Abstract
Background
Different definitions of family-centred care (FCC) exist in the newborn setting, and many FCC interventions have been tested, while a comprehensive review synthesising characteristics of existing intervention studies is still lacking.
Objective
This review aims at summarising the characteristics of randomised controlled trials (RCTs) on FCC interventions in neonatal intensive care units.
Methods
We searched PubMed, Embase, Web of Science and the Cochrane Library up to 31 January 2022, and reference lists of included studies and other reviews. Interventions were grouped into five categories according to a previous Cochrane review: (1) family support, (2) educational, (3) communication, (4) environmental interventions and (5) family-centred policies. Subgroup analyses by time period (RCTs published before vs after 2016) and by country income (based on the World Bank Classification) were conducted.
Results
Out of 6583 retrieved studies, 146 RCTs met the eligibility criteria, with 53 (36.3%) RCTs published after 2016. Overall, 118 (80.8%) RCTs were conducted in high-income countries, 28 (19.1%) in middle-income countries and none in low-income countries. Only two RCTs were multicountry. Although mothers were the most frequent caregiver involved, fathers were included in 41 RCTs (28.1%). Very few studies were conducted in at-term babies (nine RCTs); siblings (two RCTs) and other family members (two RCTs), maternity care units (two RCTs). The role of health professionals was unclear in 65 (44.5%) RCTs. A large variety of intervention combinations was tested, with 52 (35.6%) RCTs testing more than 1 category of interventions, and 24 (16.4%) RCTs including all 5 categories.
Conclusion
There is a large and rising number of RCTs on FCC interventions in neonatal intensive care units, with specific research gaps. The large variety of FCC interventions, their high complexity, the need to tailor them to the local context and major gaps in implementation suggest that implementation research is the current priority.
Keywords: Neonatology, Caregivers
WHAT IS ALREADY KNOWN ON THIS TOPIC
The benefits of family-centred care (FCC) in neonatal intensive care units (NICUs) have been tested in several randomised controlled trials (RCTs). A previous Cochrane review focused on the broad topic of FCC in children, while other existing reviews either focused on specific approaches, or specific outcomes, or specific timing of an FCC intervention.
WHAT THIS STUDY ADDS
This review identified a large (n=146) and rising number of RCTs on FCC interventions in NICUs, with increasing complexity over time, testing a large variety of interventions and combinations of different interventions, more usually (80.8%) performed in 1 or 2 facilities only and in high-income countries.
HOW THIS STUDY MIGHT AFFECT RESEARCH, PRACTICE OR POLICY
Key recommendations for research include the need for more studies in low-income countries, in at-term babies and healthy newborns, in fathers, siblings and other family members. Health professional role should be reported more clearly in future research.
Given the large variety of possible FCC interventions and intervention combinations, the critical role of context factors, and the major gaps in FCC implementation in routine practice, researchers should work together with policy-makers and implementation research projects should be prioritised.
Introduction
Babies with special needs—such as those born preterm or small for gestational age or those with congenital anomalies or postnatal infections—are usually hospitalised for a medium-term to long-term period in neonatal intensive care units (NICUs). Globally, it is estimated that every year up to 30 million newborns require some level of inpatient care.1 Substantial human potential for lifelong health and well-being is lost through newborn mortality, disability and long-term diseases.2 Therefore, it is critical that newborns during their hospitalisation receive high-quality infant-centred care aimed at reducing external stressors and at favouring newborn healthy development.
Parents and other family members play a crucial role in supporting the healthy neurological and emotional development of their babies, both during hospitalisation and after discharge. However, family members of hospitalised newborns are at high risk for long-term psychological distress, which can in turn have detrimental effects on the newborn developmental, social and cognitive growth.3 4 Consequently, effective interventions are needed to strengthen parental and caregiver coping strategies, support their psychological well-being, increase their nurturing capacity, knowledge and caregiving skills, and encourage mutual collaboration with hospital staff.5
Family-centred care (FCC) is an approach based on the concept of a mutually beneficial partnership among healthcare providers, patients and families. As such, it is deeply grounded on concepts of patient-centred participatory healthcare, promoted by many cultures and philosophies.5 6 FCC has been increasingly advocated since the late 1940s,5 6 and is supported by several institutions including the WHO, the Institute for Patient and Family-Centred Care, and the European Foundation for the Care of Newborn Infants.7,13 Its importance has been recognised by governments, such as the UK government14 and by scientific societies, such as the American Academy of Paediatrics.7 FCC is advocated based on a human and patient-rights perspective, and on increasing evidence showing that it can improve patient and family health outcomes, experience of care as well as healthcare professionals’ satisfaction and effective use of healthcare resources.7
However, FCC is a concept in continuous development, for which slightly different definitions have been provided by different authors/groups.5,711 15 Standards of FCC for newborns have been recently developed for the European Region,11 but may not be directly generalisable to all different settings and cultures.
Several previously systematic reviews reported that different groups of FCC interventions are safe and effective, with a variable level of evidence depending on the intervention and the outcome considered.516,24 A Cochrane review published in 2012 focused on the broad topic of FCC in children,5 and no other Cochrane reviews exist focusing on FCC in the newborn population.5 Other existing reviews either focused on specific approaches—such as parents’ engagement17 or effective communication18—or on specific populations—such as preterm or low birth weight infants1619,21—or on specific outcomes, such as parental satisfaction and mental health,22,24 or on specific timing of the intervention, such as the early neonatal period.19 Previous systematic reviews called for the need to list all possible interventions and outcomes types for FCC in newborns.16 19 21 However, none of the previous reviews summarised all published interventions studies related to FCC in the setting of newborn care, independently from the specific subpopulations involved, the timing of the intervention and the outcome assessed.
We conducted a set of two scoping reviews to comprehensively describe characteristics of intervention studies to implement FCC in NICUs. These two reviews had different and complementary aims: the present review aimed at describing the key characteristics of the identified randomised controlled trials (RCTs), including study design, setting, populations and type of interventions, as well as study characteristics over time and by country income groups. The second review aimed at providing a detailed description of the identified interventions, at synthesising outcomes, measurement methods and tools and at developing menus of options.25
A scoping review approach was chosen due to the broad nature of FCC, the number of expected RCTs and the need to first map interventions and outcomes before conceptualising any meta-analysis on effectiveness.26 This set of two scoping reviews may be of interest for both researchers, by providing a synthesis of the existing literature favouring the design of future studies, and for policymakers, by providing a synthesis relevant to the decision on implementation.
Methods
Study design
The two scoping reviews—including the present one—were conducted following the same methodology, using the Joanna Briggs Institute methodology26,28 and the Arksey’s framework for scoping reviews and subsequent updates.29 30 An unpublished protocol for the review was agreed before starting the screening of the studies, and it was further optimised after testing the inclusion and exclusion criteria. It was not registered in PROSPERO, since PROSPERO does not accept protocols of scoping reviews.
The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension for Scoping Reviews (PRISMA-ScR)31 was followed for reporting; the PRISMA-ScR checklist is annexed as online supplemental file 1.
Identifying the research question
The research question was developed including all domains of ‘the PCC’ (population, concept and context), as recommended by Peters et al.28 The populations involved consisted of infants admitted to NICUs of any level (as reported by the author), their families and staff involved in their care. The concept included all types of FCC interventions, as further defined in the following paragraph ‘study selection’. The context was research, specifically RCTs, conducted in NICU. Given the large number of expected studies, we opted to include only RCTs, representing studies with the lower risk of bias, and we did not include grey literature.
