Summary
Children are the main susceptible group to acute lymphoblastic leukemia (ALL), and the lack of sufficient data has impeded a comprehensive understanding of its global impact. This study analyzed the annual numbers and rates of incidence, deaths, and disability-adjusted life years (DALYs) of childhood ALL from 1990 to 2021, disaggregated by age group, gender, and socio-demographic index (SDI) at the global, regional, and national levels, based on the 2021 Global Burden of Disease (GBD) database. Although global deaths and DALYs rates for childhood ALL showed declining trends, the incidence rate fluctuated. Incidence rates in high SDI regions were higher, but deaths and DALY rates were lower. Moreover, the burden in Sub-Saharan Africa and other low SDI countries was growing. The burden on boys has been higher than on girls in this period. This study underscored improving prevention and treatment measures are critical to control the persistent global burden of children ALL.
Subject areas: Medical science, Health profession, Immunology
Graphical abstract
Highlights
-
•
Global deaths and DALYs rates for childhood ALL have significantly declined from 1990 to 2021
-
•
Males experienced a greater disease burden than females across all age groups
-
•
High SDI regions showed high incidence rates but lower deaths and DALY rates
-
•
Low SDI regions, such as Southern Sub-Saharan Africa, faced rising deaths and DALY rates
Medical science, Health profession, Immunology.
Introduction
When patients with acute lymphoblastic leukemia (ALL) are diagnosed, lymphocytes at different stages of maturity in the bone marrow proliferate significantly, inhibiting normal hematopoiesis and infiltrating various organs extensively. It typically progresses rapidly and can lead to death quickly if not promptly treated. ALL is the most common childhood cancer globally, accounting for about 25% of cancer diagnoses in children under 15 years old.1 Currently, risk-stratified treatment methods adjusted based on clinical presentations and biological characteristics have increased the 5-year overall survival rate of childhood ALL to 90%.2 However, there is still a 25% relapse rate.3 It is worth noting that among patients diagnosed with ALL, 10% are still unable to achieve a complete cure, which is a leading factor in childhood cancer mortality.4 Furthermore, due to the negative effects of this disease and its treatment, survivors may face long-term risks of sequelae.5 Emerging research also indicated that genetic factors, such as MLL gene mutations in infants, play a crucial role in the prognosis of ALL.6 Although socioeconomic development and advancements in science and technology have improved survival rates in high-income regions, where 5-year survival can exceed 90%, there remained significant disparities in outcomes across different regions. Previous studies have emphasized the impact of socioeconomic disparities on healthcare access and treatment outcomes. For example, Bhakta et al. (2019) demonstrated that regions with lower socioeconomic development indices tend to have poorer healthcare infrastructure, leading to delays in diagnosing and treating childhood cancers, which directly contribute to higher mortality rates.7 Similarly, Lam et al. (2019) highlighted the barriers faced by resource-limited settings in providing adequate pediatric cancer care, including ALL, such as the lack of trained personnel and limited availability of essential drugs.8
To date, no study has revealed the changing trends in the global burden of childhood ALL. Our study built on this understanding by using the Global Burden of Disease (GBD) 2021 data to analyze trends in childhood ALL incidence, deaths, and disability-adjusted life years (DALYs) across different socio-demographic index (SDI) regions worldwide from 1990 to 2021, aiming to analyze the changing trend of the disease burden of ALL in children and provide a new perspective for policymakers to formulate relevant prevention and control measures.
Results
Global burden of acute lymphoblastic leukemia in children
Incidence
In 2021, a total of 46,304 (95% uncertainty interval [UI], 31403 to 59159) incidence cases of ALL were reported in children globally, with a decrease of 14% (95% UI,-44%–27%) compared to 1990. The decrease in the incidence number was greater for females at 25% as opposed to males at 5%. From 1990 to 2021, the global incidence rate showed a decreasing trend (EAPC, −0.48; 95% Confidence Interval [CI], −0.7 to −0.26), along with a decreasing trend in females (EAPC, −0.84; 95% CI, −1.07 to −0.61), while the EAPC in males did not change significantly (EAPC, −0.2; 95% CI, −0.41 to 0.01) (Table 1). Between 1990 and 2021, the global incidence rate of ALL was consistently higher in males than in females among children aged 0–14 years. There was a decrease in the incidence rate for all gender groups (Figure 1A).
Table 1.
Global and SDI level trends in incidence and deaths of childhood acute lymphoblastic leukemia from 1990 to 2021
| Characteristics | Incidence (95%UI) |
Deaths (95%UI) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1990 |
2021 |
1990–2021 |
1990 |
2021 |
1990–2021 |
|||||||
| Incidence number | Rate (per100000) | Incidence number | Rate (per100000) | number change | EAPC (95%CI) | Deaths number | Rate (per100000) | Deaths number | Rate (per100000) | number change | EAPC (95%CI) | |
| Global | 53978(42103–71529) | 3.1(2.42–4.11) | 46304(31403–59159) | 2.3(1.56–2.94) | −0.14(-0.44 to 0.27) | −0.48(-0.7 to −0.26) | 40207(29731–55271) | 2.31(1.71–3.18) | 18871(13211–22920) | 0.94(0.66–1.14) | −0.53(-0.68 to −0.33) | −2.79(-2.88 to −2.69) |
| Male | 29049(17537–41743) | 3.25(1.96–4.67) | 27588(17097–37470) | 2.66(1.65–3.61) | −0.05(-0.42 to 0.79) | −0.2(-0.41 to 0.01) | 21895(11840–33395) | 2.45(1.33–3.74) | 11558(7227–14980) | 1.11(0.7–1.44) | −0.47(-0.66 to −0.02) | −2.44(-2.53 to −2.35) |
| Female | 24930(17363–34294) | 2.95(2.05–4.06) | 18716(12304–23571) | 1.92(1.26–2.42) | −0.25(-0.57 to 0.2) | −0.84(-1.07 to −0.61) | 18312(12025–26244) | 2.17(1.42–3.1) | 7314(4917–8917) | 0.75(0.5–0.92) | −0.6(-0.75 to −0.39) | −3.26(-3.36 to −3.17) |
| High SDI | 7896(7476–8362) | 4.25(4.02–4.5) | 6722(5965–7553) | 3.9(3.46–4.38) | −0.15(-0.26 to −0.03) | 0.13(-0.09 to 0.34) | 1761(1657–1905) | 0.95(0.89–1.03) | 565(504–621) | 0.33(0.29–0.36) | −0.68(-0.72 to −0.64) | −3.35(-3.47 to −3.23) |
| High-middle SDI | 12707(9830–15910) | 4.64(3.59–5.81) | 12273(7258–17488) | 5.32(3.14–7.57) | −0.03(-0.43 to 0.49) | 1.49(1.08–1.91) | 8453(6315–10601) | 3.09(2.31–3.87) | 1861(1238–2373) | 0.81(0.54–1.03) | −0.78(-0.85 to −0.68) | −4.29(-4.43 to −4.15) |
| Middle SDI | 20608(15039–27789) | 3.57(2.61–4.81) | 15175(9815–20242) | 2.68(1.73–3.57) | −0.26(-0.56 to 0.12) | −0.38(-0.71 to −0.06) | 17754(12904–23890) | 3.08(2.24–4.14) | 6037(4158–7424) | 1.07(0.73–1.31) | −0.66(-0.77 to −0.51) | −3.23(-3.36 to −3.09) |
| Low-middle SDI | 8367(5331–13041) | 1.77(1.13–2.76) | 6577(4703–8040) | 1.13(0.81–1.39) | −0.21(-0.49 to 0.25) | −1.25(-1.33 to −1.18) | 7954(4985–12501) | 1.68(1.06–2.65) | 5323(3839–6497) | 0.92(0.66–1.12) | −0.33(-0.57 to 0.06) | −1.77(-1.87 to −1.66) |
| Low SDI | 4365(2206–7684) | 1.91(0.96–3.36) | 5531(3385–7303) | 1.2(0.74–1.59) | 0.27(-0.25 to 1.54) | −1.45(-1.51 to −1.39) | 4258(2129–7525) | 1.86(0.93–3.29) | 5069(3127–6697) | 1.1(0.68–1.46) | 0.19(-0.3 to 1.38) | −1.64(-1.7 to −1.58) |
EAPC: estimated annual percentage changes; SDI: socio-demographic index; CI: confidence interval; UI: uncertainty interval.
