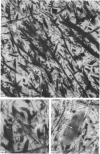
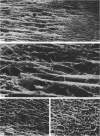
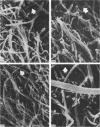
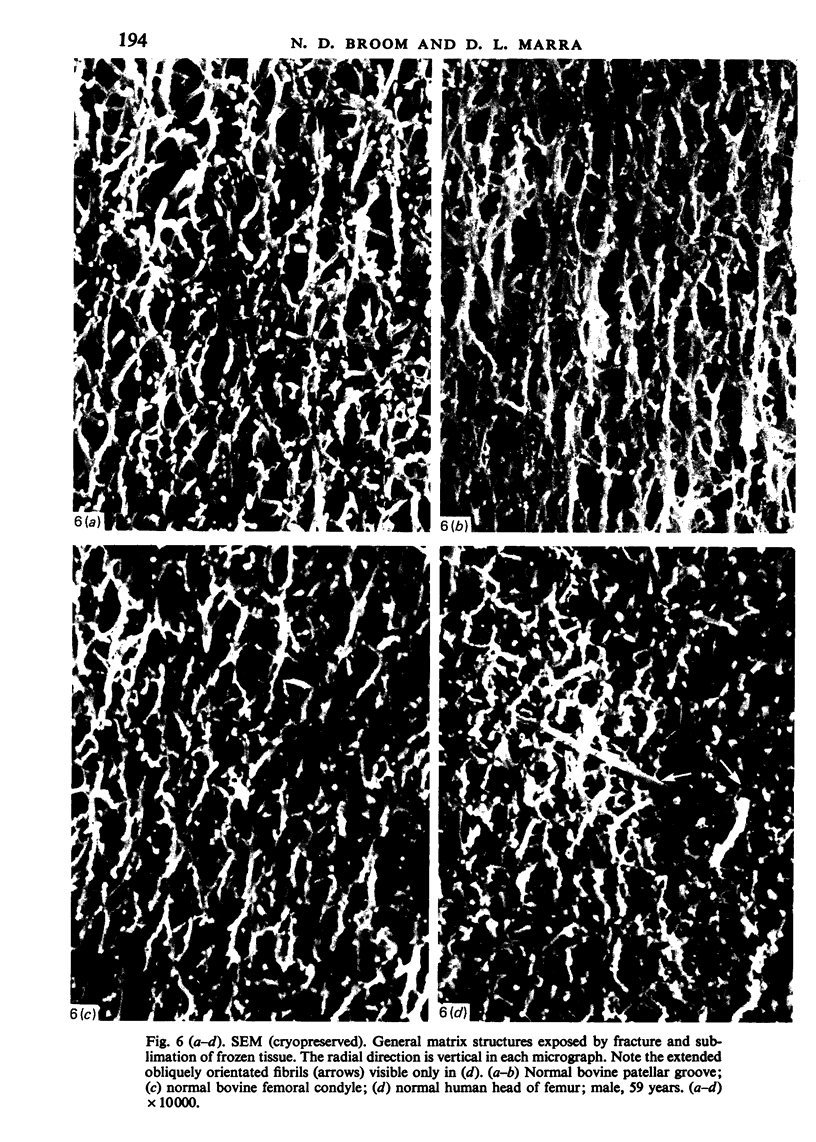
Abstract
This study presents ultrastructural evidence for the presence of a variety of fibril-to-fibril interactions or associations in the architecture of the general matrix of articular cartilage. These interactions are believed to serve a higher purpose of repeatedly constraining an overall radial arrangement of fibrils into an array of oblique interconnecting segments thus creating a three dimensional meshwork within which the hydrated ground substance is constrained. It is argued that any reduction in these interfibrillar interactions will allow the oblique fibril segments to revert to a low energy radial configuration, thus explaining the presence of such arrays prominent in various degenerate forms of articular cartilage.