Identifying relevant articles
We searched for all relevant studies published up to 31 January 2022 in four electronic databases, that is, PubMed, Embase, Web of Science and the Cochrane Library, with no language restrictions. To develop the search strategy, we first tabulated and compared the search strategies used in previous existing reviews.15 16 19 22 As second step, the search strategy was further optimised, by adding missing terms emerging from a first set of retrieved studies, and by improved use of Boolean operators. As third step, the search strategy was tested, to assess whether it retrieved all relevant studies including those resulting from previous reviews. For each database, the full final search strategy is shown in online supplemental file 2. In addition, we hand-searched reference lists of included studies. All records were imported into Mendeley software and duplicates removed.
Study selection
For this scoping review, we included RCTs and long-term extension studies of RCTs, reporting on interventions which took place or initiated in the hospital setting, in a NICU (any level), when the intervention was pertinent to FCC. Specifically, we used the five categories of interventions defined in the most recent Cochrane review on paediatric FCC,5 slightly adapted by developing more precise definitions relevant to newborns, as further detailed next.
Environmental interventions: defined as any intervention including a change in the physical structure or in their use, specifically aiming at providing an environment that maximised parental involvement and enhanced newborn recovery and/or convalescence, such as family rooms or privacy areas.
Family-centred policies: defined as any intervention including an explicit change in written policies, specifically aiming at supporting FCC, such as change in visiting hours for siblings or extended family members, or hospital guidelines/procedures to increase parental participation in newborn care (eg, baby feeding or bathing).
Communication interventions: defined as any intervention aiming at improving communication between parents and staff, for example, parental presence and participation at daily interdisciplinary ward rounds, shared medical records and local hospital-based interpreters.
Educational interventions: defined as any intervention including training of parents and/or staff specifically aiming at building knowledge and/or skills to provide FCC—such as structured educational sessions for parents, education programmes for staff to provide FCC—in any format including video sessions.
Family support interventions: defined as any intervention aiming at providing tangible support to families of newborns, such as social support, economic support (eg, flexible charging schemes for poor families), psychological support, peer-to-peer parent support.
Both studies testing single intervention or a combination of interventions were included.
Studies exploring the following specific interventions already evaluated in other systematic reviews were excluded:
breast feeding and/or skin-to-skin and/or kangaroo mother care (KMC) as single interventions, for which there is already strong evidence on their benefits32,34;
parental presence during healthcare procedures as single intervention (eg, management of procedural pain during routine examinations), summarised in other reviews35 36;
maternal voice, maternal singing, mother’s lullaby, musical therapy, sound reduction alone, or ‘Sleep Programs’ as single interventions, evaluated in previous reviews37,41;
maternal massage, either alone or in combination with KMC, covered by other reviews42,46;
purely mental health interventions, including screening or prevention of maternal depression, when administered alone, or without other specific intervention related to FCC, since already evaluated elsewhere2347,51;
Additionally, we excluded studies exploring the following interventions:
-
6 a
praying or religious support of families/mothers;
-
6 b
physical therapy interventions such as Yakson touch, M-technique methods and others kinaesthetic stimulation techniques, or physical therapy interventions specifically focusing on a specific function alone, for example, head control;
-
6 c
studies aiming at reducing parental bereavement alone;
-
6 d
studies reporting on laboratory parameters (eg, cortisol) as sole outcomes;
-
6 e
studies where data of newborns in the NICU could not be separated from data of other populations, such as the general population of children in intensive care unit.
To minimise risk of selection bias, two reviewers organised in couples (IM and ML, JB and DS, CLJV and CT, SP and MG) independently screened all study titles and abstracts using the online Abstrackr tool.52 Discrepancies were resolved through group discussion with all authors. The full-text articles of all relevant abstracts were assessed by IM, JB and CLJV to determine eligibility; any disagreement was resolved by consensus or with a third expert reviewer (ML).
Data extraction
The following data were extracted from included studies: authors, year of publication, type of RCT design, study setting (ie, country, World Bank country classification by income level,53 type and number of facilities involved), population involved in the study and samples, for example, number of newborns and parents, gestational age at birth and newborn birth weight (when available), intervention’s categories as specified earlier. A data extraction form was developed through an iterative process from a previous review,17 prepiloted on a total of 20 studies and further optimised until considered satisfactory. Data extraction was performed by two authors in parallel, organised in three couples (IM/MG and SP, JB and DS, CLJV and CT). To ensure alignment in data extraction and tabulation across couples of authors, regular discussion sessions were held. Disagreements were resolved by either consensus or through further discussion with a third senior author (ML).
This being a scoping review, it did not aim at assessing risk of bias and effectiveness of different interventions.
Data synthesis
We summarised studies’ characteristics in tables and graphs. Data were reported as absolute numbers and percentages.
Characteristics and categories of interventions were also investigated by two subgroup analyses: (1) by publication time period, comparing the most recent years to the previous (RCTs) published up to 2016 versus after 2016 (from 2017 onwards); (2) by income level, comparing RCTs published in high-income countries (HICs) versus middle-income countries (MICs). MICs included both upper-middle and lower-middle income countries as per the World Bank categorisation.53 No comparison was performed with low-income economies because no study was retrieved in that setting. To test differences, a χ2 test was performed. All tests were two tailed and a p value of <0.05 was considered statistically significant.
Two-tailed tests were performed and a p value <0.05 was considered as statistically significant. Statistical analyses were performed using Stata/SE V.14.0 (Stata Corporation) and R V.4.1.1 (R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/).
Results
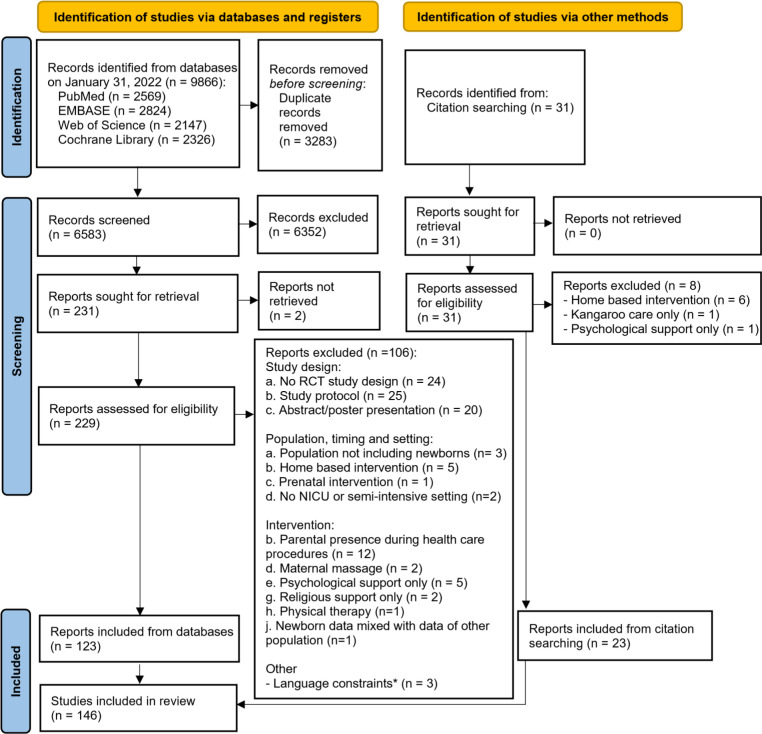
The searches yielded 9866 records (PubMed 2569, Embase 2824, Web of Science 2147, Cochrane Library 2326) and a total of 6583 records were identified for screening, after excluding duplicates. Additional 31 records were identified by hand searching of reference lists. After abstracts’ review, 260 full-text articles were assessed for eligibility. A total of 146 RCTs studies were included in the scoping review, 23 of which from citation searching (figure 1). All studies and their key characteristics are listed in online supplemental files 3 and 4.
