Full text
PDF


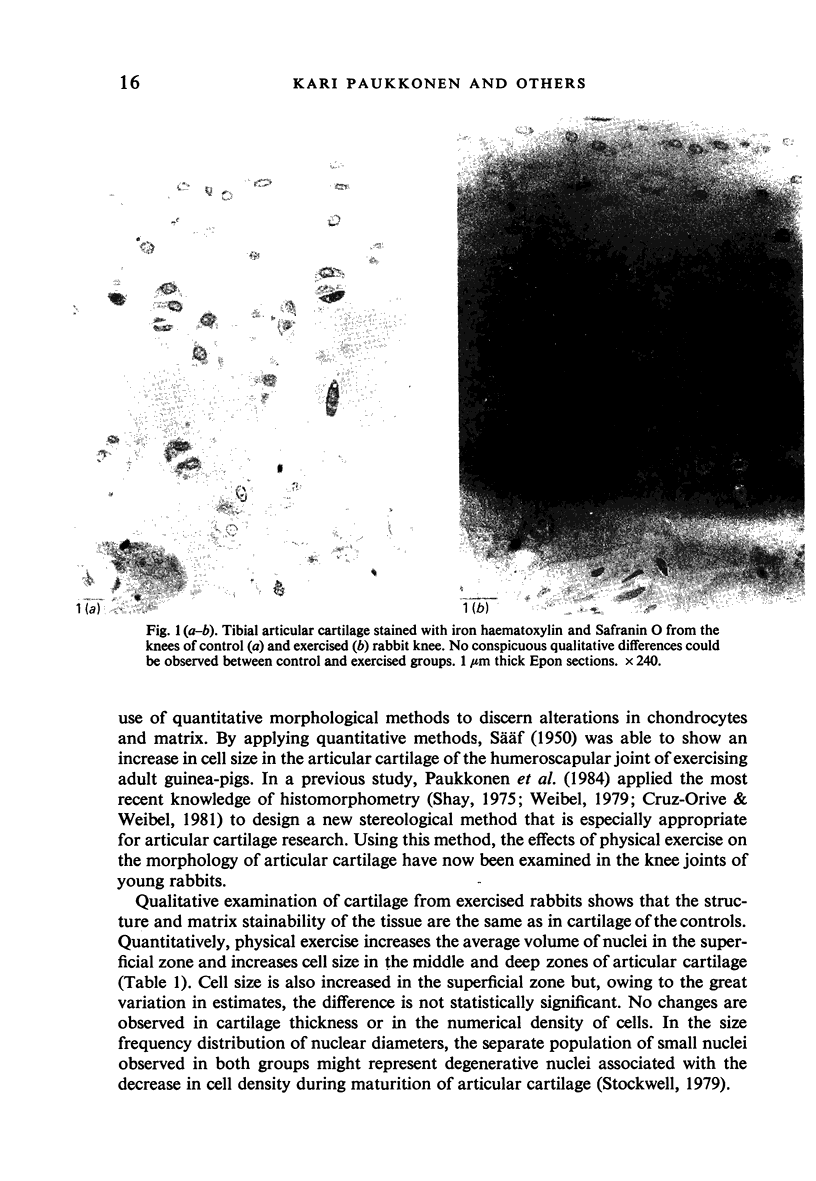
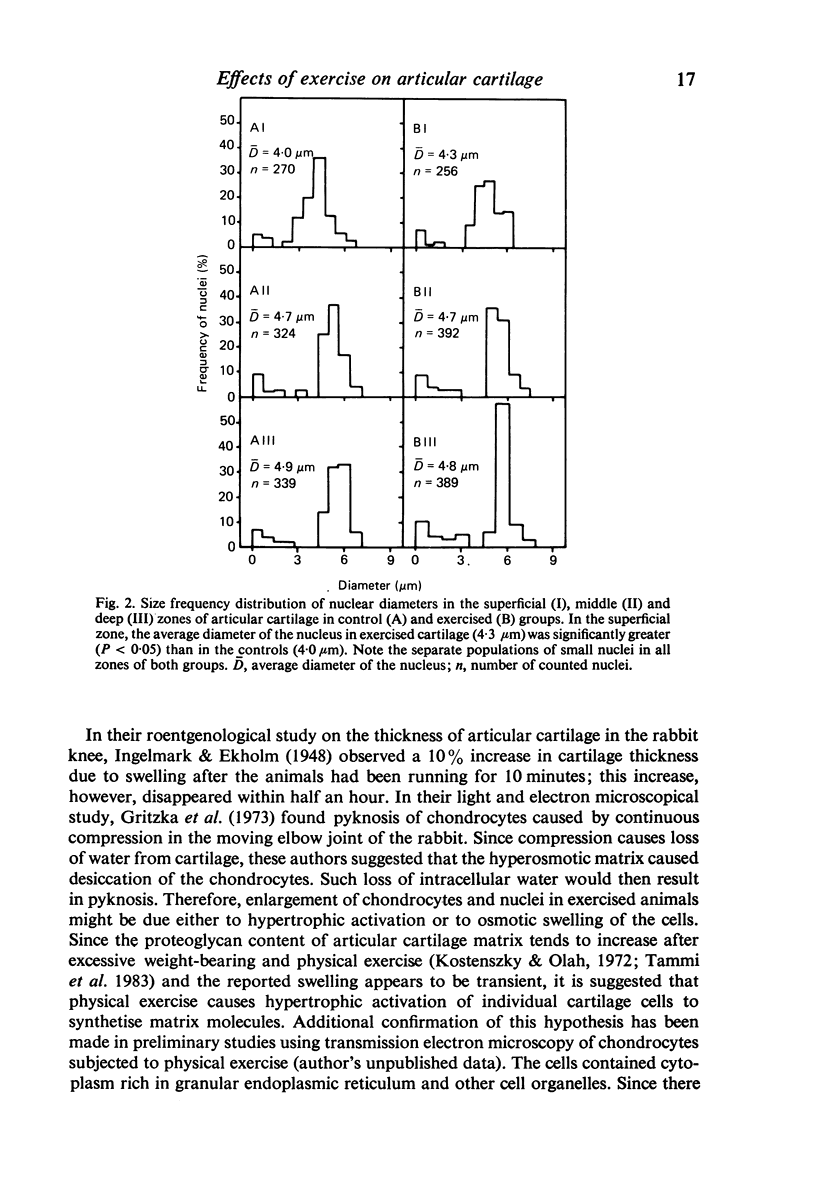



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cruz-Orive L. M., Weibel E. R. Sampling designs for stereology. J Microsc. 1981 Jun;122(Pt 3):235–257. doi: 10.1111/j.1365-2818.1981.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Dekel S., Weissman S. L. Joint changes after overuse and peak overloading of rabbit knees in vivo. Acta Orthop Scand. 1978 Dec;49(6):519–528. doi: 10.3109/17453677808993232. [DOI] [PubMed] [Google Scholar]
- Gritzka T. L., Fry L. R., Cheesman R. L., LaVigne A. Deterioration of articular cartilage caused by continuous compression in a moving rabbit joint. A light and electron microscopic study. J Bone Joint Surg Am. 1973 Dec;55(8):1698–1720. [PubMed] [Google Scholar]
- Kincaid S. A., Van Sickle D. C. Effects of exercise on the histochemical changes of articular chondrocytes in adult dogs. Am J Vet Res. 1982 Jul;43(7):1218–1226. [PubMed] [Google Scholar]
- Kostenszky K. S., Oláh E. H. Effect of increased functional demand on the glucosaminoglycan (mucopolysaccharide) content of the articular cartilage. Acta Biol Acad Sci Hung. 1972;23(1):75–82. [PubMed] [Google Scholar]
- Mankin H. J. The reaction of articular cartilage to injury and osteoarthritis (second of two parts). N Engl J Med. 1974 Dec 19;291(25):1335–1340. doi: 10.1056/NEJM197412192912507. [DOI] [PubMed] [Google Scholar]
- Oláh E. H., Kostenszky K. S. Effect of altered functional demand on the glycosaminoglycan content of the articular cartilage of dogs. Acta Biol Acad Sci Hung. 1972;23(2):195–200. [PubMed] [Google Scholar]
- Palmoski M. J., Colyer R. A., Brandt K. D. Joint motion in the absence of normal loading does not maintain normal articular cartilage. Arthritis Rheum. 1980 Mar;23(3):325–334. doi: 10.1002/art.1780230310. [DOI] [PubMed] [Google Scholar]
