Dear Editor,
In 2022, the Spanish Society of Allergology and Clinical Immunology (SEAIC), in collaboration with a multidisciplinary panel of experts in the treatment of severe asthma (SA), developed a groundbreaking tool to facilitate healthcare professionals (HCPs) and patients in managing their asthma treatment with biological therapies – BioCart© Diary.1 This tool was designed to improve patient adherence to the self-administration of biological therapy for SA in a real-life setting. Prior studies show that poor therapeutical adherence negatively affects patient's health and disease control.2, 3, 4 Therefore, a formal education program and a written action plan are recommended to prevent asthma worsening and ensuring rapid symptoms remission.2, 3 Hence the importance of tools like the BioCart© Diary which provides disease management information, practical tips on biologics self-administration, and serves as an open communication channel with HCPs.1 In this context, this study evaluates the useability and effectiveness of the Diary in real-life practice from the perspective of patients and HCPs.
Eight experts in SA (four pulmonologists and four allergologists) based across Spain participated in the survey. Eighty two patients with SA and undergoing biological therapy were invited to use the BioCart© Diary as part of usual follow-up, and participate in this survey. Both HCPs and SA patients completed two self-administered questionnaires: one before using the BioCart© Diary to evaluate expectations of its useability and utility, and another after 6 months, rating the experience of its use [PRE and POST questionnaires for HCPs and patients are available as Supplementary Material]. Regarding ethical compliance, this study is exempt from ethical committee evaluation under Spanish regulation but complies with personal data protection and deontological codes for opinion surveys.
Of the 82 patients invited, 53 completed both pre- and post-BioCart© use surveys. SA patients, 59% women and 41% men, had an average age of 54 years, with 64% having a SA diagnosis for more than 5 years, 81% using a biological drug for at least 1 year and 62% using a biological drug at home for at least 1 year [Supplementary Fig. 2].
The pre-use BioCart© Diary questionnaire revealed that patients had concerns about when and how to inject their biological therapy at home, while the HCPs did not perceive these as possible issues [Supplementary Fig. 1]. Patients were also neutral regarding knowledge about the side effects of their medication and how to act in response to these, if necessary, while HCPs again did not perceive this as a problem [Supplementary Fig. 1]. The differences in perceptions of patients and HCPs on key issues such as adherence (when to inject) and training (how to inject and identify adverse events) highlight the need for an objective tool, such as the BioCart© Diary, to provide information when needed and to support HCP–patient communication.
After using the BioCart© Diary for about 6 months, an evaluation of the post-use questionnaire revealed that 85% of patients used the Diary as explained by their HCP and completed all or part of the information; 9% used it just part of the time, completing all or part of the information and the remaining 6% did not use the Diary. The main reasons for not completing the BioCart© Diary were personal (e.g., forgetting, lack of time, using a different system to track the information) [Supplementary Fig. 2]. Overall, 88% of HCPs were positive regards the use of the BioCart© Diary and 77% of patients rated it as excellent/very good/good; 89% of patients found it easy-to-understand and 79% found the content useful, with most patients not having any problems completing it, and the 11% that did were able to resolve them satisfactorily by talking to their HCP [Supplementary Fig. 2].
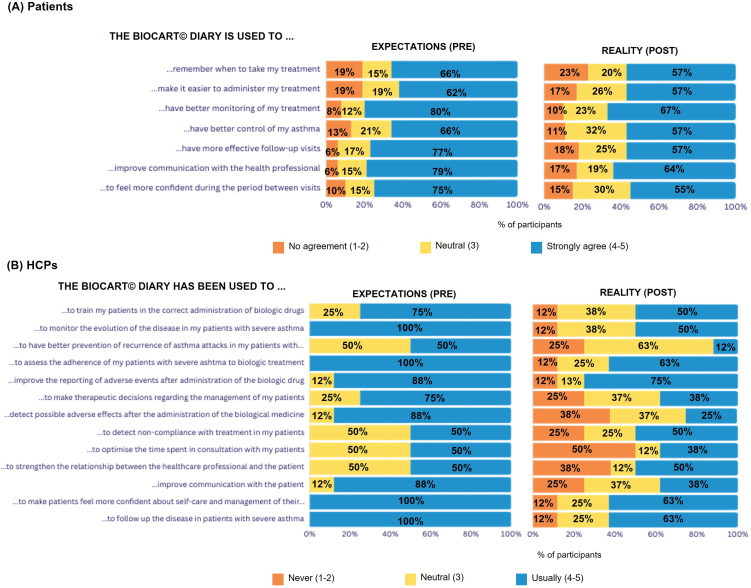
Evaluation of the pre- and post-use BioCart© Diary questionnaires indicated that patients found it easier to remember injecting, administer and monitor treatment when using the Diary, it enabled more effective follow-up visits and improved HCP–patient communication (Fig. 1A). Regarding adherence, 12% of patients have been more compliant with the Diary, while 88% remained as compliant as before [Supplementary Fig. 2]. Based on these results, HCPs found that the BioCart© Diary improved overall disease monitoring and management, facilitated training in the correct self-administration of biological therapy, improved monitoring of biological treatment adverse effects and strengthened the HCP–patient relationship (Fig. 1B). However, as not all patients responded to the survey, these results, although robust, may only be representative of the most compromised and treatment compliant patients. Therefore, our results may not represent all type of patients.