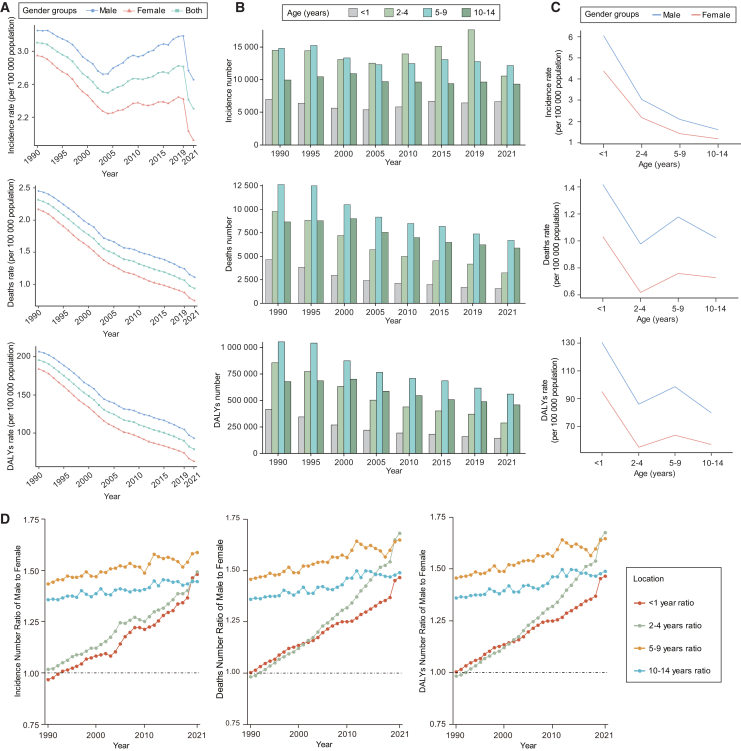
Figure 1.
The global trend of incidence, deaths, and DALYs of acute lymphoblastic leukemia in children from 1990 to 2021
(A) The rate trends of children aged 0–14 of different genders from 1990 to 2021.
(B) The trend of cases in different age groups.
(C) Comparison of disease rates between males and females in different age groups in 2021.
(D) Gender ratio of the number in each age group from 1990 to 2021. DALYs, disability-adjusted life years.
See also Table S1.
Over the period from 1990 to 2021, there has been no clear long-term upward or downward trend in the numbers of incidence for different age groups. Children aged 2–4 and 5–9 years had the higher numbers of ALL cases, while children aged 0–1 year had the fewest cases (Figure 1B). In 2021, the incidence rate of ALL among males in each age group was higher than that of females. Regardless of gender, the highest incidence rate occurred in children aged 0–1 year, while children aged 10–14 years showed the lowest (Figure 1C). We further calculated the number ratio of male-to-female (M/F) to explore the gender differences and changing trends in various ages. Regarding the burden of incidence number, all age groups exhibited a male-dominated pattern (M/F > 1), except for those aged 0–1 year before 1993. The highest ratio was shown in aged 5–9 years (Figure 1D).
Mortality
Over the past three decades, the global death rate of childhood ALL has shown a declining trend, dropping from 2.31 in 1990 to 0.94 in 2021, with an EAPC of −2.79 (95% CI, −2.88 to −2.69). Furthermore, the death rates of different genders have also shown a decreasing trend, with the male childhood ALL death rate consistently higher than that of females. The EAPC for males was −2.44 (95% CI, −2.53 to −2.35) and for females was −3.26 (95% CI, −3.36 to −3.17) (Table 1; Figure 1A). The global number of deaths due to ALL in children has also shown a decreasing trend, declining by 53% from 40,207 (29,731 to 55,271) in 1990 to 18,871 (13,211 to 22,920) in 2021. The number of deaths in each age group generally showed a decreasing trend, with the ages 5–9 years consistently having higher death numbers than other age groups (Figure 1B). Females have a lower death rate than males (Figure 1C). Similarly, death numbers across all age groups exhibited a male-dominated pattern (M/F > 1), except for aged 2–4 years, which was higher in females than males before 1992 (Figure 1D).
Disability-adjusted life years
From 1990 to 2021, the global DALYs rate in children with ALL showed a similar trend to the deaths rate, decreasing from 195.54 (95%UI, 144.32 to 270.21) in 1990 to 78.87 (95%UI, 55.08 to 96.22) in 2021. EAPC showed a downward trend of −2.79 (95%CI, −2.89 to −2.69). In terms of gender, the male DALYs rate has consistently remained higher than that of females over the past three decades (Figure 1A; Table S1). The number of DALYs declined across all age groups, with children aged 5–9 years consistently experiencing higher levels compared to other age groups (Figure 1B). In 2021, the DALYs rate was higher for males in all age groups (Figure 1C). The DALYs number ratio of male-to-female exhibited a male-dominated pattern (M/F > 1) and also increased across all age groups from 1990 to 2021. Aged 2–4 years showed the most significant increase (Figure 1D).
Burden of childhood acute lymphoblastic leukemia in socio-demographic index regions
Incidence
Overall, the incidence rate of ALL in children from high SDI, high-middle SDI, and middle SDI regions has consistently been higher than those in low-middle SDI and low SDI regions. During the COVID-19 pandemic in 2019–2020, there was a significant decrease in the incidence rate of childhood ALL across all SDI regions, with a particularly notable decrease in the high-middle SDI regions. From 1990 to 2021, only high-middle SDI areas showed an increasing trend in the EAPC of incidence rate (1.49; 95% CI, 1.08 to 1.91) (Table 1; Figure 2A). Over the past three decades, middle SDI regions showed the highest incidence number of children with ALL, and low SDI regions exhibited the lowest. When it comes to gender, the number of males with ALL has consistently been higher than females in all SDI regions. Furthermore, except for the high SDI region, the gender ratio (M/F > 1) of incidence number has been increasing in all other regions, with the highest ratio observed in the low SDI regions (Figure S1A). In terms of different age groups, those aged 0–1 and 2–4 years showed high incidence rates in high SDI, high-middle SDI, and middle SDI regions, and fluctuated at a high level, while the incidence rates of all age groups in low-middle SDI and low SDI regions were lower (Figure S2).
