Summary
Background
Depression is a severe mental disorder commonly co-morbid with diabetes, but it remains to elucidate whether depression is associated with the risks of a wide range of vascular complications in people with type 2 diabetes mellitus (T2DM) and whether metabolic biomarkers may mediate this pathway.
Methods
We conducted this prospective analysis among the participants of the UK Biobank who were diagnosed with T2DM and free of vascular complications at baseline between March 13, 2006 and September 30, 2010. Major depressive disorder (MDD) was ascertained according to the hospital admission records and self-report of doctor-diagnosed conditions, while the presence of depressive symptoms was assessed using the Patient Health Questionnaire-2. Cox proportional hazards models were performed to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) of MDD and depressive symptoms with the risks of incident heart failure (HF); total and individual atherosclerotic cardiovascular disease (ASCVD) including coronary artery disease (CAD), ischemic stroke (IS), and peripheral artery disease (PAD); total and individual microvascular complications of diabetic kidney disease (DKD), diabetic retinopathy (DR), and diabetic neuropathy (DN). Mediation analyses were conducted to quantify the potential mediation effects of circulating metabolites (involved in insulin-resistance, lipid profile, liver function, renal function, and inflammation) in the association of MDD with the outcomes.
Findings
Of the total 23,856 patients with T2DM in the UK Biobank, 13,706 participants (61% males) were eligible and included in this study. During an average of 13 years of follow-up, 2927 (21.36%) ASCVD, 1070 (7.81%) HF, and 2579 (18.82%) microvascular complications occurred. The adjusted HR (95% CI) for MDD was 1.32 (1.09–1.61) with HF, 1.17 (1.03–1.32) with ASCVD, and 1.29 (1.14–1.46) with microvascular complications, while those for depressive symptoms were 1.47 (1.20–1.79), 1.25 (1.10–1.42) and 1.20 (1.05–1.37), respectively. The HRs ranged from 1.26 (1.09–1.44) to 1.96 (1.57–2.45) for MDD with individual complications and mortality, and from 1.26 (1.08–1.47) to 1.49 (1.16–1.93) for depressive symptoms. Up to 7.8% of adverse complications were attributable to MDD and 3.8% to depressive symptoms. A series of circulating metabolites involving lipid profile, renal function, and inflammation were observed to mediate the associations of MDD with vascular complications. The identified mediators jointly accounted for 7.29%–26.87% of the disparities in incident vascular complications between patients with and without MDD.
Interpretation
Our findings highlight the role of MDD and depressive symptoms in the development of vascular complications among people with T2DM, and suggest that the effect of improving mental health on vascular outcomes in patients with T2DM should be investigated in future work.
Funding
Three-Year Public Health Action Plan of Shanghai.
Keywords: Major depressive disorder, Depressive symptoms, Type 2 diabetes mellitus, Vascular complication, Mediator metabolites
Research in context.
Evidence before this study
We searched PubMed and Google Scholar up to July 1, 2024, for published cohort studies examining the associations of depression with the risk of vascular complications among people with type 2 diabetes mellitus (T2DM) and the potential mediating effect of circulating metabolites in the health pathway. The search terms included “patients with T2DM or diabetes” AND “depression, depressive symptoms, or major depressive disorder (MDD)” AND “heart failure (HF), cardiovascular disease, atherosclerotic cardiovascular disease (ASCVD), coronary artery disease (CAD), ischemic stroke (IS), peripheral artery disease (PAD), macrovascular complications, microvascular complications, diabetic kidney disease (DKD), diabetic retinopathy (DR), or diabetic neuropathy (DN)” AND “mediating metabolites”. We found that existing cohort studies yielded inconsistent results, and large-scale prospective data is scarce on the associations of MDD and depressive symptoms with a wide spectrum of individual diabetes complications. Moreover, it remains unclear whether the circulating metabolites contribute to the development of vascular complications in people with T2DM comorbid with depression.
Added value of this study
In this prospective cohort study of 13,706 UK adults with T2DM, we found that the patients with comorbid MDD or depressive symptoms had a higher risk of multiple adverse vascular complications, including HF, total ASCVD, CAD, total microvascular complications, DKD, and DN, with up to 7.8% of the incident adverse complications attributable to MDD and 3.8% to depressive symptoms. In addition, 7.29%–26.87% of the associations between MDD and vascular complications were jointly explained by a panel of biomarkers including lipid profile, renal function, and inflammation.