Fig. 1.
Utility of the BioCart© Diary: expectations and reality for (A) patients and (B) HCPs.
Overall, despite not meeting all expectations, especially regarding the monitoring of adverse effects, HCPs and patients would recommend the BioCart© Diary to others, with most HCPs having the opinion that the Diary should be given primarily to patients about to start home administration of biological therapy. It is noteworthy that patients also considered the BioCart© Diary a useful tool for training and solving doubts, even though this was not the initial purpose of the Diary, given the relevance of correct training for the appropriate home administration of a biologic therapy, BioCart© could focus more on this direction in the future.
To the best of our knowledge, the BioCart© Diary is the first well-structured tool for monitoring and supporting the self-management of biological drugs in SA. It facilitates disease assessment for both patients and HCPs and enables precise control of treatment administration both in terms of injection site and temporal tracking. The BioCart© Diary assessment in a real clinical practice setting yielded very positive results establishing it as a user-friendly and easy-to-understand Diary for patients and HCPs, highlighting the potential role for material such as the BioCart© Diary to fill this information support gap in the home administration of biological therapy.
The utility of the BioCart© Diary might be further improved by collecting more data over longer periods of time and making it accessible via a website or an application. The journey of the BioCart© Diary is promising and is expected to have a positive impact for patients with SA and on biological therapy.
Ethics committee
Based on the project's objectives and methodology, it is determined that this is not a clinical research study but rather an opinion survey involving medical professionals and patients. Therefore, under Spanish regulations, it is exempt from evaluation as a research project. However, it must still adhere to the standard procedures for protecting personal data, as well as comply with the deontological codes governing opinion surveys.
Informed consent
Participants expressed their consent to participate in this opinion study by accepting a participation clause detailing the purpose of the study and the processing of personal data. The exact participation clauses are detailed in the Supplementary Material_PRE&POST survey patient.
Funding
This project is the result of a collaboration between GSK and the Spanish Society of Allergology and Clinical Immunology (SEAIC) and was funded by GSK.
Authors’ contributions
All authors have equally contributed to the conception, design, analysis, and interpretation of the present work. Further, all authors have contributed to drafting or critically revising this manuscript, have approved the final version and agree with its submission to Journal of Investigational Allergology & Clinical Immunology. All authors are accountable for all aspects of the work and in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of interest
GSK funded Adelphi Targis who arranged expert meetings and provided MW support (including assisting authors with the development of the initial draft and incorporation of comments), and editorial support (including figure preparation, formatting, proofreading and submission), under the direction and guidance of the expert authors, according to Good Publication Practice guidelines (GPP 2022). GSK had an opportunity to provide comments to the authors for their consideration. The authors were solely responsible for opinions, critical assessment and interpretation, and approved the final content.
Julio Delgado Romero also declares the following conflict of interests: received consulting fees from AstraZeneca and honoraria from AstraZeneca, Sanofi, and GSK for lectures, presentations, speakers’ bureaus, manuscript writing and educational events.
Lorena Soto-Retes also declares that she has received honoraria from AstraZeneca, Sanofi, and GSK for lectures, presentations, speakers’ bureaus, manuscript writing and educational events. Also grants for attending meetings and/or travel from HAL Allergy S.L.U., AstraZeneca, and Sanofi.
Carolina Cisneros Serrano also reports that she has received consulting fees from GSK, Novartis, AstraZeneca, and Sanofi. She has also received payment or honoraria for lectures from Laboratorios Gebro Pharma S.A., GSK, AstraZeneca, Chiesi, Sanofi and Novartis. She has received support for attending meetings and/or travel from Sanofi AstraZeneca, Chiesi, and Laboratorios Gebro Pharma S.A. She has participated on a Data Safety Monitoring Board or Advisory Board for GSK, AstraZeneca, Novartis, and Sanofi. Finally, she has received equipment or materials from GSK, Sanofi, and AstraZeneca.