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram. Notes: PRISMA flow diagram according to Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. doi: 10.1136/bmj.n71. RCT, randomised controlled trial.
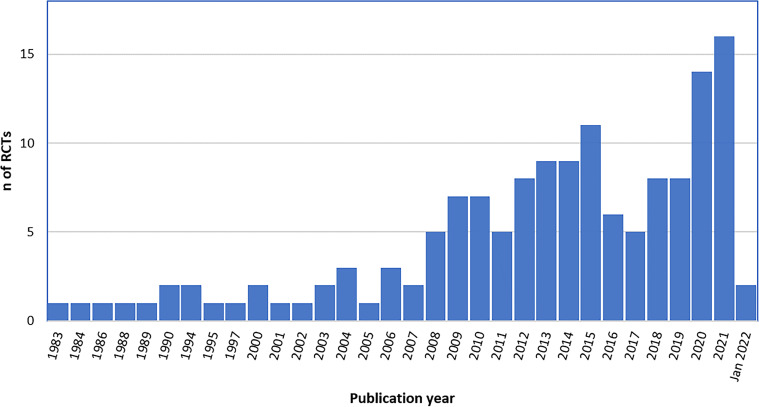
Publication year
Included RCTs were published between 1983 and January 2022 (figure 2), with an increasing trend in number of publications from 2008, and most recent years accounting for the highest number of studies. Specifically, 120 (82.2%) RCTs were published after 2008, and 32 (21.9%) in 2020–2022.
Figure 2. Number of published RCTs on FCC by year (n=146). RCT, randomised controlled trial; FCC, family-centred care.
Studies design
All but one study were parallel RCTs, the remaining was a crossover RCT (table 1). Among the 146 RCTs, 9 (6.2%) were cluster RCTs, with a significant increase in the number of cluster RCTs after 2016 (p=0.001).
Table 1. Design, characteristics and setting of the included RCTs.
| Overalln=146 | MICn=28 | HICn=118 | RCTsup to 2016n=93 | RCTs after 2016n=53 | |||
| n (%) | n (%) | n (%) | P value | n (%) | n (%) | P value | |
| RCT design | |||||||
| Parallel RCTs | 136 (93.2) | 25 (89.3) | 111 (94.1) | 0.405 | 91 (97.8) | 45 (84.9) | 0.005 |
| Parallel cluster RCTs | 9 (6.2) | 3 (10.7) | 6 (5.1) | 0.374 | 1 (1.1) | 8 (15.1) | 0.001 |
| Crossover RCTs | 1 (0.7) | 0 (0) | 1 (0.8) | >0.999 | 1 (1.1) | 0 (0) | >0.99 |
| Other characteristics of RCTs | |||||||
| Long-term extension RCTs | 27 (18.5) | 0 (0) | 27 (22.9) | 0.002 | 21 (22.6) | 6 (11.3) | 0.092 |
| Self-identified as ‘pilot’ RCTs | 9 (6.2) | 1 (3.6) | 8 (6.8) | >0.999 | 5 (5.4) | 4 (7.5) | 0.724 |
| Secondary publication of RCTs | 7 (4.8) | 0 (0) | 7 (5.9) | >0.999 | 4 (4.3) | 3 (7.5) | 0.705 |
| Interventions both in hospital and after discharge | 61 (41.8) | 7 (25.0) | 54 (45.8) | 0.074 | 47 (50.5) | 14 (26.4) | 0.008 |
| Setting | |||||||
| Type of ward | |||||||
| Only NICU | 144 (98.6) | 27 (96.4) | 117 (99.1) | 0.977 | 93 (100) | 51 (96.2) | 0.876 |
| NICU and maternity ward | 2 (1.4) | 1 (3.6) | 1 (0.8) | 0.348 | 0 (0.0) | 2 (3.8) | 0.130 |
| Number of NICUs involved | |||||||
| 1 | 91 (62.3) | 19 (67.9) | 72 (61) | 0.502 | 57 (61.3) | 34 (64.1) | 0.732 |
| 2 | 26 (17.8) | 6 (21.4) | 20 (16.9) | 0.578 | 19 (20.4) | 7 (13.2) | 0.248 |
| ≥3 | 21 (14.4) | 2 (7.1) | 19 (16.1) | 0.368 | 12 (12.9) | 9 (17.0) | 0.500 |
| Not specified | 8 (5.5) | 1 (3.6) | 7 (5.9) | >0.999 | 5 (5.4) | 3 (5.7) | >0.99 |
| World Bank country classification by income level | |||||||
| High-income country | 118 (80.8) | 0 (0) | 118 (100) | – | 87 (93.5) | 31 (58.5) | <0.001 |
| Upper middle-income country | 9 (6.2) | 19 (67.9) | 0 (0) | – | 1 (1.1) | 8 (15.1) | 0.001 |
| Lower middle-income country | 19 (13) | 9 (32.1) | 0 (0) | – | 5 (5.4) | 14 (26.4) | 0.001 |
| Low-income country | 0 (0) | 0 (0) | 0 (0) | – | 0 (0) | 0 (0) | – |
| Multicountry RCTs | 2 (1.4) | 0 (0) | 2 (1.7) | >0.999 | 0 (0) | 2 (3.8) | 0.130 |
p-value < 0.05 in bold.
HIC, high income countries; MIC, upper-middle income countries; NICU, neonatal intensive care unit; RCTs, randomised controlled trials.
Most RCTs (80.8%) were performed in one or two facilities only, with only two studies being multicountry (both conducted in Canada, Australia, New Zealand).
A total of 61 (41.8%) RCTs tested FCC interventions which extended after hospital discharge.
Studies setting
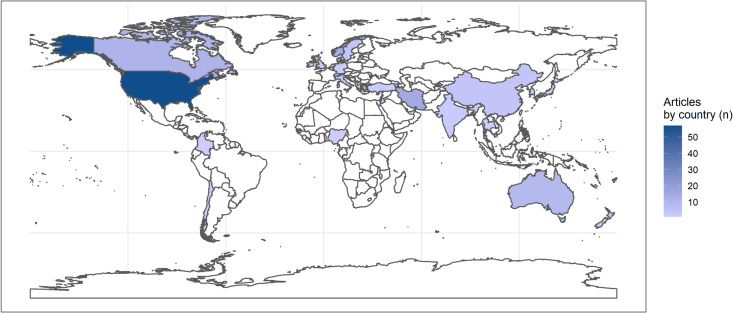
A total of 118 (80.8%) studies were conducted in HICs (table 1, figure 3) with countries with the higher numbers of studies being: USA (n=57, 39.0%), Norway (n=14, 9.6%), Australia (n=9, 6.2%), Canada (n=11, 7.6%) and the Netherlands (n=8, 5.5%).
Figure 3. Countries where RCTs on FCC were conducted (n=146). FCC, family-centred care; RCTs, randomised controlled trials.
