Abstract
Polymicrogyria (PMG) is the most common malformation of cortical development (MCD) and presents as an irregularly patterned cortical surface with numerous small gyri and shallow sulci leading to various neurological deficits including developmental delays, intellectual disability, epilepsy, and language and motor issues. The presentation of PMG varies and is often found in conjunction with other congenital anomalies. Histologically, PMG features an abnormal cortical structure and dyslamination, resulting in its classification as a defect of neuronal migration and organization. Due in part to a variety of etiologies, little is known about the molecular mechanism(s) underlining PMG. To address this gap in knowledge, a case study is presented where an elderly individual with a medical history of unspecified PMG was examined postmortem by using a combination of anatomical, magnetic resonance imaging (MRI), histopathological, and genetic techniques. The results of the study allowed the classification of this case as bifrontal PMG. The genetic screening by whole exome sequencing (WES) on the Illumina Next Generation Sequencing (NGS) platform yielded 83 rare (minor allele frequency, MAF ≤ 0.01) pathological/deleterious variants where none of the respective genes has been previously linked to PMG. However, a subsequent analysis of those variants revealed that a significant number of affected genes were associated with most of the biological processes known to be impaired in PMG thereby pointing toward a polygenic nature in the present case. One of the notable features of the WES dataset was the presence of rare pathological/deleterious variants of genes (ADGRA2, PCDHA1, PCDHA12, PTK7, TPGS1, and USP4) involved in the regulation of Wnt signaling potentially highlighting the latter as an important PMG contributor in the present case. Notably, ADGRA2 warrants a closer look as a candidate gene for PMG because it not only regulates cortical patterning but has also been recently linked to two cases of bifrontal PMG with multiple congenital anomalies through its compound heterozygous mutations.
Keywords: bifrontal, malformation of cortical development, next-generation sequencing, polymicrogyria, whole exome sequencing
Introduction
Polymicrogyria (PMG) is a malformation of cortical development (MCD) characterized primarily by overfolding of the cortical surface, producing an irregular pattern with numerous small gyri and shallow sulci [1]. With an incidence of 2.3 per 10,000 births [2], PMG is the most common form of MCD, accounting for 20% of all cases [1,3]. PMG has a remarkably heterogeneous phenotype [4] where common clinical presentations may include epilepsy, intellectual disabilities, and deficits in language and/or motor skills. To complicate this matter even further, these symptoms are often observed in conjunction with syndromes bearing multiple congenital anomalies, as well as symptoms specific to the affected cortical areas [1,4]. The phenotypical heterogeneity of PMG could be partially explained by its etiological diversity that in addition to variable genetic underpinnings [4-6] could also include environmental factors such as maternal infection during pregnancy, hypoxia/ischemia, and trauma [5]. Genetic PMG causes are diverse and could include chromosomal aberrations, copy number variations, or mutations involving a single or multiple genes [6]. The most common single-gene mutations target genes encoding cytoskeletal proteins (TUBA1A, TUBB2B, TUBB3, and TUBA8), genes regulating cell growth (PIK3R2 and FIG4) and extracellular matrix (COL18A1 and LAMC3), as well as neuronal proliferation and migration (WDR62) [4,6]. Such remarkable phenotypical and etiological diversity makes the study of addressing molecular mechanism(s) of PMG in humans extremely difficult and could explain the paucity of the available respective information.
Therefore, the main objective of this study was to gain additional insights into the mechanism(s) governing PMG development through a postmortem study with a multifaceted approach including magnetic resonance imaging (MRI), gross anatomical examination and dissection, histopathological examination, as well as genetic screening by the whole exome sequencing (WES) on the next-generation sequencing (NGS) platform. A clearer understanding of the nature of the above pathology may further advance our understanding of the mechanism(s) regulating brain development in humans.
These data were presented in part as an abstract at the Anatomy Connected Meeting on March 24, 2024.
Case presentation
Anatomical characterization
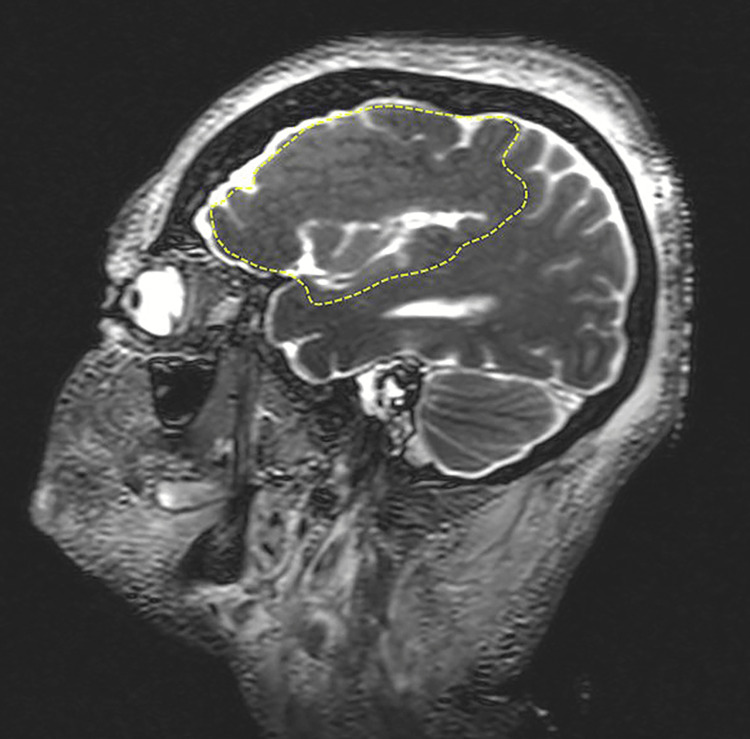
An 81-year-old female body was received through the Saint Louis University (SLU) Gift Body Program with signed informed consent. Reported medical history included PMG, intellectual disability, static encephalopathy, moderately oral pharyngeal dysphagia, osteoporosis, cerebral palsy, irritable bowel syndrome, deafness, aphasia, ptyalism, and edentia. The cause of death for this individual was acute respiratory failure from acquired pneumonia, leading to hypoxia and severe sepsis. External examination of the donor revealed no external deformities as the extremities were normal with 10 fingers and toes. Body measurements revealed a height of 155 cm or (5’1”) and a head circumference of 50.15 cm. The distinct external characteristic of the donor was a protruding tongue. MRI revealed bifrontal PMG limited to the frontal lobes with small gyri, abnormal gyral patterns, and an irregular gray-white interface (Figure 1). The subcortical areas, brainstem, and cerebellum appeared normally formed with no notable absences or hypoplasia.
Figure 1. Magnetic resonance imaging of the donor head demonstrating the presence of polymicrogyria (PMG).
Parasagittal image of a T2‐weighted MRI is shown. Note the aberrant boundary of the polymicrogyric cortex (depicted by stippled yellow lines) compared to the smooth gray‐white boundary in normal cortical areas.
