Abstract
Purpose
This systematic review aimed to assess the feasibility, safety, and efficacy of using modern external beam radiotherapy (EBRT) techniques, such as intensity-modulated radiotherapy (IMRT), volumetric modulated arc therapy (VMAT), and stereotactic body radiotherapy (SBRT) as alternative approaches to brachytherapy (BRT) in adjuvant treatment of endometrial cancer (EC).
Material and methods
A systematic review was conducted following PRISMA guidelines. The research question was framed using the PICO method, focusing on patients with EC [P] and comparing modern EBRT techniques (IMRT, VMAT, SBRT) [I] vs. BRT [C], to evaluate their feasibility, safety, and effectiveness, particularly in terms of tumor local control (LC) [O]. Both planning and clinical outcomes, including acute toxicity, late side effects, and LC were analyzed with quality assessments performed using the GRADE framework and ROBINS-I tool.
Results
Planning studies revealed that while IMRT and VMAT provided comparable or improved target coverage and dose homogeneity compared with BRT, brachytherapy was associated with lower doses to critical organs. Post-operative SBRT and SIB-VMAT studies reported high LC rates (up to 100%) with minimal acute toxicity. However, the overall quality of evidence was low to very low, with significant risks of bias, mainly related to participant selection.
Conclusions
This review highlights that, although modern EBRT techniques, such as IMRT and VMAT are feasible alternative approaches to BRT for post-operative vaginal cuff irradiation, the current evidence does not support their superiority over BRT. Brachytherapy remains a highly effective treatment modality with well-established benefits. Future research should focus on more robust comparisons between EBRT and BRT, considering not only local control and toxicity, but also psychological impact and quality of life, especially in low-resource settings, where access to BRT may be limited.
Keywords: endometrial cancer, brachytherapy, external beam radiotherapy (EBRT), intensity-modulated radiotherapy (IMRT), volumetric modulated arc therapy (VMAT), stereotactic body radiotherapy (SBRT)
Purpose
Endometrial cancer (EC) is the most common gynecological malignancy, with incidence rates rising in many countries, especially in those undergoing rapid socio-economic changes [1]. Surgery remains the standard treatment for operable EC [2, 3]. Radiotherapy is frequently employed as post-operative adjuvant treatment, either with brachytherapy (BRT) in vaginal cuff (VC), external beam radiotherapy (EBRT) in the pelvis, or a combination of both. However, the access to post-operative BRT can be limited by various factors, such as high cost, lack of facilities, or insufficient expertise, especially in low-resource settings [4].
Given these challenges, EBRT techniques replacing BRT would be valuable in some centers or specific patient populations. Various studies have explored the potential of advanced EBRT techniques, such as intensity-modulated radiotherapy (IMRT), volumetric modulated arc therapy (VMAT), and stereotactic body radiotherapy (SBRT), to replicate BRT dose distributions in EC treatment. However, no randomized trials or systematic reviews comparing BRT and EBRT in this context are currently available.
Therefore, the current systematic review aimed to evaluate the existing evidence of planning and clinical studies on the use of EBRT techniques as alternative approaches to BRT in adjuvant treatment of EC.
Material and methods
This review was conducted by a multidisciplinary team involving radiation oncologists, gynecologic oncologists, statisticians, health physicists, and radiotherapy technologists. The rationale and concept of this manuscript were developed and discussed by the authors during a meeting in June 2022. The analysis followed the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines [5], and the research question was framed using the population, intervention, comparison, and outcomes (PICO) method as follows: “In patients with endometrial carcinoma [P], are modern radiotherapy techniques (IMRT, VMAT, SBRT) [I] compared with BRT [C] feasible, safe, and effective in terms of tumor local control (LC) [O]?”.
Endpoints
The review considered both dosimetric outcomes of planning studies (target coverage, dose conformity, dose to organs at risk [OARs]) and clinical outcomes (acute toxicity, late side effects, and LC).