Marina Blanco Aparicio reports that she has no other conflicts of interests aside from the payment received from GSK to participate in the BioCart© Study.
Raúl Ferrando Piqueres reports that he has no other conflicts of interests aside from the payment received from GSK to participate in the BioCart© Study.
David Díaz-Pérez declares that he has also received honoraria from AstraZeneca, Chiesi, Sanofi, GSK, TEVA Pharmaceuticals and Boehringer Ingelheim for lectures, presentations, speakers’ bureaus, manuscript writing and educational events.
Valentín López Carrasco declares that he has also received honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events from GSK, TEVA Pharmaceuticals, AstraZeneca, Chiesi and Sanofi.
Vicente Merino Bóhorquez also declares that he has received honoraria from AstraZeneca, GSK, Sanofi and Novartis for lectures, presentations, speakers’ bureaus, manuscript writing and educational events. Also grants for attending meetings and/or travel from AstraZeneca, GSK, Sanofi, and Novartis. He has also received fees for participating on Data Safety Monitoring Board or Advisory Board from AstraZeneca, GSK, Sanofi, and Novartis.
Javier Domínguez Ortega also reports consulting fees from GSK and AstraZeneca. He has also received honoraria from ALK-Abelló A/S, AstraZeneca, Sanofi, GSK, Chiesi, LETI Pharma and TEVA Pharmaceuticals for lectures, presentations, speakers’ bureaus, manuscript writing and educational events.
Acknowledgments
We would like to thank the eight severe asthma experts who participated in the Project as well as their respective patients as their opinions contributed to the improvement of the BioCart© tool; Allergists: Mar Fernández Nieto (H.U. Fundación Jiménez Díaz), Cristina Martin García (H.C.U. of Salamanca), María Rubio Pérez (H. Infanta Sofía), María Vázquez de la Torre Gaspar (H.U. Infanta Leonor); Pulmonologists: Mª Cleofé Fernández Aracil (H.G.U. Alicante), Tamara Hermida Valverde (H.C.U. of Asturias), Inmaculada Lluch Tortajada (H.U. de la Ribera) and Gregorio Soto Campos (H.U. of Jerez).
To Beatriz Velasco-Laorga from GlaxoSmithKline (GSK) for her support to carry out this project and the manuscript.
To the strategic consultancy team of Adelphi Targis S.L., comprised of Ana Fernández and Nerea Toro, for their technical and editorial support at developing the BioCart© Diary study.
Footnotes
Supplementary data associated with this article can be found in the online version available at https://doi.org/10.1016/j.opresp.2024.100375.
Appendix B. Supplementary data
The following are the supplementary data to this article:
Supplementary Fig. 1. Questionnaire feedback from patients and HCPs before use of the BioCart.
Pre- and post-use questionnaire for HCPs.
Pre- and post-use questionnaire for patients.
References
- 1.Delgado Romero J., Blanco-Aparicio M., Cisneros Serrano C., Díaz-Pérez D., Ferrando Piqueres R., López-Carrasco V., et al. Support for home administration of biological therapy in patients with severe asthma: BioCart©. J Investig Allergol Clin Immunol. 2022;32:482–484. doi: 10.18176/jiaci.0786. [DOI] [PubMed] [Google Scholar]
- 2.Plaza V., Alobid I., Alvarez C., Blanco M., Ferreira J., García G., et al. Spanish Asthma Management Guidelines (GEMA) VERSION 5.1. Highlights and controversies. Arch Bronconeumol. 2022;58:150–158. doi: 10.1016/j.arbres.2021.05.010. [DOI] [PubMed] [Google Scholar]
- 3.Cisneros-Serrano C., Rial M.J., Gómez-Bastero-Fernández A., Igea J.M., Martínez-Meca A., Fernández-Lisón L.C., et al. Spanish multidisciplinary consensus on the characteristics of severe asthma patients on biologic treatment who are candidates for at-home administration. Rev Clin Esp. 2023;223:154–164. doi: 10.1016/j.rceng.2022.11.008. [DOI] [PubMed] [Google Scholar]
- 4.González Barcala F.J., de la Fuente-Cid R., Álvarez-Gil R., Tafalla M., Nuevo J., Caamaño-Isorna F. Factors associated with asthma control in primary care patients: the CHAS study. Arch Bronconeumol. 2010;46:358–363. doi: 10.1016/j.arbres.2010.01.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Fig. 1. Questionnaire feedback from patients and HCPs before use of the BioCart.
Pre- and post-use questionnaire for HCPs.
Pre- and post-use questionnaire for patients.