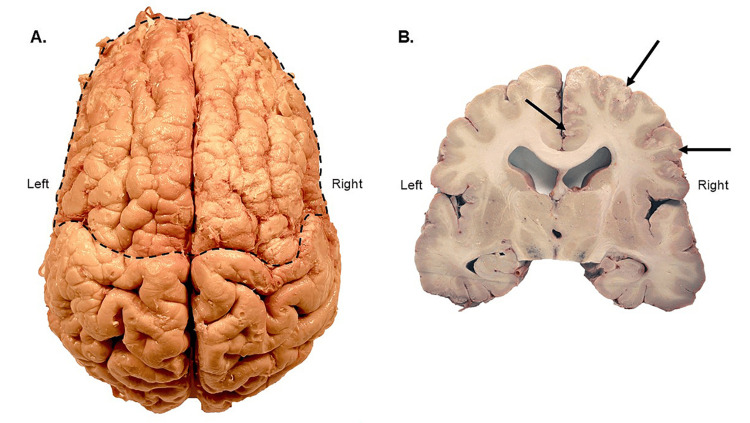
The gross brain examination confirmed the presence of bifrontal PMG (Figure 2A). Aberrant patterning of the small gyri of the frontal lobes was well defined, in addition to some asymmetrical aberrant patterning in the left parietal and right perisylvian regions, with the apparent absence of the central sulcus of Rolando and precentral gyrus. Coronal sections showed small cortical folds and shallow sulci over the frontal lobes (Figure 2B). Subcortical structures were normally present, and the lateral ventricles showed mild to moderate enlargement, indicative of age-related hydrocephalus ex-vacuo. The cortex of the right frontal lobe showed a stippled grey-white matter boundary in the anterior cingulate gyrus, extending posteriorly in the superior and inferior frontal gyri (Figure 2B).
Figure 2. Superior and coronal views of the polymicrogyria (PMG) brain.
A. Superior view of the removed brain. Polymicrogyria (PMG) is evident in the outlined area when compared to the typical gyration of the more posterior brain. B. Coronal brain section at the level of the substantia nigra. The intact and fully developed corpus callosum is evident in this section; also of note is the stippled gray matter of the right hemisphere, as indicated by the arrows. Mild to moderate enlargement of the lateral ventricles was observed.
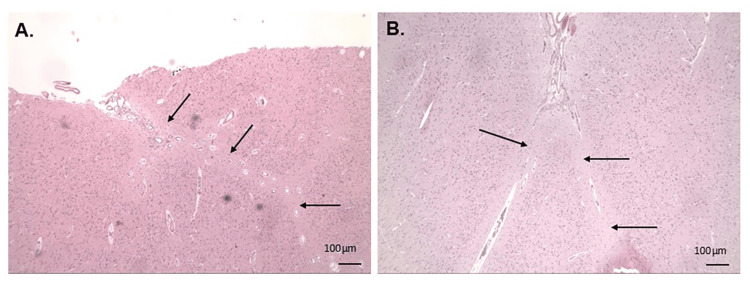
Examination of the histological images detailed multiple areas of true gyral fusion between adjacent molecular layers, with evidence of entrapped but otherwise normal, leptomeningeal blood vessels (Figure 3). Additionally, a mild decrease in cortical thickness, with focal neuronal loss in the superficial layers and neuronal dyslamination of the neocortex, was noted. No neuronal heterotopias or dysplastic neurons were noted, and pial surfaces demonstrated no specific abnormalities.
Figure 3. Hematoxylin and eosin (H&E)-stained sections of the right frontal lobe.
A. Right cingulate gyrus. The black arrows indicate areas of fused molecular layers between adjacent gyri. Leptomeningeal tissue and intracortical vessels are also evident in the upper part of this image. B. Right middle frontal gyrus. The microscopic image shows the cortical surface with fusion of the molecular layers (black arrows) which appears to extend into the underlying sulcus and includes entrapped leptomeningeal vessels.
Genetic screening
The post-mortem genetic screening by WES on the Illumina NGS platform and the respective bioinformatics analysis were performed as previously described [7]. The genetic screening revealed rare pathological/deleterious variants in 83 genes (Table 1) with none of the genes listed in the table being previously linked to PMG [6]. Interestingly, among the genes listed in Table 1, there was a group of five pleiotropic genes known to be involved in both neurogenesis and angiogenesis: ADGRA2 [8,9], JAG2 [10,11], LAMA1 [12,13], SEMA3D [14,15], and SYNM [16]. These data were consistent with a recent hypothesis linking an aberrant hypersprouting angiogenesis to PMG development [17]. To test this hypothesis in the current setting, the histopathological examination of cerebral vasculature was performed but the outcomes were unremarkable (data not shown).
Table 1. Complete list of genes with rare pathological/deleterious variants associated with the present case*.
* Gene-to-protein name conversion was performed using the GeneCards database; **BBB: blood brain barrier.