- Paukkonen K., Selkäinaho K., Jurvelin J., Helminen H. J. Morphometry of articular cartilage: a stereological method using light microscopy. Anat Rec. 1984 Dec;210(4):675–682. doi: 10.1002/ar.1092100415. [DOI] [PubMed] [Google Scholar]
- Sevier A. C., Munger B. L. The use of oxone to facilitate specific tissue stainability following osmium fixation. Anat Rec. 1968 Sep;162(1):43–52. doi: 10.1002/ar.1091620105. [DOI] [PubMed] [Google Scholar]
- Shay J. Economy of effort in electron microscope morphometry. Am J Pathol. 1975 Dec;81(3):503–512. [PMC free article] [PubMed] [Google Scholar]
- Shepard N., Mitchell N. The use of ruthenium and p-phenylenediamine to stain cartilage simultaneously for light and electron microscopy. J Histochem Cytochem. 1977 Oct;25(10):1163–1168. doi: 10.1177/25.10.915240. [DOI] [PubMed] [Google Scholar]
- Stofft E., Graf J. Rasterelektronemikroskopische Untersuchung des hyalinen Gelenkknorpels. Acta Anat (Basel) 1983;116(2):114–125. [PubMed] [Google Scholar]
- Tammi M., Sämänen A. M., Jauhiainen A., Malminen O., Kiviranta I., Helminen H. Proteoglycan alterations in rabbit knee articular cartilage following physical exercise and immobilization. Connect Tissue Res. 1983;11(1):45–55. doi: 10.3109/03008208309015010. [DOI] [PubMed] [Google Scholar]
- Videman T., Eronen I., Candolin T. Effects of motion load changes on tendon tissues and articular cartilage. A biochemical and scanning electron microscopic study on rabbits. Scand J Work Environ Health. 1979;5 Suppl 3:56–67. [PubMed] [Google Scholar]
- Warmke H. E., Lee S. L. Improved staining procedures for semithin epoxy sections of plant tissues. Stain Technol. 1976 May;51(3):179–185. doi: 10.3109/10520297609116696. [DOI] [PubMed] [Google Scholar]