Implications of all the available evidence
Our findings suggest that the effect of improving mental health on vascular outcomes in patients with T2DM should be investigated in future work.
Introduction
Type 2 diabetes mellitus (T2DM) has surged to an epidemic level globally, with 536.6 million people living with the condition in 2021.1 In individuals with T2DM, vascular complications remain the leading cause of hospitalization and mortality, accompanied by a dramatic rise in health expenditures,2 posing a significant public health challenge worldwide.
As a severe mental disorder commonly co-morbid with diabetes,3 depression is frequently accompanied by unhealthy behaviors such as smoking and physical inactivity.4 It has been reported that adults comorbid with diabetes and psychological distress were more likely to have inferior diabetes self-care and poor glycemic control, all of which may contribute to vascular dysfunction,5 and therefore a worse prognosis.6,7 A handful of studies have evaluated the associations between depression and a composite of vascular complications but yielded inconsistent results, probably due to the cross-sectional design, short-term follow-up, and relatively small sample size.7, 8, 9 Prospective evidence from large-scale and long-term follow-up cohort studies is also available for the risk of total vascular complications related to depression.10,11 However, the associations of depression with a wide spectrum of individual diabetes complications remain unclear, which has limited the development of tailored preventive strategies.
Both depression and T2DM are associated with dysregulation of the hypothalamic-pituitary-adrenal pathway, leading to increased platelet activation, elevated insulin resistance, and aggravated inflammation,12, 13, 14 The mechanisms that depression enhances the risk of diabetes complications include hyperglycemia and dyslipidemia due to chronic stress and hypercortisolism which disturb the immune system and synaptic plasticity.15 Accumulating evidence suggests that depression may trigger alternations in various circulating metabolites, such as glucose metabolism,16 serum lipid profiles,17 biomarkers of renal function,18 and systemic inflammatory factors.19 Many of the metabolites, particularly triglycerides and C-reactive protein, have served as biomarkers for adverse vascular complications.20 These observations, however, indicate a bidirectional association between depression and T2DM.21 It remains unclear whether these depression-related biomarkers contribute to the development of vascular complications in people with diabetes.
To fill these knowledge gaps, we designed this prospective study based on the UK Biobank to examine the associations of major depressive disorder (MDD) and depressive symptoms with the risks of heart failure (HF), total and individual atherosclerotic cardiovascular disease (ASCVD), and total and individual microvascular complications among individuals with T2DM. In addition, we explored the potential mediating effects of a series of circulating metabolites in the relationships between MDD and individual vascular complications among adults with diabetes with a clear temporality. This study has the potential to provide novel insight into the role of metabolic markers in the pathway from MDD to poor prognosis of diabetes.
Methods
Study population
The UK Biobank is a large-scale population-based prospective cohort study that enrolled over 500,000 adults aged 37–73 years from 22 sites across England, Scotland, and Wales during 2006–2010.22,23 Comprehensive data were collected through self-completed touch-screen questionnaires, physical and functional measures, and a range of biochemical assays of baseline samples.22 Follow-up was conducted mainly through linkages to routinely available national datasets. The disease and death outcomes were initially ascertained using electronic and semi-automated sources and supplemented by retrieval of case records, imaging data, or banked tissue samples for validation and sub-classification.