| Gene | Protein function |
| A1BG | Alpha-1-B glycoprotein |
| ABCA3 | ATP binding cassette subfamily A member 3; brain development [18]; progenitor cell regulation; neurogenesis [19]; vesicle transport [20] |
| ABHD5 | Abhydrolase domain containing 5, lysophosphatidic acid acyltransferase; skin barrier defect [21] |
| ACSS1 | Acyl-CoA synthetase short chain family member 1 |
| ADGRA2 | Adhesion G protein-coupled receptor A2; brain development [8]; cerebral angiogenesis [9]; Wnt signaling [22,23]; bifrontal PMG with multiple congenital anomalies [24] |
| ADPRHL1 | ADP-ribosylhydrolase-like 1 ROCK signaling [25] |
| BIRC7 | Baculoviral IAP repeat containing 7 |
| C19orf57 | Break repair meiotic recombinase recruitment factor 1 |
| CAPN12 | Calpain 12; angiogenesis [26]; intellectual disability [27] |
| CARMIL3 | Capping protein regulator and myosin 1 linker 3; brain ischemia [28]; cell migration [29] |
| CCDC198 | Coiled-coil domain containing 198. Neurogenesis; progenitor cell regulation; cell migration [30] |
| CHAF1A | Chromatin assembly factor 1 subunit A. Neurogenesis [31,32] |
| CHRND | Cholinergic receptor nicotinic delta subunit. Neuromuscular junction formation [33] |
| CMPK2 | Cytidine/uridine monophosphate kinase 2 |
| CYP17A1 | Cytochrome P450 family 17 subfamily A member 1; angiogenesis [34] |
| DENND1B | DENN domain containing 1B; vesicle transport [35] |
| DHRS2 | Dehydrogenase/reductase 2 |
| DNAH3 | Dynein axonemal heavy chain 3; motile cilia regulation [36] |
| FLG2 | Filaggrin 2; brain development [37]; skin disease [38] |
| GCC2 | GRIP and coiled-coil domain containing 2 |
| GLYATL3 | Glycine-N-acyltransferase like 3 |
| GUSB | Glucuronidase beta |
| HLA-DRB1 | Major histocompatibility complex, class II, DR beta 1 |
| HLA-DRB5 | Major histocompatibility complex, class II, DR beta 5 |
| HLTF | Helicase-like transcription factor; neurogenesis/brain development [39]; neuronal cell death [40] |
| IFI30 | IFI30 lysosomal thiol reductase; angiogenesis [41]; neuronal cells death [42] |
| JAG2 | Jagged canonical notch ligand 2; progenitor cell regulation [11]; angiogenesis [10] |
| KMT2C | Lysine methyltransferase 2C; neurogenesis/brain development [43,44] |
| KRT19 | Keratin 19 |
| KRT86 | Keratin 86 |
| KRTAP3-2 | Keratin associated protein 3-2 |
| LAMA1 | Laminin subunit alpha 1. Neurogenesis/brain development [12]; angiogenesis [13] |
| LONP1 | Lon Peptidase 1, mitochondrial. Mitochondrial encephalopathy [45]; neuronal cell death [46] |
| LRGUK | Leucine-rich repeats and guanylate kinase domain containing. Motile cilia regulation [47] |
| LRRC58 | Leucine-rich repeat containing 58. Neuroinflammation [48,49] |
| MDH1 | Malate dehydrogenase 1. Energy metabolism [50]; neuronal cell death [51] |
| MGST2 | Microsomal glutathione S-transferase 2. Neuronal cell death [52]; angiogenesis [53] |
| MLXIP | MLX interacting protein. Brain development/neurogenesis; progenitor cell regulation; energy metabolism [54] |
| MPEG1 | Macrophage expressed 1 |
| MRPL57 | Mitochondrial ribosomal protein L57. Energy metabolism [55] |
| MS4A15 | Membrane spanning 4-domains A15. Brain development/neurogenesis [56] |
| MTUS1 | Microtubule-associated scaffold protein 1. Rac signaling [57] |
| MYO7B | Myosin VIIB. Vesicle transport [58]. Possibly linked to brain development/neurogenesis [59] |
| NGLY1 | N-glycanase 1. Brain development/neurogenesis [60,61]; neuronal cell death [62] |
| NMU | Neuromedin U |
| OBSCN | Obscurin, cytoskeletal calmodulin, and titin-interacting. Brain development/neurogenesis [63] |
| OPN3 | Opsin 3 |
| OR2S2 | Olfactory receptor family 2 subfamily S member 2. BBB** regulation [64] |
| PAH | Phenylalanine hydroxylase |
| PCDHA1 | Protocadherin alpha 1. Brain development/neurogenesis [65,66]; Wnt signaling [67] |
| PCDHA12 | Protocadherin alpha 12. Brain development/neurogenesis [68]; Wnt signaling [69,70] |
| PGK2 | Phosphoglycerate kinase 2 |
| PHKB | Phosphorylase kinase regulatory subunit beta |
| PI15 | Peptidase inhibitor 15 |
| PLEKHG3 | Pleckstrin homology and RhoGEF domain containing G3. Brain development/neurogenesis [71,72]; Rho signaling [72]; linked to mild intellectual disability [73] |
| PSG6 | Pregnancy-specific beta-1-glycoprotein 6. Brain development/neurogenesis [74] |
| PTK7 | Protein tyrosine kinase 7 (Inactive). Brain development/neurogenesis [75,76]; Wnt signaling [75,77]; cell migration [77] |
| PTPRN2 | Protein tyrosine phosphatase receptor type N2. TGFβ signaling [78]; cerebral vasculature remodeling [79] |
| RGR | Retinal G protein coupled receptor |
| RIT2 | Ras like without CAAX 2. Brain development/neurogenesis; Ras signaling [80] |
| RSL1D1 | Ribosomal L1 domain containing 1 (cellular senescence-inhibited gene protein, CSIG). Cell proliferation and senescence [81]; linked to child cognitive development [82] |
| SEMA3D | Semaphorin 3D. Signaling [83]; linked to cognitive impairment [14]; angiogenesis [15] |
| SKIV2L | SKI2 subunit of superkiller complex |
| SLC26A1 | Solute carrier family 26 member 1 |
| SLC9C1 | Solute carrier family 9 member C1. Sperm-specific component of motile cilia [84] |
| SYNM | Synemin. Neurogenesis; progenitor cell regulation; cell migration [16] |
| TIAM2 | TIAM Rac1 associated GEF 2. Brain development/neurogenesis; Rho signaling [85] |
| TLN2 | Talin 2 |
| TMEM43 | Transmembrane protein 43 |
| TPGS1 | Tubulin polyglutamylase complex Subunit 1. Cilia regulation [86] |
| TTN | Titin |
| TUT1 | Terminal uridylyl transferase 1, U6 SnRNA-specific. Brain development/neurogenesis [87]; neuronal cell death [88] |
| TYK2 | Tyrosine kinase 2. Brain development/neurogenesis [89]. Jak/Stat signaling; neuronal cell death [90] |
| UBE4B | Ubiquitination factor E4B. Brain development/neurogenesis; progenitor cell regulation; mTOR signaling [91]; neuronal cell death [92] |
| UGT1A7 | UDP glucuronosyltransferase family 1 member A7; BBB regulation [93] |
| UNKL | Unk like zinc finger. Neurogenesis [94] |
| UPK1A | Uroplakin 1A |
| USP4 | Ubiquitin specific peptidase 4. Brain development/neurogenesis [95,96]; neuronal cell death [97]; Wnt signaling [98,99] |
| ZBED9 | SCAN domain containing 3 (SCAND3). Potential biomarker for mild cognitive impairment [100] |
| ZC3H3 | Zinc finger CCCH-type containing 3 |
| ZNF227 | Zinc finger protein 227 |
| ZNF540 | Zinc finger protein 540 |
| ZYX | Zyxin. Brain development/neurogenesis [101]; tight junction/BBB regulation [102]; Shh signaling [103]; cell migration [104] |
Discussion
The current report provides additional insights into the molecular mechanism(s) underlining PMG development. The examination of the individual’s brain by anatomical, MRI, and histological techniques allowed the classification of the observed MCD as bifrontal PMG with an absence of visible musculoskeletal defects often associated with PMG [1]. The performed genetic analysis provided several important insights into its development.