Full text
PDF











Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aspden R. M., Hukins D. W. Collagen organization in articular cartilage, determined by X-ray diffraction, and its relationship to tissue function. Proc R Soc Lond B Biol Sci. 1981 Jul 14;212(1188):299–304. doi: 10.1098/rspb.1981.0040. [DOI] [PubMed] [Google Scholar]
- Broom N. D. Abnormal softening in articular cartilage: its relationship to the collagen framework. Arthritis Rheum. 1982 Oct;25(10):1209–1216. doi: 10.1002/art.1780251010. [DOI] [PubMed] [Google Scholar]
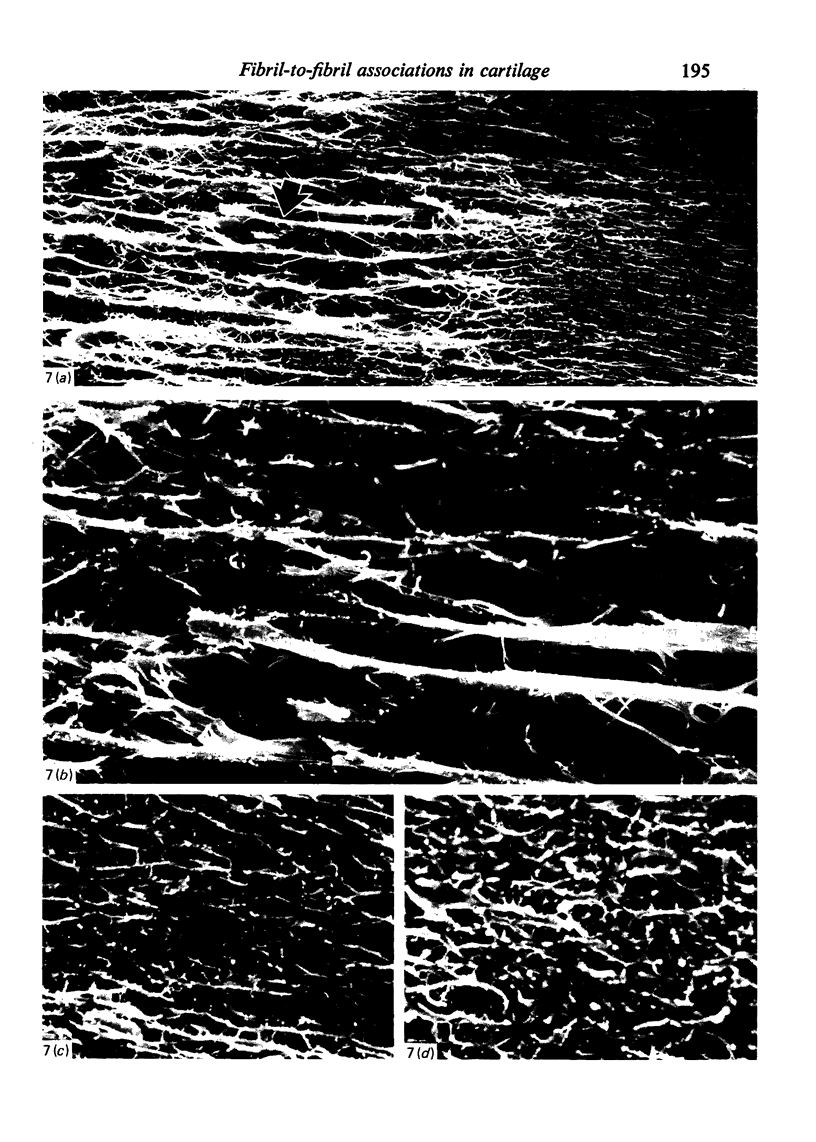
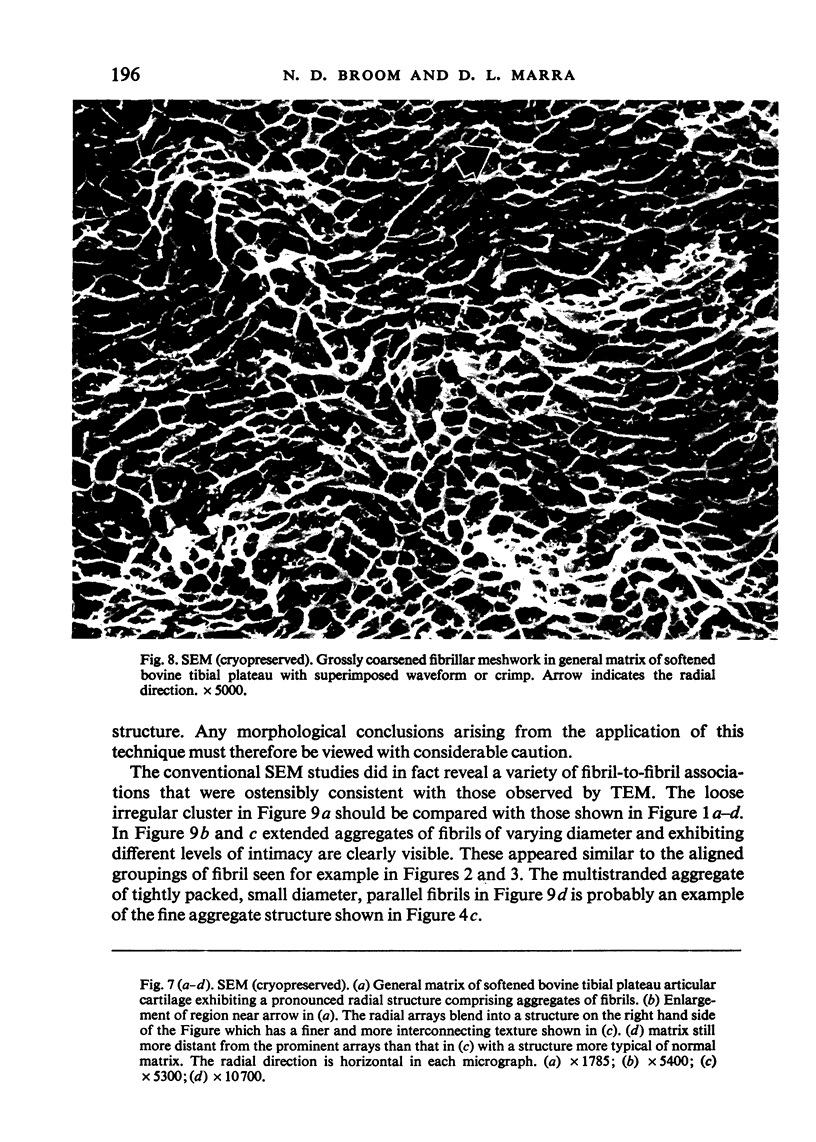
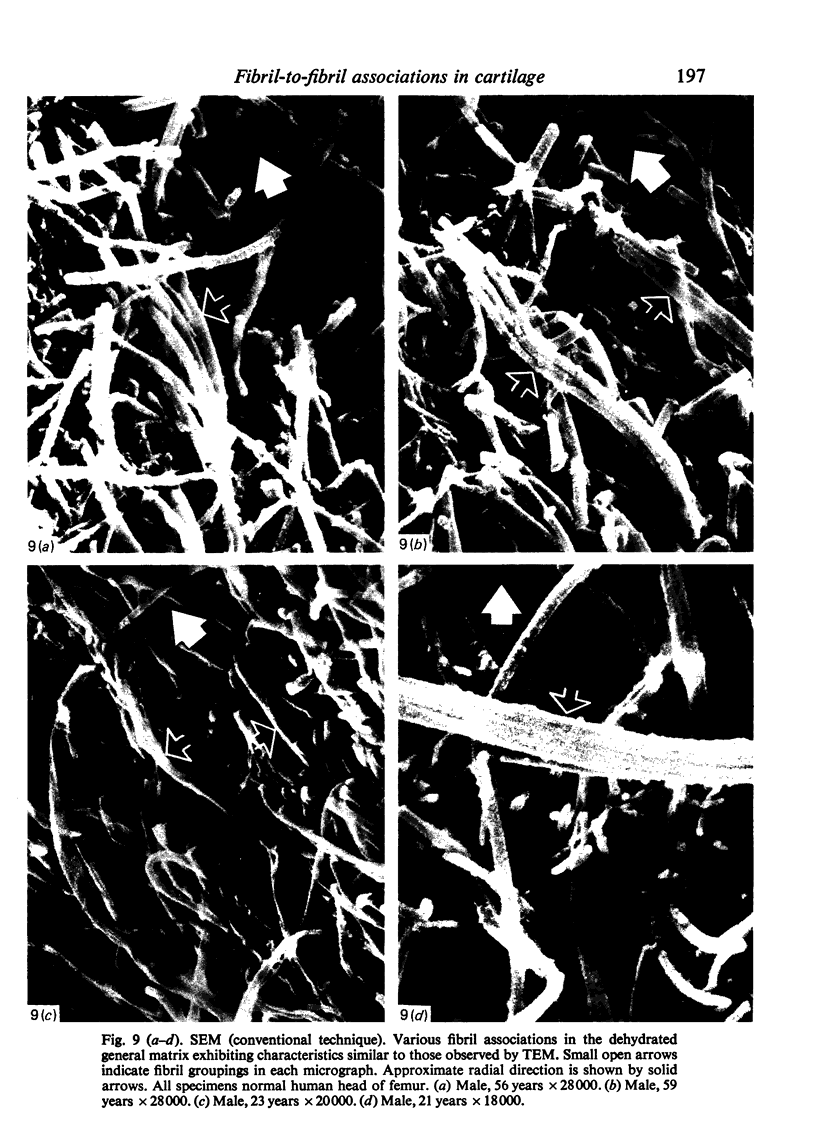
- Broom N. D. Further insights into the structural principles governing the function of articular cartilage. J Anat. 1984 Sep;139(Pt 2):275–294. [PMC free article] [PubMed] [Google Scholar]
- Broom N. D., Marra D. L. New structural concepts of articular cartilage demonstrated with a physical model. Connect Tissue Res. 1985;14(1):1–8. doi: 10.3109/03008208509089838. [DOI] [PubMed] [Google Scholar]