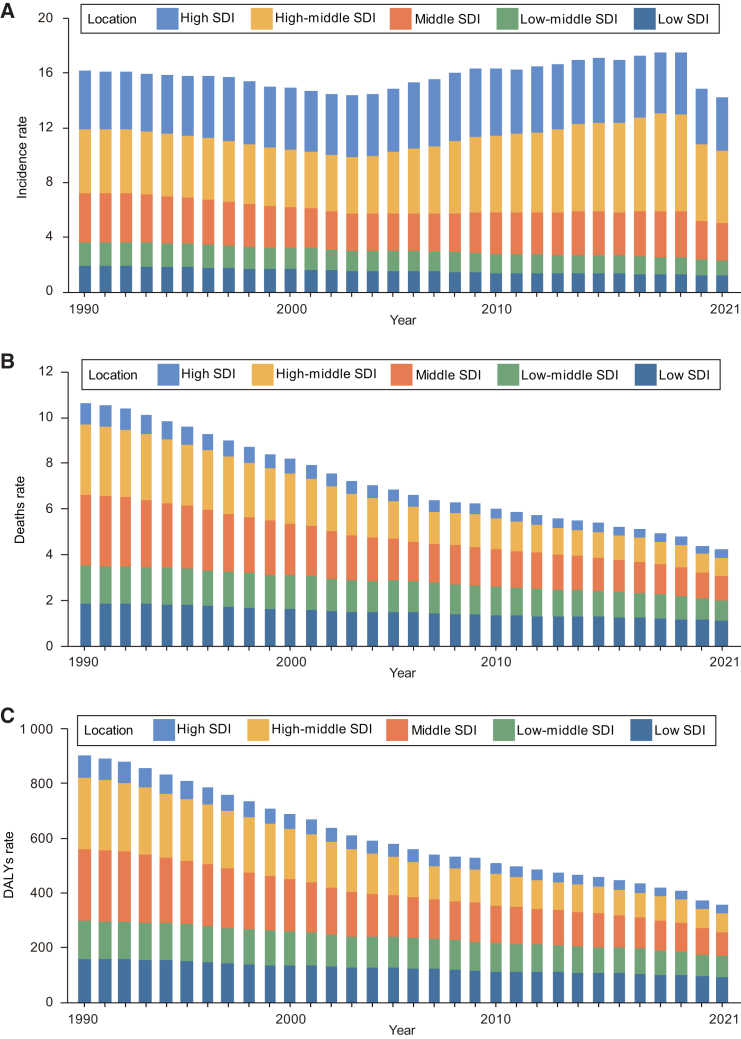
Figure 2.
Trends in the incidence, deaths, and DALYs rate of acute lymphocytic leukemia in children aged 0–14 in SDI quintiles from 1990 to 2021
(A) Trends in incidence rate (per 100 000 population).
(B) Trends in death rate (per 100 000 population).
(C) Trends in DALYs rate (per 100 000 population). DALYs, disability-adjusted life years; SDI, socio-demographic index.
See also Figures S1 and S2, and Table S1.
Mortality
From 1990 to 2021, all SDI regions experienced declines in death rate. The high SDI regions had the lowest death rate, while the high-middle SDI regions had the lowest EAPC death rate (−4.29; 95% CI, −4.43 to −4.15) (Table 1; Figure 2B). Over the past three decades, the death number of males in all SDI regions exceeded that of females. The gender ratio (M/F > 1) of deaths number has continued to increase in all regions, except in high SDI regions where the proportion has decreased (Figure S1B). Deaths rate for all age groups in high-middle SDI and middle SDI regions decreased significantly from the high level. The high SDI regions showed a lower burden of death rate for all age groups than in other regions (Figure S2).
Disability-adjusted life years
Over the past three decades, the trends in DALYs rate for children with ALL in all SDI regions have been similar to those for deaths, with both showing a decreasing trend. In 2021, the DALYs rate was lowest in high SDI regions at 29.34 (95% UI, 25.89 to 32.87), while it was highest in low SDI regions at 92.61 (95% UI, 56.7 to 122.33), with the smallest decrease in EAPC at −1.66 (95% UI, −1.72 to −1.6) (Table 1; Figure 2C). In addition, the number of DALYs for males in all SDI regions was higher than that for females, and this ratio (M/F) showed a certain downward trend in high SDI regions since 2005 (Table 1; Figure S1C). The trend of DALYs rate in each age group in different SDI regions was similar to that of death rate (Figure S2).
The burden of acute lymphoblastic leukemia in children across 21 geographic regions
Incidence
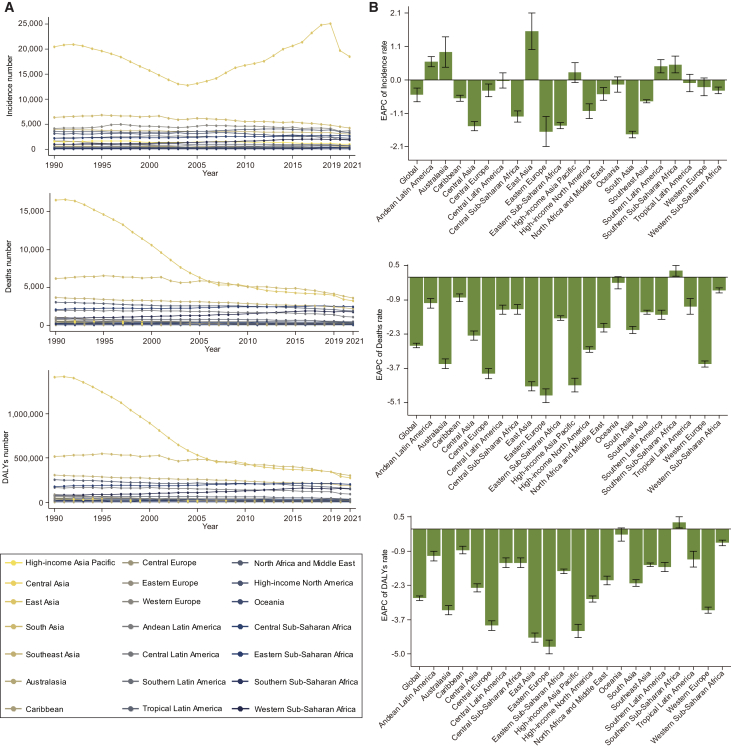
Between 1990 and 2021, in 21 geographical regions, the incidence number of ALL among children in the East Asia region was the highest, far exceeding that of other regions. In contrast, the Oceania region had the lowest number of incidence cases, with only 36 (95% UI, 18 to 67) in 2021. Furthermore, in the Western Sub-Saharan Africa region, the incidence number of ALL children in 2021 had increased by 100% compared to 1990 (Figure 3A; Table S2). Over the past three decades, one-third of the regions had childhood ALL incidence rates consistently higher than the global average, with Western Europe and East Asia having higher incidence rates. In 2021, the incidence rate in East Asia was the highest at 6.94 (95% UI, 3.75 to 10.32), followed by Western Europe at 5.36 (95% UI, 4.84 to 6); Central Sub-Saharan Africa had the lowest incidence rate at 0.61 (95% UI, 0.34 to 0.93). In addition, the EAPCs in Andean Latin America, Australasia, East Asia, Southern Latin America, and Southern Sub-Saharan Africa were increasing. The largest increase in EAPC was in East Asia at 1.56 (95% CI, 0.98 to 2.14). Children in East Asia still face a substantial burden of incidence number and rate (Figures 3B and S3A).