Selection criteria
All English written papers on the use of advanced EBRT techniques (i.e., SBRT, IMRT, VMAT) as a replacement for BRT in adjuvant treatment of any stage of EC were included. Both planning and clinical studies were considered, with no time restriction. Literature reviews, editorials, guidelines, case reports, conference abstracts, phase I trials, and studies on exclusive therapy, palliative treatment, or local/pelvic relapses were excluded.
Literature search
Literature search was conducted in August 2022 using PubMed, Scopus, and the Cochrane Library databases. Three search strategies were employed (Supplementary Material 1). Two authors (MF and AMP) independently performed the initial screening of titles and abstracts, resolving disagreements through a discussion with a third party (GM or FD). Subsequently, the selected papers were fully reviewed by the same two authors, and any discrepancy was resolved in consultation with a senior author (AGM).
Supplementary Material
Data extraction
Two authors (MF and AMP) independently extracted data from the selected studies, including radiotherapy details (dose, fractionation, target definition, immobilization devices, and timing for boosts), planning results (target coverage, dose conformity, and dose to OARs), and clinical outcomes (acute and late toxicity, LC, disease-free survival [DFS], and overall survival [OS]). Data were tabulated in Excel sheet and compared. Discrepancies were resolved through a discussion with the senior author (AGM).
Statistical analysis
The impact of total dose on grade ≥ 2 acute toxicity was analyzed using linear regression, both with and without weighting by sample size. A sensitivity analysis was planned to exclude any outliers. Due to limited number of events, no further dose-effect analyses were conducted for grade ≥ 3 acute toxicity, late toxicity, and LC.
Quality assessment
The quality of evidence from clinical studies focusing on LC as the outcome was evaluated using a checklist for quality assessment tool - study limitations (risk of bias), based on the GRADE framework [6]. Evidence quality was rated as very low, low, moderate, or high based on the number of positive responses. Since no randomized trials on this subject have been published, the risk of bias in non-randomized studies of intervention (ROBINS-I tool) [7] was employed to assess the included clinical studies. This tool evaluates bias related to confounding factors, participant selection, intervention classification, deviations from intended interventions, missing data, outcome measurement, and result selection. Two authors (MB and LF) independently rated the studies, resolving disagreements through a discussion with the senior author (AGM). The results were graphically presented using the robvis tool [8].
Results
Search results
Out of 486 publications retrieved, 8 studies [9-16] met the selection criteria and were included in the analysis (Figure 1). The characteristics and outcomes of these studies are summarized in Tables 1 and 2. The detailed data on study design, patient demographics, tumor characteristics, treatment specifics, outcomes, and toxicity are provided in Supplementary Materials (Tables S1 and S2).
Fig. 1.
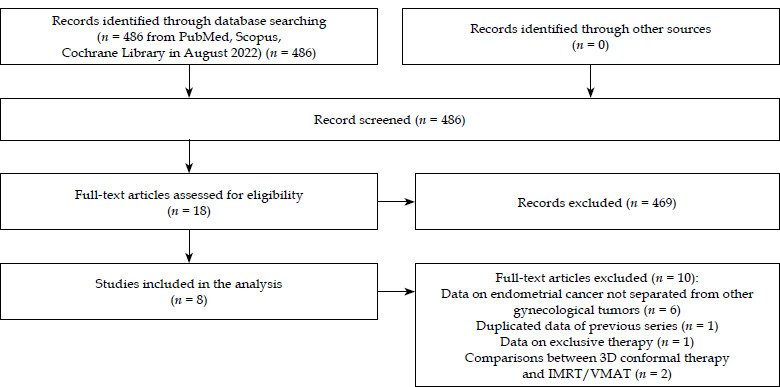
PRISMA flow chart
Table 1.