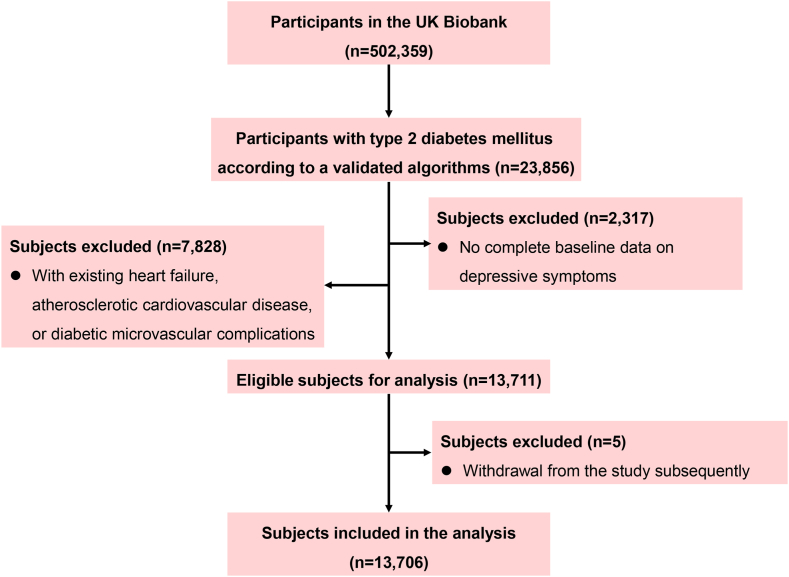
The current study included men and women diagnosed with T2DM at baseline. A total of 23,856 prevalent patients with T2DM were identified using a validated algorithm based on self-report, primary, and secondary care data.24 After the exclusion of those who had missing data on depressive symptoms (n = 2317), or prevalent HF, ASCVD, and diabetes microvascular complications identified by self-report, biological assays, or the International Classification of Diseases 10th Revision [ICD-10] code (n = 7828), or those who withdrew from the UK Biobank study subsequently (n = 5), a total of 13,706 individuals with T2DM were included in the final analysis.
The UK Biobank study was approved by the North West Multi-Centre Research Ethics Committee, and written informed consent was obtained from all participants on a touch screen using a signature capture device. This study is reported following the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guideline.
Assessment of MDD and depressive symptoms
In this study, MDD was determined based on hospital inpatient admission records using the ICD-10 codes F32 and F33,25,26 as well as self-reported diagnoses of depression from medical professionals. The depressive symptoms were assessed at baseline using the Patient Health Questionnaire-2 (PHQ-2).27 The PHQ-2 scale consists of two questions about the frequency of depressed mood and loss of interest over the past two weeks. Response options include “not at all”, “several days”, “more than half the days”, and “nearly every day” scored as 0, 1, 2, and 3, respectively. The total PHQ-2 score ranges from 0 to 6, and a score of ≥3 indicates possible depressive symptoms, which has been widely adopted in previous studies.28,29
Assessment of the circulating biomarkers
Blood samples were obtained from the consenting participants at baseline, processed into individual components, and stored at the UK Biobank's facilities at a temperature of −80 °C or in a nitrogen atmosphere until analysis. The UK Biobank confirms the reliability of blood biomarkers through rigorous quality control measures and provides comprehensive information on assay performance at https://biobank.ndph.ox.ac.uk.
The selections of biological biomarkers that might mediate the associations between MDD and vascular complications were based on the knowledge of relevant pathways.16,17,30,31 Finally, we extracted the data on circulating metabolites that involve insulin-resistance [glycated hemoglobin (HbA1c), insulin-like growth factor-1 (IGF-1)], lipid profile [total cholesterol (TC), high-density lipoprotein cholesterol (HDL-C), low-density lipoprotein cholesterol (LDL-C), triglycerides, apolipoprotein A, apolipoprotein B, and lipoprotein A], liver function [alanine aminotransferase (ALT), alkaline phosphatase (ALP), aspartate aminotransferase (AST), gamma-glutamyl transferase (GGT), total and direct bilirubin, and albumin], renal function (cystatin C, creatinine, urate, and urea), and inflammation [C-reactive protein (CRP) and white blood cell count (WBC)].
Study outcomes
The primary outcomes were the incident HF, total and individual ASCVD, as well as total and individual microvascular complications. ASCVD was defined as a composite of outcomes of coronary artery disease (CAD), ischemic stroke (IS), and peripheral artery disease (PAD), while microvascular complications included diabetic kidney disease (DKD), diabetic retinopathy (DR), and diabetic neuropathy (DN). The secondary outcomes were all-cause death and CVD-specific death. The ICD-10 code for each T2DM complication and diagnosis date are presented in Table S1. All the events and deaths were identified by linking the cohort database with the hospital inpatient admissions, the vital statistics, the primary care data in the UK, and the self-report of doctor-diagnosed conditions at repeated visits.