Figure 3.
Trends in acute lymphocytic leukemia in children aged 0–14 in GBD regions from 1990 to 2021
(A) Trends in the number of incidences, deaths, and DALYs.
(B) Trends in the EAPC of incidences, deaths, and DALY rate. DALYs, disability-adjusted life years; EAPC, estimated annual percentage changes. The error bar represents the 95% confidence interval for each data point, indicating the range within which the true population means is likely to fall. See also Figure S3 and Tables S2–S4.
Mortality
Between 1990 and 2005, the East Asia region had the highest number and the most dramatic change in deaths of children with ALL. The number of deaths in South Asia surpassed that in East Asia after 2005. The death numbers in other regions did not change much (Figure 3A; Table S3). Furthermore, the death rate of childhood ALL in East Asia has significantly decreased, from 5.01 (95% UI, 3.44 to 6.94) in 1990 to 1.21 (95% UI, 0.71 to 1.66) in 2021, with an EAPC of −4.46 (95% CI, −4.64 to −4.28). The death rates of childhood ALL in Andean Latin America and Central Latin America have also remained at relatively high levels. Except for a slight increase in EAPC of 0.27 (95% CI, 0.05 to 0.48) in Southern Sub-Saharan Africa, the EAPC decreased in the rest of the regions. The Eastern Europe region saw the most significant decrease in EAPC at −4.84 (95% CI, −5.13 to −4.56) (Figures 3B and S3B).
Disability-adjusted life years
From 1990 to 2021, the trends in DALYs number and rate across different regions were similar to those in deaths. In East Asia, the number of DALYs for children with ALL significantly decreased, from 1,409,668 (964,759 to 1,956,190) in 1990 to 277,916 (162,221 to 385,772) in 2021 (Figure 3A; Table S4). The DALYs rate in East Asia also dropped significantly, from 427.39 (95% UI, 292.5 to 593.08) in 1990 to 103.95 (95% UI, 60.68 to 144.29) in 2021. In Andean Latin America and Central Latin America, the DALY rates remained relatively high. In 2021, the highest DALYs rate for children with ALL was in Andean Latin America at 167.55 (95% UI, 109.61 to 222.65), followed by the Caribbean at 151.07 (95% UI, 94.38 to 249.01). Out of 21 regions, only Southern Sub-Saharan Africa saw an increase in EAPC of DALYs (0.27; 95% CI, 0.05 to 0.49). All other regions experienced a downward trend in EAPC, with the largest decrease observed in Eastern Europe at −4.76 (95% CI, −5.04 to −4.48) (Figures 3B and S3C).
The burden of acute lymphoblastic leukemia in children across 204 countries
Incidence
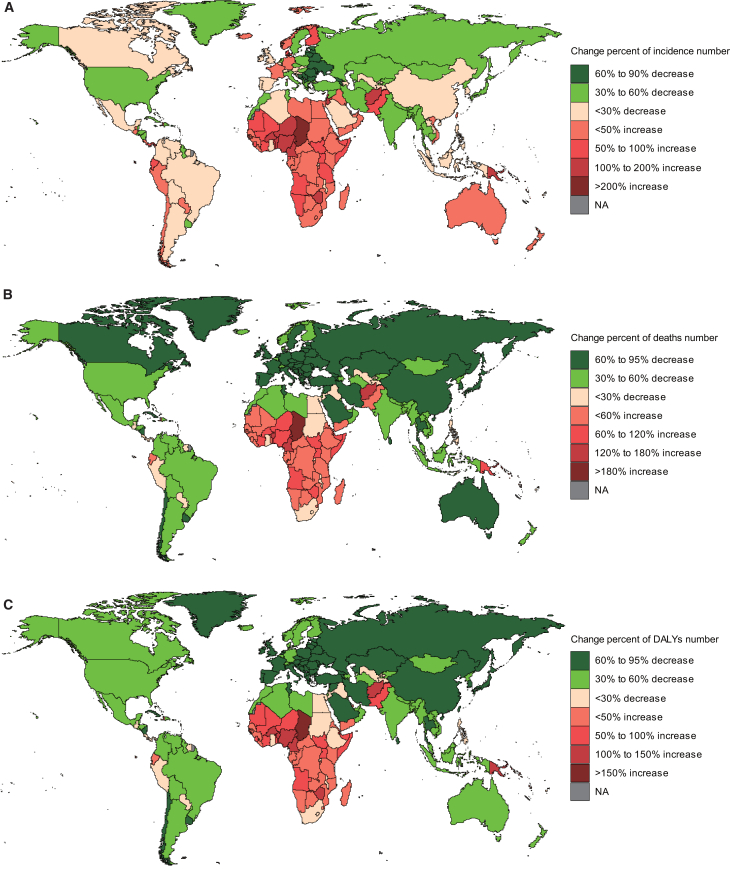
In 2021, 117 countries had fewer incidence cases of childhood ALL than in 1990, with Georgia and the Republic of Moldova showing the most significant declines, the number of cases increased in 87 countries, with the largest increase in Qatar (percent change: >400%) (Figure 4A; Table S5). In 2021, Monaco had the highest incidence rate of childhood ALL, whereas Palau had the lowest one (Figure S4A; Table S5). From 1990 to 2021, over 2/3 of countries saw a decrease in incidence rate, with 52 countries experiencing an increase; among them, Georgia showed the most significant decrease (EAPC, −4.82; 95% CI, −5.37 to −4.27), while Cyprus had the largest increase in incidence rate (EAPC, 4.16; 95% CI, 3.31 to 5.02) (Figure S5A; Table S5).
Figure 4.
Changes in the cases of children with acute lymphocytic leukemia by countries and territories between 1990 and 2021
(A) The percentage change in incident cases.
(B) The percentage change in death cases.
(C) The percentage change in DALY cases. DALYs, disability-adjusted life years.
See also Figures S4 and S5 and Tables S5, S6, and S7.
Mortality
In 204 countries, nearly 1/4 of them showed an increase in the number of children’s deaths due to ALL in 2021 compared to 1990. Chad had the largest increase in deaths number (percent change: 266%), followed by Cameroon (percent change: 151%) (Figure 4B; Table S6). Only 12 countries exhibited the increasing death rate, with Zimbabwe showing the highest increase (EAPC, 3.01; 95% CI, 2.24 to 3.78), while Luxembourg had the most significant decrease (EAPC, −5.93; 95% CI, −6.47 to −5.4) (Figure S5B; Table S6). In 2021, Haiti had the highest childhood ALL death rate, with the Cook Islands having the lowest one (Figure S4B; Table S6).