Planning studies comparing external beam radiotherapy and brachytherapy in endometrial cancer
| Authors, year [Ref.] | Methods | Main findings | Conclusions |
|---|---|---|---|
| Aydogan et al., 2006 [9] | Comparison between HDR-BRT and IMRT in post-operative VC irradiation in early EC using a BRT vaginal cylinder (10 patients) | IMRT vs. HDR-BRT:
|
IMRT may be a viable alternative to HDR-BRT in this setting |
| Grelewicz et al., 2018 [14] | Comparison between post-operative HDR-BRT and IMRT/VMAT VC boost using a BRT cylinder applicator after pelvic node irradiation in resected EC (4 patients) | IMRT/VMAT vs. HDR-BRT:
|
IMRT/VMAT boost provides acceptable OARs doses in this setting |
| Yildirim et al., 2019 [16] | Comparison between post-operative HDR-BRT and VMAT-/HT-based SBRT on VC using a BRT applicator in EC (12 patients) | VMAT-SBRT vs. HT-SBRT vs. HDR-BRT:
|
SBRT is feasible in this setting |
| Cilla et al., 2020 [15] | Comparison between PO-VMAT/FI-VMAT-based SBRT and HDR-BRT in post-operative VC irradiation using a BRT applicator in EC (8 patients) based on EUD calculation | PO-VMAT/FI-VMAT vs. HDR-BRT:
|
PO-VMAT can mimic HDR-BRT dose distribution |
3D-CRT – 3D-conformal radiotherapy, BRT – brachytherapy; Dxcc – dose to x cc of organ, Dmax – maximum dose, Dmean – mean dose, EC – endometrial cancer, EUD – equivalent uniform dose, FI-VMAT – full-inverse planning module, HDR – high-dose-rate, HT – helical tomotherapy, OAR – organ at risk, SIB – simultaneous integrated boost, PO-VMAT – anatomy-based optimization module, PTV – planning target volume, VC – vaginal cuff, VMAT – volumetric modulated arc therapy, Vx% – volume receiving x% of prescribed dose
Table 2.
Clinical studies on the use of external beam radiotherapy instead of brachytherapy in endometrial cancer
| Authors, year [Ref.] | Methods | Main findings | Conclusions |
|---|---|---|---|
| Demiral et al., 2013 [10] | Retrospective evaluation of 18 EC patients (FIGO I-III) treated with LINAC-based SBRT boost to vaginal vault (18 Gy in 3 fractions) after post-operative pelvic irradiation (45 Gy in 1.8 Gy/fraction) |
|
LINAC-based SBRT vaginal boost is feasible |
| Macchia et al., 2014 [12] | Retrospective comparison between SIB-VMAT and 3D-CRT-based concomitant boost in post-operative pelvic lymph nodes plus VC boost in high- intermediate risk EC (30 matched pairs) |
|
Reduced acute toxicity with SIB-VMAT justifies further trials in this setting |
| Alongi et al., 2015 [11] | Prospective evaluation of SIB-VMAT in post-operative pelvic lymph node irradiation (54 Gy in 30 fractions) plus VC boost (66 Gy in 30 fractions) in 50 EC patients (FIGO I-III) |
|
SIB-VMAT is feasible and well-tolerated in this setting |
| Macchia et al., 2016 [13] | Prospective phase I-II trial to determine recommended post-operative SIB-VMAT dose in 70 intermediate-/high-risk EC patients:
|
– Median follow-up: 25 months (range, 4-60)
|
SIB-VMAT is feasible and well-tolerated up to 60 Gy in 25 fractions |
3D-CRT – 3D-conformal radiotherapy, BRT – brachytherapy, DFS – disease-free survival, GTV – gross tumor volume, LC – local control, OS – overall survival, SIB – simultaneous integrated boost, VC – vaginal cuff, VMAT – volumetric modulated arc therapy, FFP – freedom from progression
Planning studies
Three studies compared post-operative treatment of VC using IMRT or VMAT vs. BRT [9, 15, 16]. One study showed similar target coverage with IMRT and BRT [9]. All three studies reported improved dose homogeneity within the target using IMRT or VMAT [9, 15, 16]. Additionally, two studies showed lower doses to the rectum [9] or to the rectum and bladder [15] with IMRT/VMAT, while another study found lower bladder (and femoral head) doses using BRT [16]. Another study compared IMRT or VMAT with HDR-BRT for VC boosting after post-operative pelvic lymph node irradiation, reporting better target coverage and dose homogeneity with EBRT, while increased irradiation of the rectum, sigmoid, bladder, and femoral heads [14].