Covariates
Covariates obtained from the baseline survey were considered as potential confounders, which included sociodemographic status (age, sex, ethnicity, Townsend deprivation index), lifestyle factors (smoking status, alcohol intake, body mass index), family history of diabetes, family history of CVD, medication (use of hypoglycemic agents, lipid-lowering agents), and duration of diabetes. All participants were dichotomised into White and non-White ethnicity and categorised into five groups according to the quintiles of the Townsend deprivation index. Body mass index (BMI) was calculated as weight (kg) divided by the square of height (m2), and classified as obese (≥30 kg/m2) or non-obese (<30 kg/m2). We further categorised participants into 3 smoking categories: never, former, and current smokers, and 3 alcohol consumption categories: never, former, and current drinkers. Duration of diabetes was defined as the difference between the year/age of the first episode of diabetes and the year/age of the baseline survey.
Statistical analysis
Baseline characteristics were presented as median (interquartile range, IQR) for continuous variables and frequency (%) for categorical variables. Person-years were calculated from the date of recruitment to the date of occurrence of events, death, end of follow-up (31 October 2022 for England sub-cohort, 31 May 2022 for Wales sub-cohort, and 31 August 2022 for Scotland sub-cohort), or loss to follow-up, whichever occurred first. Lost to follow-up was confirmed according to (1) death reported to UK Biobank by a relative; (2) NHS records indicating they were lost to follow-up; (3) NHS records indicating they left the UK; and (4) UK Biobank sources reporting they left the UK. Cox proportional hazard models were applied to estimate the hazard ratios (HRs) and 95% confidence intervals (95% CIs) of MDD and depressive symptoms with the risks of total and individual vascular complications, as well as all-cause deaths and CVD-specific deaths. The proportional hazard assumptions were tested based on scaled Schoenfeld residuals.
We fitted two statistical models: In model 1, we adjusted for age (continuous, years) and sex (male, female). In the fully adjusted model (model 2), we further adjusted for Townsend deprivation index, ethnicity, smoking status, alcohol intake, BMI, family history of CVD, family history of diabetes, diabetes duration, use of hypoglycemic, and use of lipid-lowing agents. A total of 516 participants (3.8%) had missing information on at least one of the covariates, as presented in Table S2. We imputed missing values for continuous covariates by using multiple imputations (n = 5) and created a separate response category for missing values of categorical covariates. In addition, we calculated the population-attributable fractions (PAFs) to quantify the contribution of MDD and depressive symptoms to each outcome.32
Mediation effects of metabolic biomarkers on the associations of MDD with the risks of individual vascular complications were evaluated according to the hypothesised pathway illustrated in Figure S1 and via the quasi-Bayesian Monte Carlo method with 1000 simulations using the mediation package in R.33 Considering that MDD was diagnosed before the baseline survey, while depressive symptoms were assessed simultaneously with the assays of circulating metabolites at baseline, we only used MDD as the exposure to conduct mediation analyses to ensure temporality.
We identified potential mediators from selected circulating metabolites for mediation analyses following a two-step analysis. First, we assessed the associations of MDD with the biomarkers using multiple linear regression models. Second, we evaluated the associations of the significant biomarkers in the first step with the risks of all the outcomes using the multivariable-adjusted Cox regression model. Then we chose the biomarkers significantly associated with any outcome for corresponding mediation analyses. To assess the extent to which all the mediators explain the total effect, we summarised all the mediating biomarkers in multiple principal components and then used the principal components as mediators.34
We further conducted stratified analyses by age (≤60, >60 years), sex (male, female), smoking (ever, never), diabetes duration (≤5, >5 years), and use of lipid-lowering medication (yes, no). Interactions between MDD or depressive symptoms and stratified factors in the risks of outcomes were examined using the likelihood ratio test by adding product terms in the multivariable-adjusted Cox models.