First, there was a plethora of genes linked to the biological processes that could be perturbed in PMG (Table 1) [1,105] thereby being consistent with the polygenic underlining of the present case. Second, there were several genes involved in the regulation of cilia function including both of its types, primary and motile (Table 1). Given the crucial role of motile cilia in neurodevelopment through regulation of cerebrospinal fluid (CSF) fluid flow [106,107] and ventricular development [108,109], as well as the absence of non-age related hydrocephalus pathology in the donor’s brain (Figure 2), one may conclude that PMG in the present case was not mediated by motile cilia but was rather associated with an input from the aberrant primary cilia-mediated signaling [110,111]. Such signaling aberration could be explained, at least in part, by the abnormal primary cilia formation driven by the mutated TPGS1 (Table 1) [86] and by impaired Wnt signaling, due to mutations in ADGRA2, PCDHA1, PCDHA12, PTK7, and USP4 (Table 1), which use primary cilia as a signaling platform [112].
Third, ADGRA2, also known as GPR124, was a very interesting gene because it not only positively regulates canonical Wnt signaling by increasing Wnt7 availability for Frizzled [22, 23], but by virtue of its compound heterozygous mutations, it has also been recently linked to two bifrontal PMG cases with multiple congenital anomalies [24]. It should also be noted that the other member of the same gene family, ADGRG1 (GPR56) with an autosomal recessive variant, was reported to be associated with bilateral frontoparietal PMG [113, 114]. Therefore, the results of the current report and the data presented in [24] merit a closer look at AGDRA2 as a potentially causative gene in bifrontal PMG.
Fourth, the other notable feature of the genetic screening dataset was the presence of the biallelic FLG2 variant (NM_001014342:exon3:c.C2606T:p.S869F; MAF = 4.21x10-6). FLG2 is known for its association with skin diseases including atopic dermatitis [38], as well as for its link to neurodevelopmental aberrations, leading to autism spectrum disorder (ASD) with a prenatal excessive cortical expansion frequently seen in ASD children [37]. Such a link between neuro- and ectodermal development supports a hypothesis regarding the existence of the skin-brain axis, which could interdependently regulate both processes [37]. Unfortunately, due to the condition of cadaveric tissue subjected to the embalming solution, it was impossible to correctly assess the putative epidermal pathology in the donor and, therefore, evaluate the involvement of the skin-brain axis in the current case of bifrontal PMG. However, probing similar PMG cases for the autosomal recessive FLG2 mutations and atopic syndromes antemortem would be worth pursuing.
Conclusions
The current rare case of bifrontal PMG in an elderly individual provided a unique opportunity to gain additional insights into the molecular mechanism(s) of PMG. Our results highlight the polygenic nature of PMG and the potential involvement of impaired Wnt signaling with the involvement of primary cilia deregulation and a direct Wnt signaling disruption. Our results also warrant additional studies on the ADGRA2 gene as well as probing the skin-brain axis for their participation in the development of the cerebral cortex in humans.
Acknowledgments
We are grateful to all individuals and their families for their invaluable contribution to the SLU Gift Body Program. We would also like to thank Dr. Paul Cliften (Genome Technology Access Center, Washington University in St. Louis, MO, USA) for his expert assistance with the bioinformatics analysis and Caroline Murphy (Advanced Spatial Biology and Research Histology Core, SLU SOM) for her skillful help with the histology slide preparation.
Disclosures
Human subjects: Consent for treatment and open access publication was obtained or waived by all participants in this study.
Conflicts of interest: In compliance with the ICMJE uniform disclosure form, all authors declare the following:
Payment/services info: All authors have declared that no financial support was received from any organization for the submitted work.
Financial relationships: All authors have declared that they have no financial relationships at present or within the previous three years with any organizations that might have an interest in the submitted work.
Other relationships: All authors have declared that there are no other relationships or activities that could appear to have influenced the submitted work.
Author Contributions
Concept and design: John R. Martin III, Andrey Frolov
Acquisition, analysis, or interpretation of data: John R. Martin III, Andrey Frolov, Miguel A. Guzman, Stuart G. Atwood
Drafting of the manuscript: John R. Martin III, Andrey Frolov
Supervision: John R. Martin III
Critical review of the manuscript for important intellectual content: Miguel A. Guzman, Stuart G. Atwood
References
- 1.Polymicrogyria: a common and heterogeneous malformation of cortical development. Stutterd CA, Leventer RJ. Am J Med Genet C Semin Med Genet. 2014;166C:227–239. doi: 10.1002/ajmg.c.31399. [DOI] [PubMed] [Google Scholar]
- 2.Polymicrogyria: epidemiology, imaging, and clinical aspects in a population-based cohort. Kolbjer S, Martín Muñoz DA, Örtqvist AK, Pettersson M, Hammarsjö A, Anderlid BM, Dahlin M. Brain Commun. 2023;5:0. doi: 10.1093/braincomms/fcad213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clinical and imaging features of cortical malformations in childhood. Leventer RJ, Phelan EM, Coleman LT, Kean MJ, Jackson GD, Harvey AS. Neurology. 1999;53:715–722. doi: 10.1212/wnl.53.4.715. [DOI] [PubMed] [Google Scholar]
- 4.Polymicrogyria: pathology, fetal origins and mechanisms. Squier W, Jansen A. Acta Neuropathol Commun. 2014;2:80. doi: 10.1186/s40478-014-0080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Genetics of the polymicrogyria syndromes. Jansen A, Andermann E. J Med Genet. 2005;42:369–378. doi: 10.1136/jmg.2004.023952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The genetic landscape of polymicrogyria. James J, Iype M, Surendran MO, Anitha A, Thomas SV. Ann Indian Acad Neurol. 2022;25:616–626. doi: 10.4103/aian.aian_97_22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Two cases of sporadic amyotrophic lateral sclerosis with contrasting clinical phenotypes: genetic insights. Frolov A, Guzman MA, Hayat G, Martin JR 3rd. Cureus. 2024;16:0. doi: 10.7759/cureus.56023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Adhesion G protein-coupled receptor gluing action guides tissue development and disease. Sreepada A, Tiwari M, Pal K. J Mol Med (Berl) 2022;100:1355–1372. doi: 10.1007/s00109-022-02240-0. [DOI] [PubMed] [Google Scholar]