Clinical studies
Four studies evaluated post-operative setting; one used a SBRT boost on VC after pelvic irradiation [10], and three assessed the outcomes of pelvic irradiation with a simultaneous integrated boost (SIB) to VC delivered with VMAT [11-13].
Local control
Of the four studies on post-operative radiotherapy, one did not report LC outcomes [12], while the others reported LC rates of 100% with a median follow-up of 2 years [10], 100% at 2 years [11], and 98.5% at 3 years [13].
Toxicity
In the four post-operative radiotherapy studies, grade ≥ 3 acute toxicity ranged from 0.0% to 2.8% (median, 0.0%) [11-13]. The incidence of late toxicity was 22.2% (crude rate) in a SBRT boost study [10], while in SIB-VMAT studies, it was 0.0%, with a median follow-up of 26 months [11], 7.2% (gastrointestinal) and 0.0% (genitourinary) at 3 years [13], and not reported in one study [12].
The impact of radiotherapy dose on toxicity
A linear regression analysis of grade ≥ 2 acute gastrointestinal and genitourinary toxicity incidence based on total SIB dose in three VMAT studies [11-13] in post-operative setting showed a positive correlation with increasing doses (Figure 2A, B).
Fig. 2.
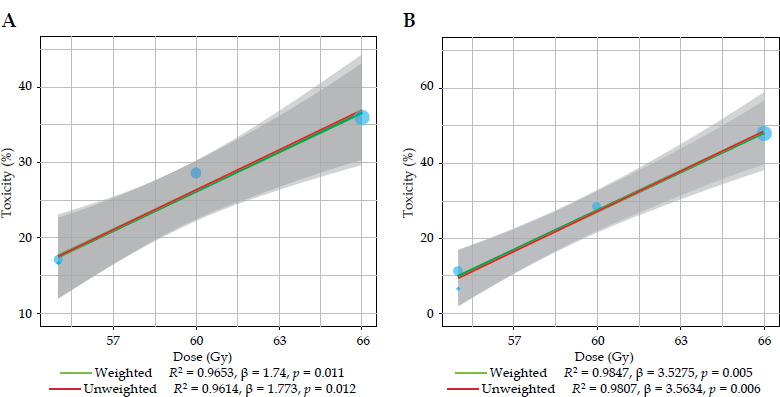
A) Linear regression on the incidence of gastrointestinal acute grade ≥ 2 toxicity. B) Linear regression on the incidence of genitourinary acute grade ≥ 2 toxicity
Quality assessment of the analyzed studies
The quality of evidence in clinical studies, as assessed by the GRADE framework and focusing on LC, was rated as very low in one study [10] and low in two studies [11, 13] (Table S2). All clinical studies included in this review demonstrated a critical (1 report) [10] or moderate (two reports) [11, 13] risk of bias. The most significant source of bias was participant selection. Traffic-light plot and summary plot based on the ROBINS-I tool are presented in Figures 3 and 4, respectively.
Fig. 3.
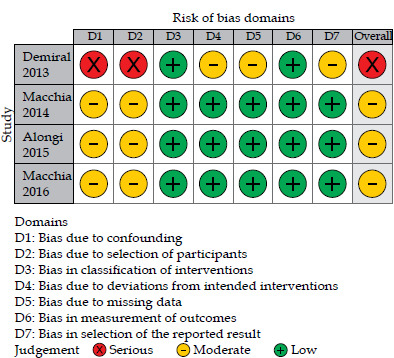
ROBINS-I traffic-light plot showing risk of bias in non-randomized studies of interventions
Fig. 4.