We performed several sensitivity analyses to test the robustness of our results. First, to minimise the potential reverse causation, we evaluated the associations of MDD and depressive symptoms with vascular complications after excluding the events that occurred within 2 years of follow-up. Second, we performed the association analysis of MDD with the outcomes by including the incident T2DM during the follow-up period as the study participants. Third, we applied the Fine–Gray proportional subdistribution hazards model by treating all-cause deaths or other-cause deaths as competing events to compute the subdistribution HR and 95% CI. Fourth, we regarded the users of antidepressant medications as potentially patients with MDD and added them to the exposed group. Finally, considering the potential interactions between MDD and the mediators, we used the four-way effect decomposition approach to classify the total effect of MDD compared to non-MDD into four components: controlled direct effect (neither mediation nor interaction), pure indirect effect (mediation only), reference interaction effect (interaction only), and mediated interaction effect (both mediation and interaction),35 as illustrated in Figure S2.
All statistical analyses were performed using SAS 9.4 (SAS Institute, Cary, NC, USA) and R software (4.3.1). A two-tailed P < 0.05 was considered statistically significant. P-values were adjusted for a false discovery rate (FDR) of 0.05 using the Benjamini-Hochberg method for multiple comparisons in analyses of circulating biomarkers.
Role of the funding source
The funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. All authors had full access to the data in the study, and W.H.X. had final responsibility for the decision to submit for publication.
Results
Baseline characteristics
Of 13,706 participants with T2DM (61.0% male; mean age of 59.5 years), 1336 (9.75%) were diagnosed with MDD, and 1246 (9.09%) had depressive symptoms, of whom 361 individuals (2.63%) had MDD and depressive symptoms simultaneously. The flow chart for recruitment of study participants is shown in Fig. 1.
Fig. 1.
Flowchart of the selection of study participants from the UK Biobank.
The baseline characteristics of the participants with and without MDD or depressive symptoms are shown in Table 1. The individuals with depressive symptoms or MDD were more likely to be female, younger, deprived, smokers, and obese, but less likely to be drinkers. In addition, the individuals with MDD were less likely to use hypoglycemic agents and have a family history of diabetes than those without.
Table 1.
Baseline characteristics of the study participants by major depressive disorder or depressive symptoms.
| Major depressive disorder | Non- major depressive disorder | P-values | Depressive symptoms | Non-depressive symptoms | P-values | |
|---|---|---|---|---|---|---|
| Count (%) | 1336 (9.75) | 12,370 (90.25) | 1246 (9.09) | 12,460 (90.91) | ||
| Sex of male (%) | 660 (49.40) | 7705 (62.29) | <0.0001 | 651 (52.25) | 7714 (61.91) | <0.0001 |
| Age (years) | 59 (53, 63) | 61 (55, 65) | <0.0001 | 56 (50, 62) | 61 (56, 65) | <0.0001 |
| Ethnic (%) | <0.0001 | <0.0001 | ||||
| White | 1233 (92.29) | 10,862 (87.81) | 907 (72.79) | 11,188 (89.79) | ||
| Others | 95 (7.11) | 1453 (11.75) | 331 (26.57) | 1217 (9.77) | ||
| Townsend deprivation index | <0.0001 | <0.0001 | ||||
| 1 (least deprived) | 218 (16.32) | 2525 (20.41) | 141 (11.32) | 2602 (20.88) | ||