Disability-adjusted life years
From 1990 to 2021, the number of DALYs for childhood ALL increased in 49 countries. Leading the list were Chad (percent change: 262%) and Cameroon (percent change: 148%). Conversely, Latvia, the Republic of Moldova, Georgia, and Estonia saw a significant decrease in the number of DALYs (percent change: −95% to −90%) (Figure 4C and Table S7). This trend was similar to changes in death rate. Additionally, only 11 regions showed an upward trend in children’s ALL DALYs rate, with Zimbabwe having the highest increase (EAPC, 2.99; 95% CI, 2.23 to 3.76). On the other hand, Luxembourg experienced the most significant decrease in DALYs rate (EAPC, −5.52; 95% CI, −6.03 to −5.01) (Figure S5C and Table S7). Notably, countries such as Botswana, Burkina Faso, Cameroon, Chad, Eswatini, Lesotho, Namibia, Niue, South Sudan, and Zimbabwe saw both DALYs rate and number increase. As of 2021, Haiti had the highest rate of children’s ALL DALYs, while the Cook Islands showed the lowest one (Figure S4C and Table S7).
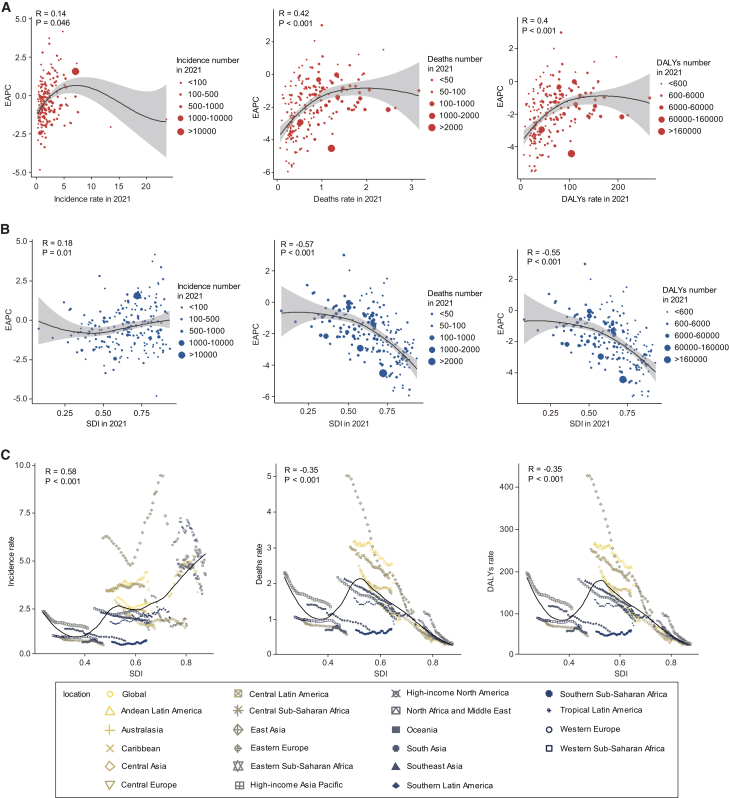
Correlation of EAPC with incidence rate, deaths rate, disability-adjusted life year rate, and socio-demographic index
In 2021, in 204 countries, the deaths rate, DALYs rate, and their corresponding EAPCs showed a moderate positive correlation (R = 0.42, p < 0.001; R = 0.4, p < 0.001); while the incidence rate was weakly correlated with its corresponding EAPC (R = 0.14, p = 0.046) (Figure 5A). The EAPCs (in deaths rate and in DALYs rate) showed a strong negative correlation with the SDI (R = −0.57, p < 0.001; R = −0.55, p < 0.001), while the incidence rate EAPC showed a weak correlation with the SDI (R = 0.18, p = 0.01) (Figure 5B).
Figure 5.
Correlation between EAPC and SDI, EAPC and rate, and rate and SDI in childhood acute lymphoblastic leukemia
(A) Correlation between incidence, deaths, DALYs rate, and EAPC in 204 countries and territories in 2021.
(B) Correlation between SDI and EAPC of incidence, deaths, and DALY rate in 204 countries and territories in 2021.
(C) Correlation of global and regional SDI with incidence, deaths, and DALY rates, 1990–2021. DALYs, disability-adjusted life years; EAPC, estimated annual percentage changes; SDI, socio-demographic index.
See also Figure S6, Tables S2–S4, Tables S5, S6, and S7.
Relation between socio-demographic index levels and incidence, deaths, and disability-adjusted life year rates
From 1990 to 2021, in 21 regions within the GBD geographic area, SDI was positively correlated with incidence rate and negatively correlated with death rate and DALYs rate (R = 0.58, p < 0.001; R = −0.35, p < 0.001; R = −0.35, p < 0.001) (Figure 5C). Among 204 countries, SDI was positively correlated with incidence rate and negatively correlated with death rate and DALYs rate (R = 0.41, p < 0.001; R = −0.42, p < 0.001; R = −0.42, p < 0.001) (Figure S6).
Discussion
In this study, the deaths and DALYs rates for childhood ALL have shown a gradual decline globally, while the incidence rate significantly decreased between 2019 and 2021, likely due to delays in cancer diagnoses caused by the COVID-19 pandemic.9,10 The incidence, deaths, and DALYs rates of ALL were higher in males compared to females, possibly due to differences in hormone levels, genetics, and the immune system.11 It was observed that children under 1 year old have the highest death rate, incidence rate, and DALYs rate for ALL across all age groups. Research has shown that the majority of cases originated prenatally, with infant (<1 year) ALL often associated with MLL gene rearrangement.12,13 In addition, undergoing background radiation and X-ray examinations while in the uterus also increases the risk of disease.14
The methodology used in this study was based on established epidemiological approaches, similar to those used in previous studies, such as Fitzmaurice et al. (2019),15 and employs EAPCs to assess trends over time while linking these trends to the SDI to better understand how socioeconomic factors influence disease outcomes. This approach has been supported by studies in global health disparities and cancer epidemiology,7 with significant potential to guide healthcare resource allocation and intervention strategies.