- Broom N. D. The altered biomechanical state of human femoral head osteoarthritic articular cartilage. Arthritis Rheum. 1984 Sep;27(9):1028–1039. doi: 10.1002/art.1780270910. [DOI] [PubMed] [Google Scholar]
- Echlin P. Low temperature scanning electron microscopy: a review. J Microsc. 1978 Jan;112(1):47–61. doi: 10.1111/j.1365-2818.1978.tb01153.x. [DOI] [PubMed] [Google Scholar]
- FESSLER J. H. A structural function of mucopolysaccharide in connective tissue. Biochem J. 1960 Jul;76:124–132. doi: 10.1042/bj0760124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner D. L., O'Connor P., Oates K. Low temperature scanning electron microscopy of dog and guinea-pig hyaline articular cartilage. J Anat. 1981 Mar;132(Pt 2):267–282. [PMC free article] [PubMed] [Google Scholar]
- Meachim G. Cartilage fibrillation on the lateral tibial plateau in Liverpool necropsies. J Anat. 1976 Feb;121(Pt 1):97–106. [PMC free article] [PubMed] [Google Scholar]
- Mow V. C., Holmes M. H., Lai W. M. Fluid transport and mechanical properties of articular cartilage: a review. J Biomech. 1984;17(5):377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- Mow V. C., Mak A. F., Lai W. M., Rosenberg L. C., Tang L. H. Viscoelastic properties of proteoglycan subunits and aggregates in varying solution concentrations. J Biomech. 1984;17(5):325–338. doi: 10.1016/0021-9290(84)90027-7. [DOI] [PubMed] [Google Scholar]
- Parry D. A., Barnes G. R., Craig A. S. A comparison of the size distribution of collagen fibrils in connective tissues as a function of age and a possible relation between fibril size distribution and mechanical properties. Proc R Soc Lond B Biol Sci. 1978 Dec 18;203(1152):305–321. doi: 10.1098/rspb.1978.0107. [DOI] [PubMed] [Google Scholar]
- Serafini-Fracassini A., Smith J. W. Observations on the morphology of the proteinpolysaccharide complex of bovine nasal cartilage and its relationship to collagen. Proc R Soc Lond B Biol Sci. 1966 Oct 11;165(1001):440–449. doi: 10.1098/rspb.1966.0076. [DOI] [PubMed] [Google Scholar]
- Weiss C. Ultrastructural characteristics of osteoarthritis. Fed Proc. 1973 Apr;32(4):1459–1466. [PubMed] [Google Scholar]
- Yarker Y. E., Aspden R. M., Hukins D. W. Birefringence of articular cartilage and the distribution on collagen fibril orientations. Connect Tissue Res. 1983;11(2-3):207–213. doi: 10.3109/03008208309004857. [DOI] [PubMed] [Google Scholar]