- 9.Defective adgra2 (gpr124) splicing and function in zebrafish ouchless mutants. Bostaille N, Gauquier A, Stainier DY, Raible DW, Vanhollebeke B. Development. 2017;144:8–11. doi: 10.1242/dev.146803. [DOI] [PubMed] [Google Scholar]
- 10.Reducing Jagged 1 and 2 levels prevents cerebral arteriovenous malformations in matrix Gla protein deficiency. Yao Y, Yao J, Radparvar M, Blazquez-Medela AM, Guihard PJ, Jumabay M, Boström KI. Proc Natl Acad Sci U S A. 2013;110:19071–19076. doi: 10.1073/pnas.1310905110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jagged2 controls the generation of motor neuron and oligodendrocyte progenitors in the ventral spinal cord. Rabadán MA, Cayuso J, Le Dréau G, et al. Cell Death Differ. 2012;19:209–219. doi: 10.1038/cdd.2011.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Laminin α1 is essential for mouse cerebellar development. Ichikawa-Tomikawa N, Ogawa J, Douet V, et al. Matrix Biol. 2012;31:17–28. doi: 10.1016/j.matbio.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lama1 mutations lead to vitreoretinal blood vessel formation, persistence of fetal vasculature, and epiretinal membrane formation in mice. Edwards MM, McLeod DS, Grebe R, Heng C, Lefebvre O, Lutty GA. BMC Dev Biol. 2011;11:60. doi: 10.1186/1471-213X-11-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cerebral Semaphorin3D is a novel risk factor for age-associated cognitive impairment. Chen CY, Chao YM, Cho CC, et al. Cell Commun Signal. 2023;21:140. doi: 10.1186/s12964-023-01158-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Semaphorin 3d signaling defects are associated with anomalous pulmonary venous connections. Degenhardt K, Singh MK, Aghajanian H, et al. Nat Med. 2013;19:760–765. doi: 10.1038/nm.3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Synemin isoforms during mouse development: multiplicity of partners in vascular and neuronal systems. Izmiryan A, Franco CA, Paulin D, Li Z, Xue Z. Exp Cell Res. 2009;315:769–783. doi: 10.1016/j.yexcr.2008.12.009. [DOI] [PubMed] [Google Scholar]
- 17.A theory for polymicrogyria and brain arteriovenous malformations in HHT. Klostranec JM, Chen L, Mathur S, McDonald J, Faughnan ME, Ratjen F, Krings T. Neurology. 2019;92:34–42. doi: 10.1212/WNL.0000000000006686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Distinct spatio-temporal expression of ABCA and ABCG transporters in the developing and adult mouse brain. Tachikawa M, Watanabe M, Hori S, Fukaya M, Ohtsuki S, Asashima T, Terasaki T. J Neurochem. 2005;95:294–304. doi: 10.1111/j.1471-4159.2005.03369.x. [DOI] [PubMed] [Google Scholar]
- 19.ABC transporters, neural stem cells and neurogenesis--a different perspective. Lin T, Islam O, Heese K. Cell Res. 2006;16:857–871. doi: 10.1038/sj.cr.7310107. [DOI] [PubMed] [Google Scholar]
- 20.The role of cholesterol in α-synuclein and Lewy body pathology in GBA1 Parkinson's disease. García-Sanz P, M F G Aerts J, Moratalla R. Mov Disord. 2021;36:1070–1085. doi: 10.1002/mds.28396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mammalian alpha beta hydrolase domain (ABHD) proteins: lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Lord CC, Thomas G, Brown JM. Biochim Biophys Acta. 2013;1831:792–802. doi: 10.1016/j.bbalip.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.A RECK-WNT7 receptor-ligand interaction enables isoform-specific regulation of Wnt bioavailability. Vallon M, Yuki K, Nguyen TD, et al. Cell Rep. 2018;25:339–349. doi: 10.1016/j.celrep.2018.09.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.A molecular mechanism for Wnt ligand-specific signaling. Eubelen M, Bostaille N, Cabochette P, et al. Science. 2018;361 doi: 10.1126/science.aat1178. [DOI] [PubMed] [Google Scholar]
- 24.The ADGRA2 gene is associated with multiple fetal brain anomalies in humans. Chong K, Keunen J, Staines A, et al. Genet Med Open. 2024;18:10. [Google Scholar]
- 25.The pseudoenzyme ADPRHL1 affects cardiac function by regulating the ROCK pathway. Tian L, Guo T, Wu F, et al. Stem Cell Res Ther. 2023;14:309. doi: 10.1186/s13287-023-03507-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novel genetic locus influencing retinal venular tortuosity is also associated with risk of coronary artery disease. Veluchamy A, Ballerini L, Vitart V, et al. Arterioscler Thromb Vasc Biol. 2019;39:2542–2552. doi: 10.1161/ATVBAHA.119.312552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Exome sequencing of Pakistani consanguineous families identifies 30 novel candidate genes for recessive intellectual disability. Riazuddin S, Hussain M, Razzaq A, et al. Mol Psychiatry. 2017;22:1604–1614. doi: 10.1038/mp.2016.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Capping protein regulator and myosin 1 linker 3 (CARMIL3) as a molecular signature of ischemic neurons in the DWI-T2 mismatch areas after stroke. Yeh SJ, Hsu PH, Yeh TY, et al. Front Mol Neurosci. 2021;14:754762. doi: 10.3389/fnmol.2021.754762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.CARMIL3 is important for cell migration and morphogenesis during early development in zebrafish. Stark BC, Gao Y, Sepich DS, et al. Dev Biol. 2022;481:148–159. doi: 10.1016/j.ydbio.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hypoxia inducible factor-2α importance for migration, proliferation, and self-renewal of trunk neural crest cells. Niklasson CU, Fredlund E, Monni E, et al. Dev Dyn. 2021;250:191–236. doi: 10.1002/dvdy.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.CHAF1A blocks neuronal differentiation and promotes neuroblastoma oncogenesis via metabolic reprogramming. Tao L, Moreno-Smith M, Ibarra-García-Padilla R, et al. Adv Sci (Weinh) 2021;8:0. doi: 10.1002/advs.202005047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Direct neuronal reprogramming: achievements, hurdles, and new roads to success. Gascón S, Masserdotti G, Russo GL, Götz M. Cell Stem Cell. 2017;21:18–34. doi: 10.1016/j.stem.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 33.Transcriptome profile of subsynaptic myonuclei at the neuromuscular junction in embryogenesis. Ohkawara B, Kurokawa M, Kanai A, et al. J Neurochem. 2024;168:342–354. doi: 10.1111/jnc.16013. [DOI] [PubMed] [Google Scholar]
- 34.In view of ovarian steroidogenesis and luteal construction to explore the effects of Bushen Huoxue recipe in mice of ovarian hyperstimulation. Song Y, Hu R, Li F, et al. J Ethnopharmacol. 2024;318:116913. doi: 10.1016/j.jep.2023.116913. [DOI] [PubMed] [Google Scholar]