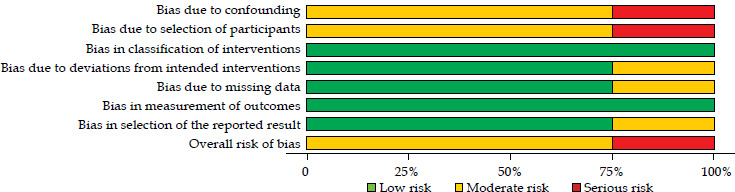
ROBINS-I summary plot showing risk of bias in non-randomized studies of interventions
Discussion
Assumptions
The current review explored the potential of replacing BRT with EBRT in the adjuvant treatment of EC patients. Interest in this topic has developed from certain disadvantages associated with BRT, especially in patients with cervical or endometrial cancer. BRT can be challenging due to patient discomfort, claustrophobia and, in some cases, outright refusal of the treatment. Additionally, in patients receiving pelvic EBRT followed by a BRT boost to VC, the overall treatment duration is significantly extended compared with SIB-based EBRT. In low-resource settings, the availability of equipment and expertise may be limited, and the cost of periodically replacing radioactive sources can be too expensive. Also, unfavorable patient anatomy can sometimes render BRT infeasible. Furthermore, the inherently inhomogeneous dose distribution of BRT can result in a much higher dose to the mucosal surface compared with deeper layers of the vaginal wall.
Conversely, BRT offers the advantage of being unaffected by organ motion, eliminating the need for margin addition to clinical target volume in planning. Clinically, BRT has shown excellent results, particularly in post-operative radiotherapy [17]. Dose inhomogeneity of BRT can even be advantageous, especially for VC irradiation. For example, a pathological study showed that 95% of vaginal lymphatic vessels lie within three millimeters of the epithelial surface [18]. Therefore, concentrating the dose on superficial layers might be beneficial. One of the analyzed planning studies aimed to identify the most appropriate EBRT technique to replicate BRT dose distribution [15].
Given these factors, a thorough analysis of the evidence supporting the use of EBRT instead of BRT is essential, as conducted in the current review of EC planning and clinical studies.
Limitations
This analysis has several limitations: 1. A small number of reports were analyzed, with only four clinical studies; 2. The studies varied in design (planning or clinical); 3. The results were reported heterogeneously, limiting the possibility of quantitative analysis, except for the impact of dose on acute toxicity in patients receiving SIB-VMAT [11-13]; 4. The quality of evidence in the clinical studies, particularly concerning LC, was very low in one study [10] and low in two [11, 13], with no clinical trial showing a low-risk of bias. Despite these limitations, certain insights can be drawn from our analysis.
Irradiation of the vaginal vault only
Clinical evidence of post-operative VC irradiation alone is lacking. However, three planning studies demonstrated that IMRT or VMAT produce a more homogeneous dose distribution compared with BRT [9, 15, 16], reducing the dose to the rectum [9, 15] and bladder [15]; nevertheless, one study noted greater bladder irradiation [16]. Modulated techniques were associated with lower dose homogeneity in VC irradiation alone [19].
Prophylactic nodal irradiation and boost on the vaginal vault
In cases of pelvic irradiation with a VC boost, one planning study using an IMRT/VMAT boost on VC after pelvic irradiation reported better target coverage and dose homogeneity, but also increased OARs’ irradiation compared with BRT [14]. A clinical study on SBRT boost to VC after EBRT reported a 0% grade ≥ 3 acute toxicity rate and 100% LC rate at a median follow-up of 24 months, but also a notable 22.2% crude rate of grade ≥ 2 late toxicity [10]. Therefore, using a sequential EBRT boost may not be as safe as BRT boost in this setting.