In regions with high SDI, the incidence rate was high, while the death rate and DALY rates were low. In 2021, low SDI regions saw the highest deaths and DALYs rates, despite having a low incidence rate. East Asia also showed a high incidence rate but with relatively lower deaths and DALY rates. In Eastern Europe, all rates of incidence, deaths, and DALYs showed a decreasing trend. However, regions such as Southern Sub-Saharan Africa, Chad, and Zimbabwe experienced rising rates in all these metrics. These findings were consistent with Bhakta et al. (2019), who observed persistent disparities in pediatric cancer outcomes between high and low SDI regions due to limited healthcare access.7 Obviously, the economic status of society has to some extent influenced the burden of diseases.16 In more economically developed regions, advanced medical technologies and abundant healthcare resources ensure accurate detection and diagnosis of diseases at early stages. In contrast, in impoverished areas, maintaining treatment becomes particularly challenging, leading to insufficient monitoring of treatment outcomes and toxicities, thereby increasing the risk of patient mortality.17 Research data indicates that in regions with relatively lower socioeconomic status, the mortality rate of children with ALL has increased by 17%–33%, and the risk of relapse in children with ALL has also increased by 1.9 times.18,19
Up to now, significant progress has been made in the treatment of ALL. The introduction of new treatment modalities such as immune therapy and targeted antibodies has increased the overall survival rate of ALL to over 90%.20 However, patients also face long-term complications and acute toxic effects from therapies such as chemotherapy. Relapse continues to be a major obstacle, particularly in T cell ALL, where post-relapse salvage rates are low, with around 20% of pediatric patients with T-ALL dying from relapse within 5 years of initial diagnosis.21,22,23 Children in impoverished regions are disproportionately affected by high relapse rates and mortality, which increases the disease burden and hinders societal progress.24
Our study highlighted the significant disparities in childhood ALL burden between high and low SDI regions, which have crucial implications for biomedical applications. High SDI regions benefit from advanced medical technologies, early diagnostic tools, and well-structured healthcare systems, leading to lower deaths and DALY rates. For example, Pui et al. (2015) demonstrated that in high-income countries, the application of minimal residual disease (MRD) monitoring and precision medicine has significantly improved treatment outcomes for childhood ALL.25 In contrast, low SDI regions face barriers to adopting these advanced technologies due to financial, infrastructural, and workforce limitations, resulting in higher death rates. Lam et al. (2019) also noted these challenges in resource-constrained settings.8 Improving healthcare access, early diagnosis, and treatment availability in low SDI regions can significantly reduce the childhood ALL burden and improve patient outcomes.
Future research should focus on investigating the underlying factors contributing to the increasing burden of childhood ALL in low- and middle-income regions. This includes exploring the role of environmental exposures, healthcare access, and genetic predispositions to better understand the geographic disparities observed. Ward et al. (2014) reported that early childhood exposure to radiation and infections is associated with an increased risk of ALL, particularly in regions with poor healthcare infrastructure.26 Moreover, studies should evaluate the effectiveness of healthcare interventions, such as early diagnosis programs and access to advanced treatments, in alleviating ALL burdens in resource-limited environments. Policies must prioritize equitable access to healthcare, improving infrastructure, and training healthcare personnel, as emphasized by Atun et al. (2020) in their analysis of healthcare systems for childhood cancer control.27 International collaboration and funding are essential to ensure low SDI regions receive adequate resources to address the increasing burden of childhood ALL.
Conclusions
From 1990 to 2021, the global deaths and DALY rates of childhood ALL significantly declined, accompanied by some fluctuations in the incidence rate. Among all age groups, children under 1 year old had the highest deaths, incidence, and DALY rates. There were significant gender differences, with males consistently experiencing a greater disease burden than females. In regions with high and high-middle SDI levels, the incidence rate of ALL remained high. In contrast, in low SDI regions and countries, such as Southern Sub-Saharan Africa, deaths and DALY rates continued to rise. These findings provide new insights for developing prevention and control policies for childhood ALL.
Limitations of the study
Despite the comprehensive nature of our study, there are several limitations that need to be acknowledged. First, while the use of the GBD 2021 database is extensive, it relies on secondary data, which may contain inherent biases due to variations in data collection quality across different countries. Disparities in data quality, particularly in low-income regions, may result in underreporting or inaccuracies, potentially affecting the reliability of disease burden estimates. Additionally, the GBD data does not provide the in-depth classification of ALL subtypes, limiting our ability to analyze differences in incidence and outcomes for specific genetic variants, such as T cell or B-cell ALL. This is important because outcomes for different ALL subtypes can vary significantly depending on available treatments and genetic predispositions. Finally, our study primarily focuses on global and regional trends, and while it provides a broad overview, it does not address local factors such as healthcare access, environmental exposures, or genetic risk factors, which could further influence the burden of ALL in certain populations. Future research should focus on collecting more granular, region-specific data to address these gaps.
Resource availability
Lead contact
Further information and requests for resources should be directed to and will be fulfilled by the lead contact, Ying Song (yingsong@sdsmu.edu.cn).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
All data reported in this article will be shared by the lead contact upon request.
-
•
This article does not report the original code.
-
•
Any additional information required to reanalyze the data reported in this article is available from the lead contact upon request.
-
•
All data in this study are publicly available based on the GBD 2021 portal (http://ghdx.healthdata.org/gbd-results-tool).
Acknowledgments
All the authors appreciate the collaborators involved in the GBD study 2021. This work was supported by the Natural Science Foundation of Shandong Province (Grant No. ZR2021QH001), the Science and Technology Support Plan for Youth Innovation of Colleges and Universities of Shandong Province of China (Grant No. 2023KJ253), and the Affiliated Hospital of Shandong Second Medical University, Principal Investigator Start-Up Fund (2020BSQ007).
Author contributions
Y. H., Investigation and data curation. Y. L., Writing – original draft and validation. J. F., Methodology and formal analysis. Y. L., Validation and software. H. W., Validation and data curation. Y. S., Project administration and editing.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Software and algorithms | ||
| R-4.3.3 | The R Foundation for Statistical Computing | https://www.r-project.org/ |
| R Studio 2024.04.1 | Posit Software | https://posit.co/downloads/ |
| Other | ||
| GBD 2021 | IHME | http://ghdx.healthdata.org/gbd-results-tool |
Experimental model and study participant details
This study did not generate experimental model or enroll subjects.
Method details
Data sources
The data for our study were sourced from the Global Burden of Disease (GBD) 2021 database, which provides comprehensive estimates of various health outcomes across 204 countries and territories. Using the standard GBD methodology, the incidence, deaths, and DALYs rates and numbers for childhood ALL were estimated across all regions and time periods through modeling approaches, with adjustments made based on differences in data quality and availability. The rates are age-standardized per 100,000 population, and each estimate is presented with a 95% uncertainty interval (UI). DALYs are calculated as the sum of years of life lost (YLLs) due to premature death and years lived with disability (YLDs). This methodology integrates epidemiological data from a wide range of sources, including vital registration, cancer registries, and survey data.15,28
Regarding disease classification, ALL was classified using the GBD-specific codes for leukemia, which are mapped to the International Classification of Diseases (ICD-10) codes for ALL. In our study, the relevant codes used for ALL classification included C91.0 (acute lymphoblastic leukemia) in ICD-10. This ensures the comparability of data across countries and years.29
In this study, we extracted global, 21 geographic regions, 204 countries and regions data on annual number and rate of incidence, deaths, and DALYs associated with childhood ALL, respectively, by genders (male and female) and age-groups (<1 year, 2–4 years, 5–9 years, and 10–14 years). In addition, we downloaded the SDI data for each region from 1990 to 2021 to examine its relationship with the disease burden of childhood ALL.