- 35.The connecdenn family, Rab35 guanine nucleotide exchange factors interfacing with the clathrin machinery. Marat AL, McPherson PS. J Biol Chem. 2010;285:10627–10637. doi: 10.1074/jbc.M109.050930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clustering of genetic anomalies of cilia outer dynein arm and central apparatus in patients with transposition of the great arteries. De Ita M, Gaytán-Cervantes J, Cisneros B, et al. Genes (Basel) 2022;13 doi: 10.3390/genes13091662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ectodermal origins of the skin-brain axis: a novel model for the developing brain, inflammation, and neurodevelopmental conditions. Jameson C, Boulton KA, Silove N, Nanan R, Guastella AJ. Mol Psychiatry. 2023;28:108–117. doi: 10.1038/s41380-022-01829-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Uncommon variants in FLG2 and TCHHL1 are associated with remission of atopic dermatitis in a large longitudinal US cohort. Berna R, Mitra N, Hoffstad O, Wubbenhorst B, Nathanson KL, Margolis DJ. Arch Dermatol Res. 2022;314:953–959. doi: 10.1007/s00403-021-02319-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rad5, HLTF, and SHPRH: a fresh view of an old story. Elserafy M, Abugable AA, Atteya R, El-Khamisy SF. Trends Genet. 2018;34:574–577. doi: 10.1016/j.tig.2018.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Role of helicase-like transcription factor (hltf) in the G2/m transition and apoptosis in brain. Helmer RA, Foreman O, Dertien JS, Panchoo M, Bhakta SM, Chilton BS. PLoS One. 2013;8:0. doi: 10.1371/journal.pone.0066799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ifi30 is required for sprouting angiogenesis during caudal vein plexus formation in zebrafish. Wang X, Ge X, Qin Y, Liu D, Chen C. Front Physiol. 2022;13:919579. doi: 10.3389/fphys.2022.919579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuromyelitis optica/Devic's disease: gene expression profiling of brain lesions. Satoh J, Obayashi S, Misawa T, Tabunoki H, Yamamura T, Arima K, Konno H. Neuropathology. 2008;28:561–576. doi: 10.1111/j.1440-1789.2008.00915.x. [DOI] [PubMed] [Google Scholar]
- 43.Regulation of histone H3K4 methylation in brain development and disease. Shen E, Shulha H, Weng Z, Akbarian S. Philos Trans R Soc Lond B Biol Sci. 2014;369 doi: 10.1098/rstb.2013.0514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Disrupted intricacy of histone H3K4 methylation in neurodevelopmental disorders. Vallianatos CN, Iwase S. Epigenomics. 2015;7:503–519. doi: 10.2217/epi.15.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.LONP1 de novo dominant mutation causes mitochondrial encephalopathy with loss of LONP1 chaperone activity and excessive LONP1 proteolytic activity. Besse A, Brezavar D, Hanson J, Larson A, Bonnen PE. Mitochondrion. 2020;51:68–78. doi: 10.1016/j.mito.2020.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bi-allelic mutations of LONP1 encoding the mitochondrial LonP1 protease cause pyruvate dehydrogenase deficiency and profound neurodegeneration with progressive cerebellar atrophy. Nimmo GA, Venkatesh S, Pandey AK, et al. Hum Mol Genet. 2019;28:290–306. doi: 10.1093/hmg/ddy351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Central apparatus, the molecular kickstarter of ciliary and flagellar nanomachines. Samsel Z, Sekretarska J, Osinka A, Wloga D, Joachimiak E. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22063013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Proteomic dissimilarities of primary microglia and BV2 cells under stimuli. Luan W, Li M, Wu C, Shen X, Sun Z. Eur J Neurosci. 2022;55:1709–1723. doi: 10.1111/ejn.15637. [DOI] [PubMed] [Google Scholar]
- 49.Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. Sousa C, Golebiewska A, Poovathingal SK, et al. EMBO Rep. 2018;19 doi: 10.15252/embr.201846171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Oxidative damage to the TCA cycle enzyme MDH1 dysregulates bioenergetic enzymatic activity in the aged murine brain. Guo X, Park JE, Gallart-Palau X, Sze SK. J Proteome Res. 2020;19:1706–1717. doi: 10.1021/acs.jproteome.9b00861. [DOI] [PubMed] [Google Scholar]
- 51.Upregulation of MDH1 acetylation by HDAC6 inhibition protects against oxidative stress-derived neuronal apoptosis following intracerebral hemorrhage. Wang M, Zhou C, Yu L, et al. Cell Mol Life Sci. 2022;79:356. doi: 10.1007/s00018-022-04341-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Comparative developmental neurotoxicity of organophosphates in vivo: transcriptional responses of pathways for brain cell development, cell signaling, cytotoxicity and neurotransmitter systems. Slotkin TA, Seidler FJ. Brain Res Bull. 2007;72:232–274. doi: 10.1016/j.brainresbull.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Orai3 surface accumulation and calcium entry evoked by vascular endothelial growth factor. Li J, Bruns AF, Hou B, et al. Arterioscler Thromb Vasc Biol. 2015;35:1987–1994. doi: 10.1161/ATVBAHA.115.305969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sex differences in gene regulatory networks during mid-gestational brain development. de Toledo VH, Feltrin AS, Barbosa AR, Tahira AC, Brentani H. Front Hum Neurosci. 2022;16:955607. doi: 10.3389/fnhum.2022.955607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Human primitive brain displays negative mitochondrial-nuclear expression correlation of respiratory genes. Barshad G, Blumberg A, Cohen T, Mishmar D. Genome Res. 2018;28:952–967. doi: 10.1101/gr.226324.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Omics analysis of mouse brain models of human diseases. Paban V, Loriod B, Villard C, et al. Gene. 2017;600:90–100. doi: 10.1016/j.gene.2016.11.022. [DOI] [PubMed] [Google Scholar]
- 57.A de novo Mutation in the MTUS1 Gene Decreases the Risk of Non-compaction of Ventricular Myocardium via the Rac1/Cdc42 Pathway. Bai X, Zhou Y, Ouyang N, Liu L, Huang X, Tian J, Lv T. Front Pediatr. 2019;7:247. doi: 10.3389/fped.2019.00247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.A myosin-7B-dependent endocytosis pathway mediates cellular entry of α-synuclein fibrils and polycation-bearing cargos. Zhang Q, Xu Y, Lee J, et al. Proc Natl Acad Sci U S A. 2020;117:10865–10875. doi: 10.1073/pnas.1918617117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Clinical and molecular evaluation of 13 Brazilian patients with Gomez-López-Hernández syndrome. Perrone E, Perez AB, D'Almeida V, et al. Am J Med Genet A. 2021;185:1047–1058. doi: 10.1002/ajmg.a.62059. [DOI] [PubMed] [Google Scholar]