| 2 | 225 (16.84) | 2490 (20.13) | 172 (13.80) | 2543 (20.41) | ||
| 3 | 250 (18.71) | 2511 (20.30) | 201 (16.13) | 2560 (20.55) | ||
| 4 | 299 (22.38) | 2438 (19.71) | 304 (24.40) | 2433 (19.53) | ||
| 5 (most deprived) | 344 (25.75) | 2406 (19.45) | 428 (34.35) | 2322 (18.64) | ||
| Obese (%) | 855 (64.00) | 6674 (53.95) | <0.0001 | 745 (59.79) | 6784 (54.45) | 0.0002 |
| Alcohol intake (%) | <0.0001 | <0.0001 | ||||
| Never | 93 (6.96) | 959 (7.75) | 175 (14.04) | 877 (7.04) | ||
| Past | 127 (9.51) | 729 (5.89) | 132 (10.59) | 724 (5.81) | ||
| Current | 1112 (83.23) | 10,673 (86.28) | 935 (75.04) | 10,850 (87.08) | ||
| Smoking status (%) | 0.0004 | <0.0001 | ||||
| Never | 581 (43.49) | 5828 (47.11) | 637 (51.12) | 5772 (46.32) | ||
| Past | 583 (43.64) | 5238 (42.34) | 426 (34.19) | 5395 (43.30) | ||
| Current | 171 (12.80) | 1237 (10.00) | 179 (14.37) | 1229 (9.86) | ||
| Duration of diabetes (years) | 3.00 (1.29, 6.00) | 3.43 (1.39, 7.00) | 0.024 | 3.00 (1.33, 7.00) | 3.35 (1.37, 7.00) | 0.90 |
| Glycated hemoglobin (mmol/mol) | 47.70 (41.60, 56.40) | 48.70 (42.30, 56.80) | 0.014 | 48.95 (41.90, 59.10) | 48.60 (42.20, 56.60) | 0.061 |
| Use of hypoglycemic agents (%) | 794 (59.43) | 7824 (63.25) | 0.0061 | 807 (64.77) | 7811 (62.69) | 0.15 |
| Use of lipid-lowing medication (%) | 985 (73.73) | 9160 (74.05) | 0.80 | 877 (70.39) | 9268 (74.38) | 0.0022 |
| Family history of diabetes (%) | 373 (27.92) | 3986 (32.22) | 0.0013 | 433 (34.75) | 3926 (31.51) | 0.019 |
| Family history of CVD (%) | 317 (23.73) | 3206 (25.92) | 0.082 | 268 (21.51) | 3255 (26.12) | 0.0004 |
Data presented as frequency (%) or median (Q25–Q75). MDD, major depressive disorder.
P-value for Mann–Whitney U tests (continuous variables) or Chi-squared tests (categorical variables). CVD, cardiovascular disease.
Major depressive disorder determined based on hospital inpatient admission records and self-report, and depressive symptoms assessed using the Patient Health Questionnaire-2.
Incidence of vascular complications
During the median follow-up period of over 13 years, 1070 of the total 13,706 participants (7.81%) were newly diagnosed with HF, 2927 (21.36%) with ASCVD (including 2205 CAD, 615 PAD, and 570 IS), and 2579 (18.82%) with microvascular complications (including 1114 DKD, 582 DN, and 1393 DR). In addition, 2157 (15.74%) participants died by the end of the follow-up, with 465 (3.39%) dying from CVD. Participants with MDD or depressive symptoms had significantly higher cumulative incidences or age-standardised incidences of HF, ASCVD, CAD, microvascular complications, and DN compared with those without during the follow-up period (Figures S3 and S4 and Table S3).
Associations of MDD and depressive symptoms with outcomes
After adjusting for potential confounders as in model 2, higher risks of most complications were observed in patients with MDD, with HRs (95% CI) being 1.32 (1.09–1.61) for HF, 1.17 (1.03–1.32) for ASCVD, 1.26 (1.10–1.45) for CAD, 1.29 (1.14–1.46) for total microvascular complications, 1.31 (1.09–1.59) for DKD, and 1.96 (1.57–2.45) for DN (Table 2). The estimated PAFs of MDD to the complications ranged from 1.1% to 7.8%. No significant association was observed between MDD and the risks of PAD, IS, and DR.
Table 2.
Associations of major depressive disorder and depressive symptoms with the risk of total and individual vascular complications and death among individuals with type 2 diabetes mellitus.