A total of 26 (17.8%) studies were conducted in MICs. Among these, 9 (6.2%) were held in upper-middle income countries, with the most frequent being China (n=4, 2.7%), and 19 (13.0%) were performed in lower-middle income countries, mostly in Iran (n=15, 10.3%). No study was identified from low-income country.
The number of studies conducted in MICs significantly increased after 2016 (p=0.001), though no long-term extension study were identified in MICs. A postdischarge extension of the intervention was more common in RCTs published up to 2016 rather than after 2016 (50.5 % vs 26.4%, p=0.008).
Most RCTs included NICU only (n=144, 98.6%) while two studies (1.4%) included both a NICU and a maternity ward (table 1). No study made explicit that a neonatal ward was included, in addition to the intensive care unit.
Studies populations and sample size
The majority of RCTs included ≤300 caregivers (n=106, 72.6%) (table 2) and ≤300 newborns (n=119, 81.5%) (table 3) and, although the number of studies including >300 caregivers and >300 newborns significantly increased after 2016 (p=0.009 and p=0.027, respectively).
Table 2. Characteristics of randomised parents/caregivers in the included RCTs.
| Overalln=146 | MICn=28 | HICn=118 | RCTsup to 2016n=93 | RCTs after 2016n=53 | |||
| n (%) | n (%) | n (%) | P value | n (%) | n (%) | P value | |
| Characteristics of randomised parents/caregivers | |||||||
| Number of randomised parents/caregivers | |||||||
| ≤50 | 27 (18.5) | 9 (32.1) | 18 (15.3) | 0.039 | 17 (18.3) | 10 (18.9) | 0.930 |
| 51–100 | 31 (21.2) | 11 (39.3) | 20 (16.9) | 0.009 | 16 (17.2) | 15 (28.3) | 0.115 |
| 101–200 | 38 (26) | 6 (21.4) | 32 (27.1) | 0.537 | 26 (28) | 12 (22.6) | 0.482 |
| 201–300 | 10 (6.8) | 0 (0) | 10 (8.5) | 0.209 | 10 (10.8) | 0 (0) | 0.014 |
| >300 | 7 (4.8) | 1 (3.6) | 6 (5.1) | >0.999 | 1 (1.1) | 6 (11.3) | 0.009 |
| Not specified | 33 (22.6) | 1 (3.6) | 32 (27.1) | 0.005 | 23 (24.7) | 10 (18.9) | 0.415 |
| Type of parents/caregivers | |||||||
| Mothers only | 74 (50.7) | 23 (82.1) | 51 (43.2) | <0.001 | 41 (44.1) | 33 (62.3) | 0.035 |
| Both mothers and fathers | 37 (25.3) | 4 (14.3) | 33 (28) | 0.155 | 27 (29) | 10 (18.9) | 0.175 |
| Mothers and fathers and other caregivers (grandparents, relatives other) | 2 (1.4) | 1 (3.6) | 1 (0.8) | 0.348 | 1 (1.1) | 1 (1.9) | >0.99 |
| Siblings only | 2 (1.4) | 0 (0) | 2 (1.7) | >0.999 | 2 (2.2) | 0 (0.0) | 0.534 |
| Fathers only | 1 (0.7) | 0 (0) | 1 (0.8) | >0.999 | 1 (1.1) | 0 (0.0) | >0.99 |
| Not specified | 30 (20.5) | 0 (0) | 30 (25.4) | <0.001 | 21 (22.6) | 9 (17.0) | 0.421 |
p-value < 0.05 in bold.
HIC, high-income countries; MIC, upper-middle income countries; RCTs, randomised controlled trials
Table 3. Characteristics of randomised newborns in the included RCTs and information on health professionals’ involvement.
| Overalln=146 | MICn=28 | HICn=118 | RCTsup to 2016n=93 | RCTs after 2016n=53 | |||
| n (%) | n (%) | n (%) | Pvalue | n (%) | n (%) | Pvalue | |
| Characteristics of randomised newborns | |||||||
| Number of randomised newborns | |||||||
| ≤50 | 34 (23.3) | 726 | 27 (22.9) | 0.812 | 23 (24.7) | 11 (20.8) | 0.585 |
| 51–100 | 28 (19.2) | 7 (25) | 21 (17.8) | 0.384 | 15 (16.1) | 13 (24.5) | 0.215 |
| 101–200 | 45 (30.8) | 4 (14.3) | 41 (34.7) | 0.041 | 33 (35.5) | 12 (22.6) | 0.106 |
| 201–300 | 12 (8.2) | 1 (3.6) | 11 (9.3) | 0.463 | 10 (10.8) | 2 (3.8) | 0.140 |
| >300 | 8 (5.5) | 1 (3.6) | 7 (5.9) | >0.999 | 2 (2.2) | 6 (11.3) | 0.027 |
| Not specified | 19 (13) | 8 (28.6) | 11 (9.3) | 0.012 | 10 (10.8) | 9 (17.0) | 0.282 |
| Classification by gestational age* | n=127 | n=25 | n=102 | n=77 | n=50 | ||
| Term (≥37 weeks) | 9 (7.3) | 3 (12.0) | 94.1) | 0.378 | 6 (7.8) | 3 (6.4) | 0.535 |
| Late preterm (34–36+6 weeks) | 53 (41.7) | 16 (60.0) | 37 (36.3) | 0.012 | 35 (45.5) | 18 (36.0) | 0.291 |
| Moderate preterm (32–33+6 weeks) | 87 (68.5) | 22 (88.0) | 65 (63.8) | 0.029 | 53 (86.8) | 34 (68.0) | 0.922 |
| Very preterm (28–31+6 weeks) | 118 (92.9) | 23 (92.0) | 95 (93.1) | >0.999 | 72 (93.5) | 46 (92.0) | 0.747 |
| Extremely preterm (<28 weeks) | 95 (74.8) | 12 (48.0) | 83 (81.4) | 0.001 | 61 (79.2) | 34 (68.0) | 0.155 |
| Classification by birth weight* | n=49 | n=9 | n=40 | n=38 | n=11 | ||
| LBW (1500–2500 g) | 25 (51.0) | 7 (77.8) | 18 (45.0) | 0.138 | 18 (47.4) | 7 (63.6) | 0.342 |
| VLBW (1000–1499 g) | 40 (81.6) | 6 (66.7) | 34 (85.0) | 0.336 | 32 (84.2) | 8 (72.7) | 0.386 |
| ELBW (<1000 g) | 40 (81.6) | 5 (55.6) | 35 (87.5) | 0.046 | 33 (86.8) | 7 (63.6) | 0.179 |
| RCTs involving health professionals | |||||||
| Involved in the intervention delivery† | 137 (93.8) | 26 (92.9) | 111 (94.1) | 0.683 | 87 (93.5) | 50 (94.3) | >0.999 |
| Involved as receiver of an educational component‡ | 81 (55.5) | 6 (21.4) | 75 (63.6) | <0.001 | 56 (60.2) | 25 (47.2) | 0.176 |
In most RCTs the involvement of health professionals was not detailed clearly distinguishing between those involved in the delivery of a FCC intervention and those receiving it.
p-value < 0.05 in bold.
Only a subset of studies provided this information, specifically, gestational age was available for 127 studies and birth weight for 49 studies; each RCT could include more than one category of gestational age/birth weight category; classifications by gestational age and by birth weight are taken from UpToDate, available at https://www.uptodate.com/contents/image?imageKey=PEDS%2F119362 (accessed on 10 May 2023).