- 60.Reversibility of motor dysfunction in the rat model of NGLY1 deficiency. Asahina M, Fujinawa R, Hirayama H, Tozawa R, Kajii Y, Suzuki T. Mol Brain. 2021;14:91. doi: 10.1186/s13041-021-00806-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Deficiency of N-glycanase 1 perturbs neurogenesis and cerebral development modeled by human organoids. Lin VJ, Hu J, Zolekar A, et al. Cell Death Dis. 2022;13:262. doi: 10.1038/s41419-022-04693-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ngly1 -/- rats develop neurodegenerative phenotypes and pathological abnormalities in their peripheral and central nervous systems. Asahina M, Fujinawa R, Nakamura S, Yokoyama K, Tozawa R, Suzuki T. Hum Mol Genet. 2020;29:1635–1647. doi: 10.1093/hmg/ddaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Targeted deletion of the zebrafish obscurin A RhoGEF domain affects heart, skeletal muscle and brain development. Raeker MO, Bieniek AN, Ryan AS, Tsai HJ, Zahn KM, Russell MW. Dev Biol. 2010;337:432–443. doi: 10.1016/j.ydbio.2009.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.The senses of the choroid plexus. Santos CR, Duarte AC, Costa AR, Tomás J, Quintela T, Gonçalves I. Prog Neurobiol. 2019;182:101680. doi: 10.1016/j.pneurobio.2019.101680. [DOI] [PubMed] [Google Scholar]
- 65.New candidates for autism/intellectual disability identified by whole-exome sequencing. Bruno LP, Doddato G, Valentino F, et al. Int J Mol Sci. 2021;22 doi: 10.3390/ijms222413439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Defining the human brain proteome using transcriptomics and antibody-based profiling with a focus on the cerebral cortex. Sjöstedt E, Fagerberg L, Hallström BM, et al. PLoS One. 2015;10:0. doi: 10.1371/journal.pone.0130028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.FZD7 drives in vitro aggressiveness in Stem-A subtype of ovarian cancer via regulation of non-canonical Wnt/PCP pathway. Asad M, Wong MK, Tan TZ, et al. Cell Death Dis. 2014;5:0. doi: 10.1038/cddis.2014.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.DNA methylation associated with mitochondrial dysfunction in a South African autism spectrum disorder cohort. Stathopoulos S, Gaujoux R, Lindeque Z, Mahony C, Van Der Colff R, Van Der Westhuizen F, O'Ryan C. Autism Res. 2020;13:1079–1093. doi: 10.1002/aur.2310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berberine potently attenuates intestinal polyps growth in ApcMin mice and familial adenomatous polyposis patients through inhibition of Wnt signalling. Zhang J, Cao H, Zhang B, et al. J Cell Mol Med. 2013;17:1484–1493. doi: 10.1111/jcmm.12119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Critical genomic networks and vasoreactive variants in idiopathic pulmonary arterial hypertension. Hemnes AR, Zhao M, West J, et al. Am J Respir Crit Care Med. 2016;194:464–475. doi: 10.1164/rccm.201508-1678OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.A de novo 1.5 Mb microdeletion on chromosome 14q23.2-23.3 in a patient with autism and spherocytosis. Griswold AJ, Ma D, Sacharow SJ, et al. Autism Res. 2011;4:221–227. doi: 10.1002/aur.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Function of SYDE C2-RhoGAP family as signaling hubs for neuronal development deduced by computational analysis. Kouchi Z, Kojima M. Sci Rep. 2022;12:4325. doi: 10.1038/s41598-022-08147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.A 2.1 Mb deletion adjacent but distal to a 14q21q23 paracentric inversion in a family with spherocytosis and severe learning difficulties. Lybaek H, Øyen N, Fauske L, Houge G. Clin Genet. 2008;74:553–559. doi: 10.1111/j.1399-0004.2008.01072.x. [DOI] [PubMed] [Google Scholar]
- 74.A study of the genomic variations associated with autistic spectrum disorders in a Russian cohort of patients using whole-exome sequencing. Gibitova EA, Dobrynin PV, Pomerantseva EA, et al. Genes (Basel) 2022;13 doi: 10.3390/genes13050920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.PTK7 recruits dsh to regulate neural crest migration. Shnitsar I, Borchers A. Development. 2008;135:4015–4024. doi: 10.1242/dev.023556. [DOI] [PubMed] [Google Scholar]
- 76.The novel mouse mutant, chuzhoi, has disruption of Ptk7 protein and exhibits defects in neural tube, heart and lung development and abnormal planar cell polarity in the ear. Paudyal A, Damrau C, Patterson VL, et al. BMC Dev Biol. 2010;10:87. doi: 10.1186/1471-213X-10-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.PTK7 faces the Wnt in development and disease. Berger H, Wodarz A, Borchers A. Front Cell Dev Biol. 2017;5:31. doi: 10.3389/fcell.2017.00031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Host genetic predictors of the kynurenine pathway of tryptophan catabolism among treated HIV-infected Ugandans. Lee SA, Mefford JA, Huang Y, et al. AIDS. 2016;30:1807–1815. doi: 10.1097/QAD.0000000000001124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Proteome profiling of brain vessels in a mouse model of cerebrovascular pathology. Haqqani AS, Mianoor Z, Star AT, et al. Biology (Basel) 2023;12 doi: 10.3390/biology12121500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.RIT2: responsible and susceptible gene for neurological and psychiatric disorders. Daneshmandpour Y, Darvish H, Emamalizadeh B. Mol Genet Genomics. 2018;293:785–792. doi: 10.1007/s00438-018-1451-4. [DOI] [PubMed] [Google Scholar]
- 81.Ribosomal L1 domain and lysine-rich region are essential for CSIG/ RSL1D1 to regulate proliferation and senescence. Ma L, Zhao W, Zheng Q, Chen T, Qi J, Li G, Tong T. Biochem Biophys Res Commun. 2016;469:593–598. doi: 10.1016/j.bbrc.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 82.Cognitive development and brain gray matter susceptibility to prenatal adversities: moderation by the prefrontal cortex brain-derived neurotrophic factor gene co-expression network. de Mendonça Filho EJ, Barth B, Bandeira DR, et al. Front Neurosci. 2021;15:744743. doi: 10.3389/fnins.2021.744743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Semaphorin 3d and semaphorin 3e direct endothelial motility through distinct molecular signaling pathways. Aghajanian H, Choi C, Ho VC, Gupta M, Singh MK, Epstein JA. J Biol Chem. 2014;289:17971–17979. doi: 10.1074/jbc.M113.544833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dissecting the signaling pathways involved in the function of sperm flagellum. Vyklicka L, Lishko PV. Curr Opin Cell Biol. 2020;63:154–161. doi: 10.1016/j.ceb.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Cloning and characterization of T-cell lymphoma invasion and metastasis 2 (TIAM2), a novel guanine nucleotide exchange factor related to TIAM1. Chiu CY, Leng S, Martin KA, Kim E, Gorman S, Duhl DM. Genomics. 1999;61:66–73. doi: 10.1006/geno.1999.5936. [DOI] [PubMed] [Google Scholar]