| Incidence (per 1000 person-years) | Cases/deaths | Major depressive disorder |
PAF, % | Depressive symptoms |
PAF, % | |||
|---|---|---|---|---|---|---|---|---|
| HR1 (95% CI) | HR2 (95% CI) | HR1 (95% CI) | HR2 (95% CI) | |||||
| ASCVD | 18.00 | 2927 | 1.21 (1.08–1.37) | 1.17 (1.03–1.32) | 1.1 | 1.37 (1.21–1.55) | 1.25 (1.10–1.42) | 1.4 |
| CAD | 13.26 | 2205 | 1.30 (1.13–1.49) | 1.26 (1.10–1.45) | 1.9 | 1.39 (1.20–1.60) | 1.27 (1.10–1.47) | 1.7 |
| PAD | 3.52 | 615 | 1.19 (0.91–1.55) | 1.07 (0.82–1.40) | – | 1.47 (1.13–1.92) | 1.30 (0.99–1.69) | – |
| IS | 3.26 | 570 | 0.93 (0.67–1.27) | 0.90 (0.66–1.23) | – | 1.10 (0.80–1.50) | 0.97 (0.71–1.34) | – |
| HF | 6.16 | 1070 | 1.43 (1.18–1.74) | 1.32 (1.09–1.61) | 2.5 | 1.69 (1.39–2.06) | 1.47 (1.20–1.79) | 3.1 |
| Microvascular complications | ||||||||
| Overall | 15.42 | 2579 | 1.30 (1.15–1.47) | 1.29 (1.14–1.46) | 2.0 | 1.31 (1.15–1.50) | 1.20 (1.05–1.37) | 1.2 |
| DKD | 6.42 | 1114 | 1.36 (1.13–1.65) | 1.31 (1.09–1.59) | 2.3 | 1.22 (0.99–1.51) | 1.09 (0.88–1.36) | – |
| DN | 3.33 | 582 | 2.06 (1.66–2.57) | 1.96 (1.57–2.45) | 7.8 | 1.62 (1.26–2.09) | 1.49 (1.16–1.93) | 3.8 |
| DR | 8.10 | 1393 | 0.96 (0.80–1.16) | 0.99 (0.83–1.20) | – | 1.25 (1.04–1.49) | 1.13 (0.94–1.35) | – |
| All-cause death | 12.16 | 2157 | 1.39 (1.21–1.59) | 1.26 (1.09–1.44) | 2.0 | 1.38 (1.18–1.60) | 1.26 (1.08–1.47) | 1.6 |
| CVD-specific death | 2.62 | 465 | 1.36 (1.01–1.83) | 1.26 (0.93–1.70) | – | 1.12 (0.79–1.59) | 1.01 (0.71–1.44) | – |
HR1 (95% CI): adjusted for age and sex.
HR2 (95% CI): adjusted for age, sex, ethnicity, Townsend deprivation index, smoking status, alcohol intake, body mass index, family history of cardiovascular disease, family history of diabetes, diabetes duration, use of hypoglycemic agents, and use of lipid-lowing medication.
Major depressive disorder determined based on hospital inpatient admission records and self-report, and depressive symptoms assessed using the Patient Health Questionnaire-2.
ASCVD, atherosclerotic cardiovascular disease; CAD, coronary artery disease; DKD, diabetic kidney disease; DN, diabetic neuropathy; DR, diabetic retinopathy; HF, heart failure; HR, hazard ratio; IS, ischemic stroke; PAD, peripheral artery disease; PAF, population attributable fraction.
A similar association pattern was observed for depressive symptoms. The HR (95% CI) was 1.47 (1.20–1.79) with HF, 1.25 (1.10–1.42) with ASCVD, 1.27 (1.10–1.47) with CAD, 1.20 (1.05–1.37) with total microvascular complications, and 1.49 (1.16–1.93) with DN, and the PAFs to the outcomes ranged from 1.2% to 3.8%. The associations of depressive symptoms with PAD, IS, DKD, and DR were not significant in this population.
The individuals with MDD or depressive symptoms also demonstrated a higher risk of all-cause death, with HR (95% CI) of 1.26 (1.09–1.44) for MDD and 1.26 (1.08–1.47) for depressive symptoms, and PAF of 2.0% for MDD and 1.6% for depressive symptoms. However, no excess risk of CVD-specific death was observed in the individuals with MDD or depressive symptoms.
