR, Rücker G: Meta-analysis with R. Springer; 2015.
- 75.Rouse B, Chaimani A, Li T. Network meta-analysis: an introduction for clinicians. Intern Emerg Med. 2017;12:103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Fig. S1. Supplementary appendix, sections 1.1–1.6. [NMA methods – additional information and details of illustrative example data simulation.] Table S1. [Illustrative example: frequentist data.] Table S2. [Illustrative example: Bayesian data.] Table S3. [Illustrative example: Bucher ITC data.] Table S4. [Illustrative example: pairwise results.] Table S5. [Illustrative example: probability of being better than comparator – Bayesian analysis.] Table S6. [Illustrative example: indirect comparison of intervention X versus comparator A] Figure S1. [LSR model.]
Data Availability Statement
No datasets were generated or analyzed during the current study.