- 86.Regulators of tubulin polyglutamylation control nuclear shape and cilium disassembly by balancing microtubule and actin assembly. Wang L, Paudyal SC, Kang Y, et al. Cell Res. 2022;32:190–209. doi: 10.1038/s41422-021-00584-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Genes that affect brain structure and function identified by rare variant analyses of mendelian neurologic disease. Karaca E, Harel T, Pehlivan D, et al. Neuron. 2015;88:499–513. doi: 10.1016/j.neuron.2015.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.USP15 deubiquitinates TUT1 associated with RNA metabolism and maintains cerebellar homeostasis. Kim J, Nakamura J, Hamada C, et al. Mol Cell Biol. 2020;40 doi: 10.1128/MCB.00098-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jak2 and Tyk2 are necessary for lineage-specific differentiation, but not for the maintenance of self-renewal of mouse embryonic stem cells. Chung BM, Kang HC, Han SY, et al. Biochem Biophys Res Commun. 2006;351:682–688. doi: 10.1016/j.bbrc.2006.10.081. [DOI] [PubMed] [Google Scholar]
- 90.Tyk2/STAT3 signaling mediates beta-amyloid-induced neuronal cell death: implications in Alzheimer's disease. Wan J, Fu AK, Ip FC, et al. J Neurosci. 2010;30:6873–6881. doi: 10.1523/JNEUROSCI.0519-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fine-tuning of mTOR signaling by the UBE4B-KLHL22 E3 ubiquitin ligase cascade in brain development. Kong X, Shu X, Wang J, et al. Development. 2022;149 doi: 10.1242/dev.201286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.UBE4B: a promising regulatory molecule in neuronal death and survival. Zeinab RA, Wu H, Sergi C, Leng R. Int J Mol Sci. 2012;13:16865–16879. doi: 10.3390/ijms131216865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.The UDP-glucuronosyltransferases of the blood-brain barrier: their role in drug metabolism and detoxication. Ouzzine M, Gulberti S, Ramalanjaona N, Magdalou J, Fournel-Gigleux S. Front Cell Neurosci. 2014;8:349. doi: 10.3389/fncel.2014.00349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Control of a neuronal morphology program by an RNA-binding zinc finger protein, Unkempt. Murn J, Zarnack K, Yang YJ, et al. Genes Dev. 2015;29:501–512. doi: 10.1101/gad.258483.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Effects of deubiquitylases on the biological behaviors of neural stem cells. Zhao Q, Li Y, Du X, Chen X, Jiao Q, Jiang H. Dev Neurobiol. 2021;81:847–858. doi: 10.1002/dneu.22844. [DOI] [PubMed] [Google Scholar]
- 96.Genome-wide analysis of genes encoding core components of the ubiquitin system during cerebral cortex development. Bouron A, Fauvarque MO. Mol Brain. 2022;15:72. doi: 10.1186/s13041-022-00958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Increased expression of ubiquitin-specific protease 4 participates in neuronal apoptosis after intracerebral hemorrhage in adult rats. Liu C, Liu C, Liu H, et al. Cell Mol Neurobiol. 2017;37:427–435. doi: 10.1007/s10571-016-0375-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.The ubiquitin specific protease 4 (USP4) is a new player in the Wnt signalling pathway. Zhao B, Schlesiger C, Masucci MG, Lindsten K. J Cell Mol Med. 2009;13:1886–1895. doi: 10.1111/j.1582-4934.2008.00682.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ubiquitin specific protease 4 positively regulates the WNT/β-catenin signaling in colorectal cancer. Yun SI, Kim HH, Yoon JH, et al. Mol Oncol. 2015;9:1834–1851. doi: 10.1016/j.molonc.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Association of peripheral blood DNA methylation level with Alzheimer's disease progression. Li QS, Vasanthakumar A, Davis JW, Idler KB, Nho K, Waring JF, Saykin AJ. Clin Epigenetics. 2021;13:191. doi: 10.1186/s13148-021-01179-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Transcriptional factor Xanf1 interacts with the focal adhesion protein zyxin in the early development of the Xenopus laevis brain. Martynova NIu, Ermolina LV, Eroshkin FM, Gioeva FK, Zaraĭskiĭ AG. Bioorg Khim. 2008;34:573–576. doi: 10.1134/s1068162008040183. [DOI] [PubMed] [Google Scholar]
- 102.Zyxin modulates the transmigration of Haemophilus influenzae to the central nervous system. Miyazaki Y, Yusa T, Matsuo S, Terauchi Y, Miyazaki S. Virulence. 2014;5:665–672. doi: 10.4161/viru.29786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.The cytoskeletal protein Zyxin inhibits Shh signaling during the CNS patterning in Xenopus laevis through interaction with the transcription factor Gli1. Martynova NY, Ermolina LV, Ermakova GV, Eroshkin FM, Gyoeva FK, Baturina NS, Zaraisky AG. Dev Biol. 2013;380:37–48. doi: 10.1016/j.ydbio.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 104.Zyxin mediates vascular repair via endothelial migration promoted by forskolin in mice. Kang X, Deng Y, Cao Y, Huo Y, Luo J. Front Physiol. 2021;12:741699. doi: 10.3389/fphys.2021.741699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deconstructing cortical folding: genetic, cellular and mechanical determinants. Llinares-Benadero C, Borrell V. Nat Rev Neurosci. 2019;20:161–176. doi: 10.1038/s41583-018-0112-2. [DOI] [PubMed] [Google Scholar]
- 106.The role of motile cilia in the development and physiology of the nervous system. Ringers C, Olstad EW, Jurisch-Yaksi N. Philos Trans R Soc Lond B Biol Sci. 2020;375:20190156. doi: 10.1098/rstb.2019.0156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.The regulatory roles of motile cilia in CSF circulation and hydrocephalus. Kumar V, Umair Z, Kumar S, Goutam RS, Park S, Kim J. Fluids Barriers CNS. 2021;18:31. doi: 10.1186/s12987-021-00265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ciliary beating compartmentalizes cerebrospinal fluid flow in the brain and regulates ventricular development. Olstad EW, Ringers C, Hansen JN, et al. Curr Biol. 2019;29:229–241. doi: 10.1016/j.cub.2018.11.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Diversity and function of motile ciliated cell types within ependymal lineages of the zebrafish brain. D'Gama PP, Qiu T, Cosacak MI, et al. Cell Rep. 2021;37:109775. doi: 10.1016/j.celrep.2021.109775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.RTTN mutations link primary cilia function to organization of the human cerebral cortex. Kheradmand Kia S, Verbeek E, Engelen E, et al. Am J Hum Genet. 2012;91:533–540. doi: 10.1016/j.ajhg.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Primary cilia in the developing and mature brain. Guemez-Gamboa A, Coufal NG, Gleeson JG. Neuron. 2014;82:511–521. doi: 10.1016/j.neuron.2014.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cilia as Wnt signaling organelles. Niehrs C, Da Silva F, Seidl C. Trends Cell Biol. 2024 doi: 10.1016/j.tcb.2024.04.001. [DOI] [PubMed] [Google Scholar]
- 113.G protein-coupled receptor-dependent development of human frontal cortex. Piao X, Hill RS, Bodell A, et al. Science. 2004;303:2033–2036. doi: 10.1126/science.1092780. [DOI] [PubMed] [Google Scholar]
- 114.Genotype-phenotype analysis of human frontoparietal polymicrogyria syndromes. Piao X, Chang BS, Bodell A, et al. Ann Neurol. 2005;58:680–687. doi: 10.1002/ana.20616. [DOI] [PubMed] [Google Scholar]