Here we included those RCTs where an ‘involvement’ of any health worker in the intervention delivery was explicitly stated.
Here we included RCTs where an “involvement” of health worker as receivers of an educational interventions was explicitly stated.
ELBWextremely low birth weightHICshigh-income countriesLBWlow birth weightRCTsrandomised controlled trialsVLBWvery low birth weight
About 1 out of 5 RCTs (n=30, 20.5%) did not made explicit which caregiver (whether the mother, the father or both) was involved. Among those specifying it, mothers were the caregivers more frequently involved (n=113, 97.4%). Fathers were involved in 40 (34.5%) RCTs, mostly together with mothers, with only 1 RCT (0.9%) focusing on an intervention specific for fathers alone. Siblings and other family members were included in 2 RCTs (1.4%) each (table 2).
Information on newborn gestational age was provided in 127 RCTs (87%); among these, 118 (92.9%) included very preterm newborns (28–31+6 weeks), 95 (74.8%) extremely preterm (<28 weeks) and 87 (68.5%) moderate preterm (32–33+6 weeks), while only 9 (7.1%) were on term infants. Information on birth weight was available in 49 RCTs, and very low birth weight (1000–1499 g) and extremely low birth weight (<1000 g) were the most common categories involved (each n=40, 81.6%) (table 3).
A total of 137 (93.8%) RCTs clearly stated an involvement of health professionals, with 81 (55.5%) RCTs explicitly describing that health workers were the receiver of an educational FCC intervention. This frequency was significantly higher in HICs compared (n=75, 63.6%) compared with MICs (n=6, 21.4%, p<0.001).
In the remaining 65 (44.5%) RCTs, the involvement of health professionals was not further detailed, and it was unclear in which role the health professionals were involved, either in the delivery or as receiver of a component of the FCC intervention (such as education), or both (table 3).
Categories of intervention tested
When interventions were classified into the five categories of FCC, a large variety of interventions and intervention combinations was observed (figure 4 and online supplemental file 5).
Figure 4. Number of RCTs testing each category of FCC interventions (n=146). Notes: in addition to the RCTs shown in the figure, 2 RCTs (1.4%) tested environmental interventions as single interventions and 2 RCTs (1.4%) tested family-centred policies as single-category interventions. FCC, family-centred care; RCT, randomised controlled trial.
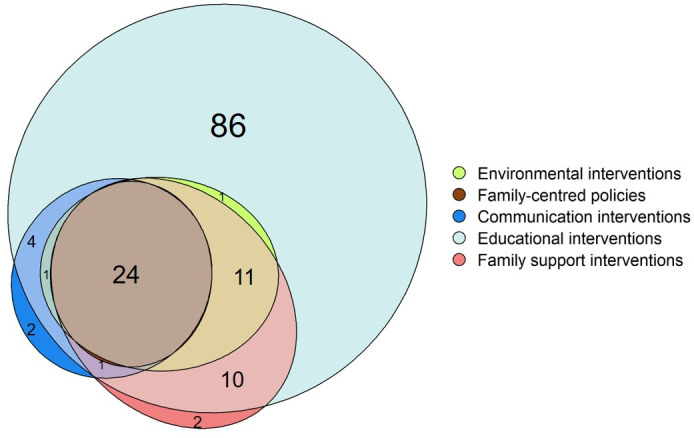
Overall, when considering both single-category or multiple-category FCC interventions, educational interventions were the most common intervention category (138 RCTs, 94.5%), followed by family support interventions (48 RCTs, 32.9%), environmental interventions (39 RCTs, 26.7%) and communication interventions (32 RCTs, 21.9%) and family-centred policies (26 RCTs, 17.8%).
A total of 52 RCTs (35.6%) tested more than 1 category of FCC interventions, with 24 (16.4%) including all 5 categories. All multiple-category interventions tested included an educational component. More than half of the included RCTs (n=86, 58.9%) tested educational interventions as single intervention (figure 4 and online supplemental file 5) while all the other categories were rarely tested alone (each one in 2 studies, 1.4%).
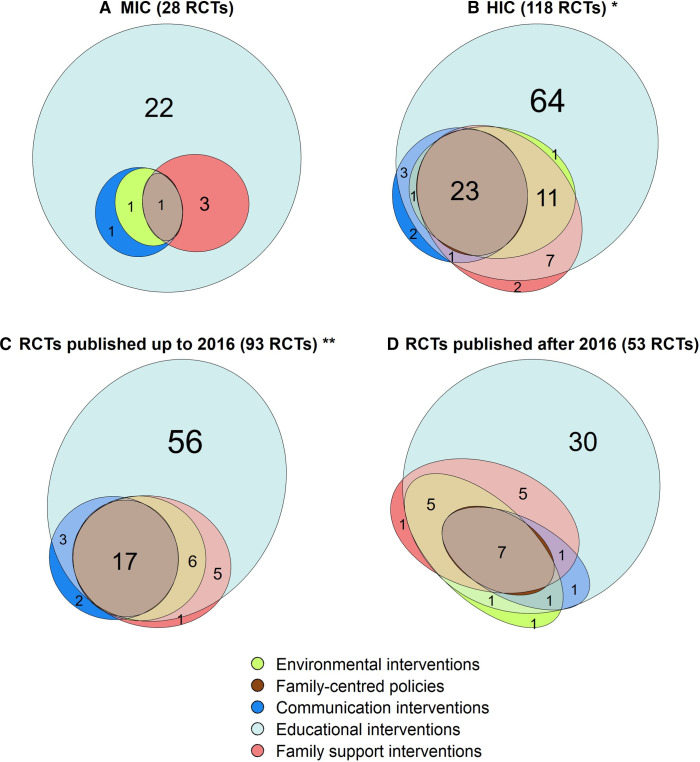
Subgroup analyses on categories of interventions tested
In the subgroup analysis (figure 5 and online supplemental file 5), family support interventions and environmental interventions were significantly more frequent in HICs compared with MICs (37.3% vs 14.3%, p=0.035; 31.4% vs 7.1%, p=0.018, respectively). A trend for more studies on family-centred policies intervention in HICs compared with MICs was also found (21.2% vs 3.6%, p=0.055).
Figure 5. Frequency of different categories of FCC interventions by subgroups (n=146). Notes: * in addition to the RCTs shown in the figure, two RCTs tested environmental interventions as single intervention and two RCTs tested family-centred policies as single component interventions; ** in addition to the RCTs shown in the figure, two RCTs tested family-centred policies as single intervention. Data are presented by income (A-B) and year of publication (C-D). FCC, family-centred care; HIC, high-income country; MIC, middle-income country; RCT, randomised clinical trial.
No significant difference in intervention categories was found by time period.
Discussion
This scoping review brings new evidence in comparison with previous existing reviews.56 16,23 32 It identified 146 RCTs representing an important body of evidence on interventions to promote FCC in the NICU setting. The increasing number of RCTs published in the last years indicates an increasing interest in FCC, and the increasing recognition of its importance in NICU settings.
In terms of recommendations for research, the most relevant gap in current research on FCC interventions appears to be the lack of RCTs in low-income countries, though several RCTs could be identified in MICs, with a significant increase in number in the last years. Second, included RCTs focused primarily on very preterm and very/extremely low birth weight infants even though infants with gestational age above 34 weeks and birth weight ≥2000 g represent the most common NICU population in many settings.54 55 Third, only about a quarter of studies included fathers, and extremely few included siblings or other family members. Existing evidence highlighted that fathers of newborns hospitalised in the NICU frequently suffer from mental distress56 57 and they may benefit from a more individualised approach and involvement in newborn care.56 Similarly, evidence suggests that siblings perceive the NICU hospitalisation of their brother or sister as stressful and therefore would benefit to be more actively involved in FCC interventions.58,60 Therefore, both populations should be more involved in FCC interventions. Finally, future research should better report the health professional role, both in delivering FCC interventions and as receiver/beneficiary on the intervention.