Definitions
SDI. The socio-demographic index (SDI) is an indicator that measures per capita income, average education level, and fertility rate. The SDI value range is 0–1; the higher the value, the higher the level of socioeconomic development.30,31 Based on SDI, these regions and countries were divided into 5 categories (high, high-middle, middle, low-middle, low).32
DALYs. Disability-adjusted life years (DALYs) provides a comprehensive assessment of disease burden, representing the cumulative loss of healthy life years due to premature death and disability.33
Patient engagement and protocol approvals
This study utilized openly collected data and conducted in-depth analysis without involving any personally identifiable information. Patients did not participate in determining the research questions, designing and executing experiments, or measuring results, hence patient consent was not necessary. Furthermore, this study strictly adhered to the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER) in every step of analyzing and reporting the global burden of childhood ALL, and the research protocol has been formally approved by the Ethics Committee of Shandong Second Medical University.
Quantification and statistical analysis
Describe the burden of childhood ALL using indicators such as incidence, deaths, DALYs. Data on number and rate are reported with a 95% uncertainty interval(95%UI). The 95% uncertainty interval (UI) is presented as the 2.5th and 97.5th percentiles of the mean estimate of 1000 draws.34 By applying the log-linear regression model, we calculated the estimated annual percentage change (EAPC) and its corresponding 95% confidence intervals (CIs) to depict the changing patterns of childhood ALL burden.35 The formula for calculation is EAPC = 100%×(eβ-1).36 If the lower limit of the 95% confidence interval exceeds 0, it indicates an increasing trend; when the upper limit is below 0, the trend shows a gradual decrease; if the interval includes 0, the trend is not significant. Through Pearson correlation analysis, we estimated the interrelationships between EAPC and rate, EAPC and SDI, and rate and SDI, with two-tailed p < 0.05 indicating statistical significance. Data analysis was conducted using R-4.3.3 and RStudio-2024.04.1, allowing for in-depth analysis and visual representation of the data.
Published: November 23, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111356.
Contributor Information
Haiying Wang, Email: wanghaiying1121@126.com.
Ying Song, Email: yingsong@sdsmu.edu.cn.
Supplemental information
References
- 1.Tran T.H., Hunger S.P. The genomic landscape of pediatric acute lymphoblastic leukemia and precision medicine opportunities. Semin. Cancer Biol. 2022;84:144–152. doi: 10.1016/j.semcancer.2020.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Dixon S.B., Chen Y., Yasui Y., Pui C.H., Hunger S.P., Silverman L.B., Ness K.K., Green D.M., Howell R.M., Leisenring W.M., et al. Reduced Morbidity and Mortality in Survivors of Childhood Acute Lymphoblastic Leukemia: A Report From the Childhood Cancer Survivor Study. J. Clin. Oncol. 2020;38:3418–3429. doi: 10.1200/jco.20.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barz M.J., Hof J., Groeneveld-Krentz S., Loh J.W., Szymansky A., Astrahantseff K., von Stackelberg A., Khiabanian H., Ferrando A.A., Eckert C., Kirschner-Schwabe R. Subclonal NT5C2 mutations are associated with poor outcomes after relapse of pediatric acute lymphoblastic leukemia. Blood. 2020;135:921–933. doi: 10.1182/blood.2019002499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schüz J., Erdmann F. Environmental Exposure and Risk of Childhood Leukemia: An Overview. Arch. Med. Res. 2016;47:607–614. doi: 10.1016/j.arcmed.2016.11.017. [DOI] [PubMed] [Google Scholar]
- 5.Andrés-Jensen L., Attarbaschi A., Bardi E., Barzilai-Birenboim S., Bhojwani D., Hagleitner M.M., Halsey C., Harila-Saari A., van Litsenburg R.R.L., Hudson M.M., et al. Severe toxicity free survival: physician-derived definitions of unacceptable long-term toxicities following acute lymphocytic leukaemia. Lancet. Haematol. 2021;8:e513–e523. doi: 10.1016/s2352-3026(21)00136-8. [DOI] [PubMed] [Google Scholar]
- 6.Pieters R., Schrappe M., De Lorenzo P., Hann I., De Rossi G., Felice M., Hovi L., LeBlanc T., Szczepanski T., Ferster A., et al. A treatment protocol for infants younger than 1 year with acute lymphoblastic leukaemia (Interfant-99): an observational study and a multicentre randomised trial. Lancet. 2007;370:240–250. doi: 10.1016/s0140-6736(07)61126-x. [DOI] [PubMed] [Google Scholar]
- 7.Bhakta N., Force L.M., Allemani C., Atun R., Bray F., Coleman M.P., Steliarova-Foucher E., Frazier A.L., Robison L.L., Rodriguez-Galindo C., Fitzmaurice C. Childhood cancer burden: a review of global estimates. Lancet Oncol. 2019;20:e42–e53. doi: 10.1016/s1470-2045(18)30761-7. [DOI] [PubMed] [Google Scholar]
- 8.Lam C.G., Howard S.C., Bouffet E., Pritchard-Jones K. Science and health for all children with cancer. Science. 2019;363:1182–1186. doi: 10.1126/science.aaw4892. [DOI] [PubMed] [Google Scholar]
- 9.Yabroff K.R., Wu X.C., Negoita S., Stevens J., Coyle L., Zhao J., Mumphrey B.J., Jemal A., Ward K.C. Association of the COVID-19 Pandemic With Patterns of Statewide Cancer Services. J. Natl. Cancer Inst. 2022;114:907–909. doi: 10.1093/jnci/djab122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desai A., Mohammed T.J., Duma N., Garassino M.C., Hicks L.K., Kuderer N.M., Lyman G.H., Mishra S., Pinato D.J., Rini B.I., et al. COVID-19 and Cancer: A Review of the Registry-Based Pandemic Response. JAMA Oncol. 2021;7:1882–1890. doi: 10.1001/jamaoncol.2021.4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Murray C.J.L. The Global Burden of Disease Study at 30 years. Nat. Med. 2022;28:2019–2026. doi: 10.1038/s41591-022-01990-1. [DOI] [PubMed] [Google Scholar]
- 12.Dong Y., Shi O., Zeng Q., Lu X., Wang W., Li Y., Wang Q. Leukemia incidence trends at the global, regional, and national level between 1990 and 2017. Exp. Hematol. Oncol. 2020;9:14. doi: 10.1186/s40164-020-00170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francis S.S., Wallace A.D., Wendt G.A., Li L., Liu F., Riley L.W., Kogan S., Walsh K.M., de Smith A.J., Dahl G.V., et al. In utero cytomegalovirus infection and development of childhood acute lymphoblastic leukemia. Blood. 2017;129:1680–1684. doi: 10.1182/blood-2016-07-723148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nikkilä A., Raitanen J., Lohi O., Auvinen A. Radiation exposure from computerized tomography and risk of childhood leukemia: Finnish register-based case-control study of childhood leukemia (FRECCLE) Haematologica. 2018;103:1873–1880. doi: 10.3324/haematol.2018.187716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Global Burden of Disease Cancer Collaboration. Fitzmaurice C., Abate D., Abbasi N., Abbastabar H., Abd-Allah F., Abdel-Rahman O., Abdelalim A., Abdoli A., Abdollahpour I. Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-Years for 29 Cancer Groups, 1990 to 2017: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749–1768. doi: 10.1001/jamaoncol.2019.2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bona K., Li Y., Winestone L.E., Getz K.D., Huang Y.S., Fisher B.T., Desai A.V., Richardson T., Hall M., Naranjo A., et al. Poverty and Targeted Immunotherapy: Survival in Children's Oncology Group Clinical Trials for High-Risk Neuroblastoma. J. Natl. Cancer Inst. 2021;113:282–291. doi: 10.1093/jnci/djaa107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Anandasabapathy S., Asirwa C., Grover S., Mungo C. Cancer burden in low-income and middle-income countries. Nat. Rev. Cancer. 2024;24:167–170. doi: 10.1038/s41568-023-00659-2. [DOI] [PubMed] [Google Scholar]