In contrast, three clinical trials using SIB-VMAT concurrently with post-operative pelvic irradiation reported LC rates of 98.5-100% [11-13], grade ≥ 3 acute toxicity rates of 0.0-2.8% [11-13], an overall late toxicity rate of 0% [11], and 0% late genitourinary toxicity and 7.2% late gastrointestinal toxicity [13]. These findings suggest that SIB offers advantages in patients undergoing pelvic irradiation with a VC boost compared with a sequential boost. This benefit likely stems from dosimetric advantages of SIB combined with radiobiological benefits of a reduced overall treatment time, as reflected in high LC rates observed in these trials.
Conclusions
Based on the above outlined considerations, we conclude that:
Post-operative EBRT of VC is dosimetrically feasible, particularly with IMRT/VMAT techniques [9, 15, 16], but dosimetric impact on the bladder remains unclear [15, 16].
No clinical data are available on adjuvant EBRT of VC alone.
In post-operative prophylactic lymph node irradiation combined with a VC boost, using EBRT for the boost appears effective and safe only with SIB techniques. Based on our dose/effect analysis, a recommended SIB dose is 55-60 Gy in 25 fractions or a radiobiologically equivalent regimen.
Finally, our findings align with previous similar literature review on cervical cancers [20]. Campitelli et al. found that while planning studies on EBRT boost in locally advanced cervical cancers yielded contradictory results, clinical evidence was very limited [20].
In conclusion, our review indicates no evidence for the superiority of EBRT-based techniques over BRT in EC patients. Therefore, like in other tumors, BRT is likely to remain a valuable treatment modality [21]. However, in the context of VC irradiation (alone or as a boost following prophylactic nodal irradiation), the present analysis suggests that further studies are warranted. Indeed, in patients undergoing pelvic irradiation with a boost to VC, local control rates observed in our review (range, 98.5-100%) are comparable to those reported after BRT (98.5%) [22]. Moreover, the rate of late gastrointestinal toxicity (7.2%) in these patients is lower than that reported in patients receiving BRT following pelvic EBRT, with toxicity rate of 14.5% [22].
Future research can focus on 1. Comparing EBRT and BRT not only in terms of LC and toxicity, but also regarding psychological impact and quality of life; 2. Comparing different total doses and fractionation schemes in patients receiving EBRT, both for VC-only irradiation and VC boosts; 3. Assessing the feasibility and outcomes of EBRT instead of BRT in EC treatment in low-resource settings.
Footnotes
The authors declare no conflict of interest.
Supplementary material is available on the journal’s website.
Funding
This research received no external funding.
Disclosures
Approval of the Bioethics Committee was not required.
References
- 1.Lortet-Tieulent J, Ferlay J, Bray F, Jemal A. International patterns and trends in endometrial cancer incidence, 1978-2013. J Natl Cancer Inst 2018; 110: 354-361. [DOI] [PubMed] [Google Scholar]
- 2.Concin N, Matias-Guiu X, Vergote Iet al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int J Gynecol Cancer 2021; 31: 12-39. [DOI] [PubMed] [Google Scholar]
- 3.NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®), Uterine Neoplasms Version 1.2022 – November 4, 2021 . Available online: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf (accessed on 10th June 2022)
- 4.Chuang L, Rainville N, Byrne M, et al. Cervical cancer screening and treatment capacity: A survey of members of the African Organisation for Research and Training in Cancer (AORTIC). Gynecol Oncol Rep 2021; 38: 100874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009, 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meader N, King K, Llewellyn Aet al. A checklist designed to aid consistency and reproducibility of GRADE assessments: Development and pilot validation. Syst Rev 2014; 3: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sterne JA, Hernán MA, Reeves BCet al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016; 355: i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGuinness LA, Higgins JPT. Risk-of-bias VISualization (robvis): An R package and Shiny web app for visualizing risk-of-bias assessments. Res Synth Methods 2021; 12: 55-61. [DOI] [PubMed] [Google Scholar]