This review also clearly shows that there is a large variety of possible FCC interventions options in the NICU setting (multiple types of interventions, and intervention combinations into multiple populations). The most comprehensive intervention packages are highly complex, combining different components such as activities aimed at improving family support and communication among the family and the health professionals, interventions on the physical structures, change in policies. Besides, this review highlights a large variety of FCC-related trainings for families and staff.
Additionally, we found a very limited number of multicountry RCTs (n=2) studies, suggesting that it may be difficult to adopt and implement exactly the same intervention packages in different settings. There is a plausible need to tailor the FCC interventions to the local setting (eg, existing culture, needs, priorities, but also available resources), to be sustainable, resulting in difficulty in standardising the intervention across different settings. In particular, complex interventions have high dependency from multiple context factors (eg, local commitment) as well as delivery factors (how the intervention is actually delivered, and by whom), affecting reproducibility and interpretation of research results. Evidence shows that their success depends from multiple factors, and some of the key drivers of success—such as the institutional culture, good leadership, teamwork, competent and motivated staff stably available over time, peer pressure61—are very difficult to measure and report in an RCT format.
Moreover, FCC multifaceted interventions may require a long time to be effectively implemented, thus limiting measurability in the context of the typical time frame of an RCT, which is usually few years. They require multiple changes at multiple levels—structure, organisation of care, technical skills and attitudes, and multiple resources, including a lot of staff, for a long time, in already very busy clinical departments such as NICUs, and they also represent a major cultural shift.62 As such, FCC is difficult to operationalise and measure.
Notably, despite in theory, the principles of FCC are widely accepted,63 64 practical initiatives to operationalise them in the routine of NICU care continue to be only sporadically implemented.6 16 Evidence shows that parent involvement in the care of newborns is still limited, while gaps remain in many aspects of newborn routine care, such as physician–parent communications, even in HICs.65 66 High rates of psychological distress among NICU parents50 51 and lifelong medical and neurodevelopmental problems among many preterm and low birthweight infants66 further call for the urgent need to translate the FCC principles into actual policies, procedures and practice.
Therefore, our recommendation for the future is for policy-makers to work together with researchers and health professionals to implement FCC within medium-term to long-term projects, and make available good documentation that can help other groups to embed FCC in real practice. As recommended for implementation research, studies should report measures of acceptability, adoption, cost, penetration and sustainability,67 together with information on the context.
We acknowledge some limitations of this review. Given the large variety of interventions potentially ascribable to FCC, this review may have failed to capture some. However, a very broad search strategy was used, drawing on previous reviews,16,1922 and further tested and optimised. Multiple databases were searched without language barrier and hand-searching was also performed. Although the last search was conducted in 2022, we think it is improbable that the addition of most recent studies will change the key conclusions of this review.
Assessment of the risk of bias of included studies was not performed as not strictly recommended in scoping reviews.27 28
The assessment of the effectiveness of the FCC intervention was outside the scope of this review.
More details on the interventions identified, on the outcomes measured and on tools used for this purpose are provided in the other paired review, together with the resulting menus of intervention options and outcome methods.25
supplementary material
Footnotes
Funding: This study was supported by the Ministry of Health, Rome, Italy, in collaboration with the Institute for Maternal and Child Health IRCCS Burlo Garofolo, Trieste, Italy. The study was also supported by “Chiesi Foundation research grant 2019 in Neonatology”. As per the contract undersigned among parties, “The Grant does not constitute, directly or indirectly, a fee for services provided or to be provided in favour of the Foundation”.
Patient consent for publication: Not applicable.
Ethics approval: Not applicable.
Provenance and peer review: Not commissioned; externally peer reviewed.
Map disclaimer: The inclusion of any map (including the depiction of any boundaries therein), or of any geographic or locational reference, does not imply the expression of any opinion whatsoever on the part of BMJ concerning the legal status of any country, territory, jurisdiction or area or of its authorities. Any such expression remains solely that of the relevant source and is not endorsed by BMJ. Maps are provided without any warranty of any kind, either express or implied.
Data availability free text: All data are reported in the review.
Contributor Information
Marzia Lazzerini, Email: marzia.lazzerini@burlo.trieste.it.
Jenny Bua, Email: jennybua@gmail.com.
Cecilia Laure Juliette Vuillard, Email: cecilialaurejuliette.vuillard@burlo.trieste.it.
Domenica Squillaci, Email: domenica.squillaci@gmail.com.
Cristina Tumminelli, Email: cristina.tumminelli@gmail.com.
Silvia Panunzi, Email: silvia.panunzi@gmail.com.
Martina Girardelli, Email: martina.girardelli@burlo.trieste.it.
Ilaria Mariani, Email: ilaria.mariani@burlo.trieste.it.
References
- 1.World Health Organization Survive and thrive: transforming care for every small and sick newborn 2019. https://www.who.int/publications-detail-redirect/9789241515887 Available.
- 2.Lawn JE, Ohuma EO, Bradley E, et al. Small babies, big risks: global estimates of prevalence and mortality for vulnerable newborns to accelerate change and improve counting. Lancet. 2023;401:1707–19.:S0140-6736(23)00522-6. doi: 10.1016/S0140-6736(23)00522-6. [DOI] [PubMed] [Google Scholar]
- 3.Al Maghaireh DF, Abdullah KL, Chan CM, et al. Systematic review of qualitative studies exploring parental experiences in the neonatal intensive care unit. J Clin Nurs. 2016;25:2745–56. doi: 10.1111/jocn.13259. [DOI] [PubMed] [Google Scholar]
- 4.Provenzi L, Santoro E. The lived experience of fathers of Preterm infants in the neonatal intensive care unit: a systematic review of qualitative studies. J Clin Nurs. 2015;24:1784–94. doi: 10.1111/jocn.12828. [DOI] [PubMed] [Google Scholar]
- 5.Shields L, Zhou H, Pratt J, et al. Family‐Centred care for hospitalised children aged 0‐12 years. Cochrane Database of Systematic Reviews. 2012 doi: 10.1002/14651858.CD004811.pub3. http://dx.doi.org/10.1002/14651858.CD004811.pub3 Available. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gooding JS, Cooper LG, Blaine AI, et al. Family support and family-centered care in the neonatal intensive care unit: origins, advances, impact. Semin Perinatol. 2011;35:20–8. doi: 10.1053/j.semperi.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 7.Committee On Hospital Care and Institute For Patient-And Family-Centered Care. Patient- and family-centered care and the pediatrician’s role. Pediatrics. 2012;129(2):394–404. doi: 10.1542/peds.2011-3084. [DOI] [PubMed] [Google Scholar]
- 8.Brazelton TB, Nugent JK. Neonatal behavioral assessment scale. Cambridge University Press; 1995. [Google Scholar]
- 9.Harrison H. The principles for family-centered neonatal care. Pediatrics. 1993;92:643–50. [PubMed] [Google Scholar]
- 10.Shelton TL. Family-centered care for children with special health care needs. ERIC; 1987. https://eric.ed.gov/?id=ED288321 Available. [Google Scholar]
- 11.European Standards of Care for Newborn Health. https://newborn-health-standards.org/standards/standards-english/infant-family-centred-developmental-care/ Available.