- 18.Petridou E.T., Sergentanis T.N., Perlepe C., Papathoma P., Tsilimidos G., Kontogeorgi E., Kourti M., Baka M., Moschovi M., Polychronopoulou S., et al. Socioeconomic disparities in survival from childhood leukemia in the United States and globally: a meta-analysis. Ann. Oncol. 2015;26:589–597. doi: 10.1093/annonc/mdu572. [DOI] [PubMed] [Google Scholar]
- 19.Wadhwa A., Chen Y., Hageman L., Hoppmann A., Angiolillo A., Dickens D.S., Neglia J.P., Ravindranath Y., Ritchey A.K., Termuhlen A., et al. Poverty and relapse risk in children with acute lymphoblastic leukemia: a Children's Oncology Group study AALL03N1 report. Blood. 2023;142:221–229. doi: 10.1182/blood.2023019631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brivio E., Bautista F., Zwaan C.M. Naked antibodies and antibody-drug conjugates: targeted therapy for childhood acute lymphoblastic leukemia. Haematologica. 2024;109:1700–1712. doi: 10.3324/haematol.2023.283815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jensen K.S., Oskarsson T., Lähteenmäki P.M., Flaegstad T., Jónsson Ó.G., Svenberg P., Schmiegelow K., Heyman M., Norén-Nyström U., Schrøder H., Albertsen B.K. Temporal changes in incidence of relapse and outcome after relapse of childhood acute lymphoblastic leukemia over three decades; a Nordic population-based cohort study. Leukemia. 2022;36:1274–1282. doi: 10.1038/s41375-022-01540-1. [DOI] [PubMed] [Google Scholar]
- 22.Reismüller B., Attarbaschi A., Peters C., Dworzak M.N., Pötschger U., Urban C., Fink F.M., Meister B., Schmitt K., Dieckmann K., et al. Long-term outcome of initially homogenously treated and relapsed childhood acute lymphoblastic leukaemia in Austria--a population-based report of the Austrian Berlin-Frankfurt-Münster (BFM) Study Group. Br. J. Haematol. 2009;144:559–570. doi: 10.1111/j.1365-2141.2008.07499.x. [DOI] [PubMed] [Google Scholar]
- 23.Cordo' V., van der Zwet J.C.G., Canté-Barrett K., Pieters R., Meijerink J.P.P. T-cell Acute Lymphoblastic Leukemia: A Roadmap to Targeted Therapies. Blood Cancer Discov. 2021;2:19–31. doi: 10.1158/2643-3230.Bcd-20-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newman H., Li Y., Liu H., Myers R.M., Tam V., DiNofia A., Wray L., Rheingold S.R., Callahan C., White C., et al. Impact of poverty and neighborhood opportunity on outcomes for children treated with CD19-directed CAR T-cell therapy. Blood. 2023;141:609–619. doi: 10.1182/blood.2022017866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pui C.H., Yang J.J., Hunger S.P., Pieters R., Schrappe M., Biondi A., Vora A., Baruchel A., Silverman L.B., Schmiegelow K., et al. Childhood Acute Lymphoblastic Leukemia: Progress Through Collaboration. J. Clin. Oncol. 2015;33:2938–2948. doi: 10.1200/jco.2014.59.1636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ward E., DeSantis C., Robbins A., Kohler B., Jemal A. Childhood and adolescent cancer statistics, 2014. CA A Cancer J. Clin. 2014;64:83–103. doi: 10.3322/caac.21219. [DOI] [PubMed] [Google Scholar]
- 27.Atun R., Bhakta N., Denburg A., Frazier A.L., Friedrich P., Gupta S., Lam C.G., Ward Z.J., Yeh J.M., Allemani C., et al. Sustainable care for children with cancer: a Lancet Oncology Commission. Lancet Oncol. 2020;21:e185–e224. doi: 10.1016/s1470-2045(20)30022-x. [DOI] [PubMed] [Google Scholar]
- 28.GBD 2013 DALYs and HALE Collaborators. Murray C.J.L., Barber R.M., Foreman K.J., Abbasoglu Ozgoren A., Abd-Allah F., Abera S.F., Aboyans V., Abraham J.P., Abubakar I. Global, regional, and national disability-adjusted life years (DALYs) for 306 diseases and injuries and healthy life expectancy (HALE) for 188 countries, 1990-2013: quantifying the epidemiological transition. Lancet. 2015;386:2145–2191. doi: 10.1016/s0140-6736(15)61340-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GBD 2016 Causes of Death Collaborators Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/s0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.GBD 2021 Forecasting Collaborators Burden of disease scenarios for 204 countries and territories, 2022-2050: a forecasting analysis for the Global Burden of Disease Study 2021. Lancet. 2024;403:2204–2256. doi: 10.1016/s0140-6736(24)00685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.GBD 2017 DALYs and HALE Collaborators Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1859–1922. doi: 10.1016/s0140-6736(18)32335-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.GBD 2019 Adolescent Young Adult Cancer Collaborators The global burden of adolescent and young adult cancer in 2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 2022;23:27–52. doi: 10.1016/s1470-2045(21)00581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.GBD 2019 Diseases and Injuries Collaborators Global burden of 369 diseases and injuries in 204 countries and territories, 1990-2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396:1204–1222. doi: 10.1016/s0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.GBD 2019 North Africa and the Middle East Neurology Collaborators The burden of neurological conditions in north Africa and the Middle East, 1990-2019: a systematic analysis of the Global Burden of Disease Study 2019. Lancet Global Health. 2024;12:e960–e982. doi: 10.1016/s2214-109x(24)00093-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ding Q., Liu S., Yao Y., Liu H., Cai T., Han L. Global, Regional, and National Burden of Ischemic Stroke, 1990-2019. Neurology. 2022;98:e279–e290. doi: 10.1212/wnl.0000000000013115. [DOI] [PubMed] [Google Scholar]
- 36.Zhang N., Wu J., Wang Q., Liang Y., Li X., Chen G., Ma L., Liu X., Zhou F. Global burden of hematologic malignancies and evolution patterns over the past 30 years. Blood Cancer J. 2023;13:82. doi: 10.1038/s41408-023-00853-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
All data reported in this article will be shared by the lead contact upon request.
-
•
This article does not report the original code.
-
•
Any additional information required to reanalyze the data reported in this article is available from the lead contact upon request.
-
•
All data in this study are publicly available based on the GBD 2021 portal (http://ghdx.healthdata.org/gbd-results-tool).