- 9.Aydogan B, Mundt AJ, Smith BDet al. A dosimetric analysis of intensity-modulated radiation therapy (IMRT) as an alternative to adjuvant high-dose-rate (HDR) brachytherapy in early endometrial cancer patients. Int J Radiat Oncol Biol Phys 2006; 65: 266-273. [DOI] [PubMed] [Google Scholar]
- 10.Demiral S, Beyzadeoglu M, Uysal Bet al. Evaluation of stereotactic body radiotherapy (SBRT) boost in the management of endometrial cancer. Neoplasma 2013; 60: 322-327. [DOI] [PubMed] [Google Scholar]
- 11.Alongi F, Mazzola R, Ricchetti Fet al. Volumetric-modulated arc therapy with vaginal cuff simultaneous integrated boost as an alternative to brachytherapy in adjuvant irradiation for endometrial cancer: a prospective study. Anticancer Res 2015; 35: 2149-2155. [PubMed] [Google Scholar]
- 12.Macchia G, Cilla S, Morganti AGet al. Adjuvant volumetric-modulated arc therapy with simultaneous integrated boost in endometrial cancer. Planning and toxicity comparison. Acta Oncol 2014; 53: 251-258. [DOI] [PubMed] [Google Scholar]
- 13.Macchia G, Cilla S, Deodato Fet al. Simultaneous integrated boost volumetric modulated arc therapy in the postoperative treatment of high-risk to intermediate-risk endometrial cancer: Results of ADA II phase 1-2 trial. Int J Radiat Oncol Biol Phys 2016; 96: 606-613. [DOI] [PubMed] [Google Scholar]
- 14.Grelewicz Z, Zerrusen B, Sathiaseelan V, Zhang H. A feasibility study of using advanced external beam techniques to create a vaginal cuff brachytherapy-like endometrial boost plan. Med Dosim 2018; 43: 30-38. [DOI] [PubMed] [Google Scholar]
- 15.Cilla S, Macchia G, Mattiucci Get al. Optimized stereotactic volumetric modulated arc therapy as an alternative to brachytherapy for vaginal cuff boost. A dosimetric study. Med Dosim 2020; 45: 352-358. [DOI] [PubMed] [Google Scholar]
- 16.Yildirim BA, Dolek Y, Guler OCet al. Dosimetric comparison of vaginal vault brachytherapy vs applicator-guided stereotactic body radiotherapy with volumetric modulated arc therapy and helical tomotherapy for endometrium cancer patients. Med Dosim 2019; 44: 332-338. [DOI] [PubMed] [Google Scholar]
- 17.Nout RA, Smit VT, Putter Het al.; PORTEC Study Group. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet 2010; 375: 816-823. [DOI] [PubMed] [Google Scholar]
- 18.Choo JJ, Scudiere J, Bitterman Pet al. Vaginal lymphatic channel location and its implication for intracavitary brachytherapy radiation treatment. Brachytherapy 2005; 4: 236-240. [DOI] [PubMed] [Google Scholar]
- 19.Cilla S, Macchia G, Digesù Cet al. 3D-Conformal versus intensity-modulated postoperative radiotherapy of vaginal vault: A dosimetric comparison. Med Dosim 2010; 35: 135-142. [DOI] [PubMed] [Google Scholar]
- 20.Campitelli M, Lazzari R, Piccolo Fet al. Brachytherapy or external beam radiotherapy as a boost in locally advanced cervical cancer: a Gynaecology Study Group in the Italian Association of Radiation and Clinical Oncology (AIRO) review. Int J Gynecol Cancer 2021; 31: 1278-1286. [DOI] [PubMed] [Google Scholar]
- 21.Sorbe BG, Horvath G, Andersson Het al. External pelvic and vaginal irradiation versus vaginal irradiation alone as postoperative therapy in medium-risk endometrial carcinoma: a prospective, randomized study–quality-of-life analysis. Int J Gynecol Cancer 2012; 22: 1281-1288. [DOI] [PubMed] [Google Scholar]
- 22.Major T, Fröhlich G, Ágoston Pet al. The value of brachytherapy in the age of advanced external beam radiotherapy: a review of the literature in terms of dosimetry. Strahlenther Onkol 2022; 198: 93-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