- 12.Institute for Patient- and Family-Centered Care Frequently asked questions. http://www.ipfcc.org Available.
- 13.World Health Organization WHO recommendations for care of the preterm or low-birth-weight infant 2022. https://www.who.int/publications/i/item/9789240058262 Available. [PubMed]
- 14.Department of Health Getting the right start: National service framework for children, young people and maternity services: Standard for hospital services. https://assets.publishing.service.gov.uk/media/5a7b2f9640f0b66a2fc05bde/Getting_the_right_start_-_National_Service_Framework_for_Children_Standard_for_Hospital_Services.pdf Available.
- 15.Ramezani T, Hadian Shirazi Z, Sabet Sarvestani R, et al. Family-centered care in neonatal intensive care unit: a concept analysis. Int J Community Based Nurs Midwifery. 2014;2:268–78. [PMC free article] [PubMed] [Google Scholar]
- 16.Ding X, Zhu L, Zhang R, et al. Effects of family-centred care interventions on Preterm infants and parents in neonatal intensive care units: A systematic review and meta-analysis of randomised controlled trials. Aust Crit Care. 2019;32:63–75.:S1036-7314(18)30152-8. doi: 10.1016/j.aucc.2018.10.007. [DOI] [PubMed] [Google Scholar]
- 17.McAndrew NS, Jerofke-Owen T, Fortney CA, et al. Systematic review of family engagement interventions in neonatal, Paediatric, and adult Icus. Nurs Crit Care. 2022;27:296–325. doi: 10.1111/nicc.12564. [DOI] [PubMed] [Google Scholar]
- 18.Brett J, Staniszewska S, Newburn M, et al. A systematic mapping review of effective interventions for communicating with, supporting and providing information to parents of Preterm infants. BMJ Open. 2011;1:e000023. doi: 10.1136/bmjopen-2010-000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Benzies KM, Magill-Evans JE, Hayden KA, et al. Key components of early intervention programs for Preterm infants and their parents: a systematic review and meta-analysis. BMC Pregnancy Childbirth. 2013;13 Suppl 1(Suppl 1):Suppl. :S10. doi: 10.1186/1471-2393-13-S1-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu X, Zhang J. Family-centred care for hospitalized Preterm infants: A systematic review and meta-analysis. Int J Nurs Pract. 2019;25:e12705. doi: 10.1111/ijn.12705. [DOI] [PubMed] [Google Scholar]
- 21.Brecht C, Shaw RJ, Horwitz SM, et al. Effectiveness of therapeutic behavioral interventions for parents of low birth weight premature infants: A review. Infant Ment Health J. 2012;33:651–65. doi: 10.1002/imhj.21349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segers E, Ockhuijsen H, Baarendse P, et al. The impact of family centred care interventions in a neonatal or Paediatric intensive care unit on parents’ satisfaction and length of stay: A systematic review. Intensive Crit Care Nurs. 2019;50:63–70.:S0964-3397(18)30002-8. doi: 10.1016/j.iccn.2018.08.008. [DOI] [PubMed] [Google Scholar]
- 23.Sabnis A, Fojo S, Nayak SS, et al. Reducing parental trauma and stress in neonatal intensive care: systematic review and meta-analysis of hospital interventions. J Perinatol. 2019;39:375–86. doi: 10.1038/s41372-018-0310-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maleki M, Mardani A, Harding C, et al. Nurses’ strategies to provide emotional and practical support to the mothers of Preterm infants in the neonatal intensive care unit: A systematic review and meta-analysis. Womens Health (Lond Engl) 2022;18:174550572211046. doi: 10.1177/17455057221104674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mariani I, Vuillard CLJ, Bua J, et al. Family-centred care interventions in neonatal intensive care units: a scoping review of randomised controlled trials providing a menu of interventions, outcomes and measurement methods. Bmjpo. 2024;8:e002537. doi: 10.1136/bmjpo-2024-002537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The Joanna Briggs Institute The Joanna Briggs Institute reviewers’ manual 2015: methodology for JBI scoping reviews. 2015. https://reben.com.br/revista/wp-content/uploads/2020/10/Scoping.pdf Available.
- 27.Peters MDJ, Godfrey CM, Khalil H, et al. Guidance for conducting systematic Scoping reviews. Int J Evid Based Healthc. 2015;13:141–6. doi: 10.1097/XEB.0000000000000050. [DOI] [PubMed] [Google Scholar]
- 28.Peters MDJ, Marnie C, Tricco AC, et al. Updated methodological guidance for the conduct of Scoping reviews. JBI Evid Synth . 2020;18:2119–26. doi: 10.11124/JBIES-20-00167. [DOI] [PubMed] [Google Scholar]
- 29.Arksey H, O’Malley L. Scoping studies: towards a methodological framework. International Journal of Social Research Methodology. 2005;8:19–32. doi: 10.1080/1364557032000119616. [DOI] [Google Scholar]
- 30.Levac D, Colquhoun H, O’Brien KK. Scoping studies: advancing the methodology. Implement Sci. 2010;5:69. doi: 10.1186/1748-5908-5-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for Scoping reviews (PRISMA-SCR): checklist and explanation. Ann Intern Med. 2018;169:467–73. doi: 10.7326/M18-0850. [DOI] [PubMed] [Google Scholar]
- 32.Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev . 2014:CD002771. doi: 10.1002/14651858.CD002771.pub3. https://www.embase.com/search/results?subaction=viewrecord&id=L620551159&from=export Available. [DOI] [PubMed] [Google Scholar]
- 33.Moore ER, Bergman N, Anderson GC, et al. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2016;11:CD003519. :CD003519. doi: 10.1002/14651858.CD003519.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shah PS, Herbozo C, Aliwalas LL, et al. Breastfeeding or breast milk for procedural pain in neonates. Cochrane Database Syst Rev. 2012;12:CD004950. :CD004950. doi: 10.1002/14651858.CD004950.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hatfield LA, Murphy N, Karp K, et al. A systematic review of behavioral and environmental interventions for procedural pain management in Preterm infants. J Pediatr Nurs. 2019;44:22–30.:S0882-5963(18)30118-0. doi: 10.1016/j.pedn.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 36.Francisco ASPG, Montemezzo D, Ribeiro SNDS, et al. Positioning effects for procedural pain relief in NICU. Pain Manag Nurs . 2021;22:121–32.:S1524-9042(20)30167-3. doi: 10.1016/j.pmn.2020.07.006. [DOI] [PubMed] [Google Scholar]
- 37.Provenzi L, Broso S, Montirosso R. Do mothers sound good? A systematic review of the effects of maternal voice exposure on Preterm infants’ development. Neurosci Biobehav Rev. 2018;88:42–50.:S0149-7634(17)30199-9. doi: 10.1016/j.neubiorev.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Williamson S, McGrath JM. What are the effects of the maternal voice on Preterm infants in the NICU. Adv Neonatal Care. 2019;19:294–310. doi: 10.1097/ANC.0000000000000578. [DOI] [PubMed] [Google Scholar]
- 39.Costa VS, Bündchen DC, Sousa H, et al. Clinical benefits of music-based interventions on Preterm infants’ health: A systematic review of randomised trials. Acta Paediatr. 2022;111:478–89. doi: 10.1111/apa.16222. [DOI] [PubMed] [Google Scholar]
- 40.Almadhoob A, Ohlsson A. Sound reduction management in the neonatal intensive care unit for Preterm or very low birth weight infants. Cochrane Database Syst Rev. 2020;1:CD010333. doi: 10.1002/14651858.CD010333.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.van den Hoogen A, Teunis CJ, Shellhaas RA, et al. How to improve sleep in a neonatal intensive care unit: A systematic review. Early Hum Dev. 2017;113:78–86.:S0378-3782(17)30324-9. doi: 10.1016/j.earlhumdev.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Álvarez MJ, Fernández D, Gómez-Salgado J, et al. The effects of massage therapy in hospitalized Preterm neonates: A systematic review. Int J Nurs Stud. 2017;69:119–36.:S0020-7489(17)30043-3. doi: 10.1016/j.ijnurstu.2017.02.009. [DOI] [PubMed] [Google Scholar]
- 43.Mollà-Casanova S, Sempere-Rubio N, Muñoz-Gómez E, et al. Effects of massage therapy alone or together with passive Mobilisations on weight gain and length of Hospitalisation in Preterm infants: systematic review and meta-analysis. Early Hum Dev. 2023;182:105790.:S0378-3782(23)00086-5. doi: 10.1016/j.earlhumdev.2023.105790. [DOI] [PubMed] [Google Scholar]
- 44.Lu L-C, Lan S-H, Hsieh Y-P, et al. Massage therapy for weight gain in Preterm neonates: A systematic review and meta-analysis of randomized controlled trials. Complement Ther Clin Pract. 2020;39:101168.:S1744-3881(19)30257-9. doi: 10.1016/j.ctcp.2020.101168. [DOI] [PubMed] [Google Scholar]
- 45.Niemi A-K. Review of randomized controlled trials of massage in Preterm infants. Children (Basel) 2017;4:21. doi: 10.3390/children4040021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vickers A, Ohlsson A, Lacy JB, et al. Massage for promoting growth and development of Preterm and/or low birth-weight infants. Cochrane Database Syst Rev. 2004;2004:CD000390. doi: 10.1002/14651858.CD000390.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mendelson T, Cluxton-Keller F, Vullo GC, et al. NICU-based interventions to reduce maternal depressive and anxiety symptoms: a meta-analysis. Pediatrics. 2017;139:e20161870. doi: 10.1542/peds.2016-1870. [DOI] [PubMed] [Google Scholar]
- 48.Cherak SJ, Rosgen BK, Amarbayan M, et al. Mental health interventions to improve psychological outcomes in informal Caregivers of critically ill patients: A systematic review and meta-analysis. Crit Care Med. 2021;49:1414–26. doi: 10.1097/CCM.0000000000005011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caporali C, Pisoni C, Gasparini L, et al. A global perspective on parental stress in the neonatal intensive care unit: a meta-analytic study. J Perinatol. 2020;40:1739–52. doi: 10.1038/s41372-020-00798-6. [DOI] [PubMed] [Google Scholar]
- 50.Staver MA, Moore TA, Hanna KM. An integrative review of maternal distress during neonatal intensive care hospitalization. Arch Womens Ment Health. 2021;24:217–29. doi: 10.1007/s00737-020-01063-7. [DOI] [PubMed] [Google Scholar]
- 51.Roque ATF, Lasiuk GC, Radünz V, et al. Scoping review of the mental health of parents of infants in the NICU. J Obstet Gynecol Neonatal Nurs. 2017;46:576–87.:S0884-2175(17)30082-5. doi: 10.1016/j.jogn.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 52.Software for Semi-Automatic Citation Screening. 2012. http://abstrackr.cebm.brown.edu/account/login Available.
- 53.World Bank World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups Available.
- 54.Braun D, Braun E, Chiu V, et al. Trends in neonatal intensive care unit utilization in a large integrated health care system. JAMA Netw Open . 2020;3:e205239. doi: 10.1001/jamanetworkopen.2020.5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lazzerini M, Barcala Coutinho do Amaral Gomez D, Azzimonti G, et al. Parental stress, depression, anxiety and participation to care in neonatal intensive care units: results of a prospective study in Italy, Brazil and Tanzania. BMJ Paediatr Open. 2024;8:e002539. doi: 10.1136/bmjpo-2024-002539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Baldoni F, Ancora G, Latour JM. Being the Father of a Preterm-Born Child: Contemporary Research and Recommendations for NICU Staff. Front Pediatr. 2021;9:724992. doi: 10.3389/fped.2021.724992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bua J, Dalena P, Mariani I, et al. Parental stress, depression, anxiety and participation in care in neonatal intensive care unit: a cross-sectional study in Italy comparing mothers versus fathers. BMJ Paediatr Open. 2024;8:e002429. doi: 10.1136/bmjpo-2023-002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Greisen G, Mirante N, Haumont D, et al. Parents, siblings and grandparents in the neonatal intensive care unit. A survey of policies in eight European countries. Acta Paediatr. 2009;98:1744–50. doi: 10.1111/j.1651-2227.2009.01439.x. [DOI] [PubMed] [Google Scholar]
- 59.Aita M, Héon M, Savanh P, et al. Promoting family and siblings’ adaptation following a Preterm birth: A quality improvement project of a family-centered care nursing educational intervention. J Pediatr Nurs. 2021;58:21–7.:S0882-5963(20)30657-6. doi: 10.1016/j.pedn.2020.11.006. [DOI] [PubMed] [Google Scholar]
- 60.Savanh P, Aita M, Héon M. A review of siblings’ needs and interventions supporting their adaptation in the neonatal intensive care unit. Infants Young Child. 2020;33:332–51. doi: 10.1097/IYC.0000000000000178. [DOI] [Google Scholar]
- 61.Zanoni P, Scime NV, Benzies K, et al. Facilitators and barriers to implementation of Alberta family integrated care (Ficare) in level II neonatal intensive care units: a qualitative process evaluation Substudy of a Multicentre cluster-randomised controlled trial using the Consolidated framework for implementation research. BMJ Open. 2021;11:e054938. doi: 10.1136/bmjopen-2021-054938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuttini MFE, Zeitlin J. What drives change in neonatal intensive care units? results of the qualitative Epice study with neonatal physicians and nurses in six European countries. J Neonatal Perinatal Med. 2017;10:223–4. [Google Scholar]
- 63.Angelmar R, Berman PC. Patient empowerment and efficient health outcomes. Financ Sustain Healthc Eur New Approaches New Outcomes. 2007;1:3. [Google Scholar]
- 64.Organization WH. Framework on integrated people-centred health services. report by the Secretariat document number A67/39. 2016. https://iris.who.int/handle/10665/252698 Available.
- 65.Hagen IH, Iversen VC, Nesset E, et al. Parental satisfaction with neonatal intensive care units: a quantitative cross-sectional study. BMC Health Serv Res. 2019;19:37. doi: 10.1186/s12913-018-3854-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Health Resources and Services Administration National survey of children with special health care needs. Chartbook. [19-Jul-2020]. http://mchb.hrsa.gov/cshcn05/ Available. Accessed.
- 67.Wolfenden L, Foy R, Presseau J, et al. Designing and undertaking randomised implementation trials: guide for researchers. BMJ. 2021;372:m3721. doi: 10.1136/bmj.m3721. [DOI] [PMC free article] [PubMed] [Google Scholar]