Mediation analyses
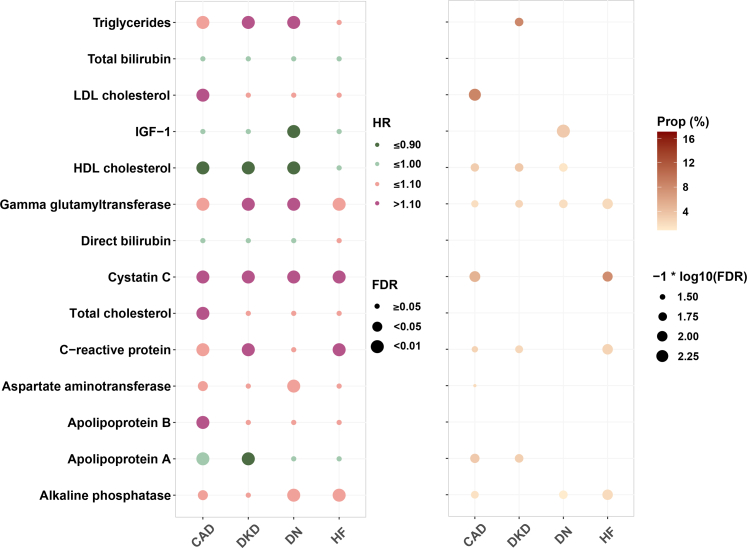
MDD was significantly associated with 14 of 22 circulating metabolites involving lipid profile, liver function, renal function, IGF-1, and inflammation at FDR <0.05. As shown in Table S4, the adjusted regression coefficients (95% CIs) ranged from −0.14 (−0.20, −0.09) to 0.17 (0.11, 0.22). The associations between selected biomarkers and all outcomes are shown in Table S5. A total of 11 significant mediators were identified in the association of MDD with CAD and 4 in the association with HF, mainly involving lipid profile, renal function, and inflammation. The mediators accounted for 1.6%–9.8% of the MDD-CAD association and 2.1%–7.8% of the MDD-HF association. Specifically, the relationship between MDD and the risk of DKD was mediated by cystatin C, GGT, HDL-C, triglycerides, CRP, and apolipoprotein A, with the proportions of mediation effect of 2.3%–17.0%. The association between MDD and DN explained by the selected metabolites was relatively low (up to 4.6% by triglycerides) (Fig. 2).
Fig. 2.
Associations and mediation effects of MDD-related metabolic biomarkers with incident CAD, HF, DKD, and DN among people with type 2 diabetes Models adjusted for age, sex, ethnicity, Townsend deprivation index, smoking status, alcohol intake, body mass index, family history of cardiovascular disease, family history of diabetes, duration of diabetes, use of hypoglycemic agents, and use of lipid-lowing medication. CAD, coronary artery disease; DKD, diabetic kidney disease; DN, diabetic neuropathy; FDR, false discovery rate; HDL, high-density lipoprotein; HF, heart failure; IGF-1, insulin-like growth factor-1; LDL, low-density lipoprotein; MDD, major depressive disorder.
After summarizing the mediating metabolites in principal components, we found that the mediators jointly explained 21.39% (95% CI: 12.18–46.91), 10.49% (95% CI: 3.31–31.61), 26.87% (95% CI: −10.56 to 109.94), and 7.29% (95% CI: 3.72–11.92) of the associations of MDD with CAD, HF, DKD, and DN, respectively (Table S6). The decomposed effects of MDD on vascular complications mediated by selected circulating metabolites are shown in Table S7.
After excluding the users of lipid-lowering agents, we found that the associations remained significant for MDD with 10 of 16 significant biomarkers identified in all individuals (Table S4), and for 8 selected circulating metabolites with at least one vascular complication (Table S5). Eight significant mediators were detected in the associations of MDD with the risk of vascular complications: ALT, apolipoprotein A, apolipoprotein B, AST, TC, IGF-1, LDL-C, and triglycerides (Table S8).
Subgroup and sensitive analyses
Generally, the associations of MDD and depressive symptoms with vascular complications were consistently observed across subgroups stratified by sex (male, female) (Figure S5), age (≤60, >60 years) (Figure S6), diabetes duration (≤5, >5 years) (Figure S7), smoking status (never, ever) (Figure S8), and use of lipid-lowing agents (yes, no) (Figure S9). The associations between MDD or depressive symptoms and the outcomes were seldom modified by the factors. We only observed significant interactions between MDD and smoking on DN, between depressive symptoms and use of lipid-lowing agents or duration of diabetes on DN, and between MDD and use of lipid-lowing agents on IS (all p for interaction <0.05).
Sensitivity analyses did not show substantial changes in the mediation effects of the selected biomarkers in the associations of MDD with the outcomes by excluding the events within 2 years of follow-up, including incident T2D patients during the follow-up, using Fine–Gray models to perform competing risk analyses, or by regarding the users of antidepressant medications as individuals with MDD (Tables S9–S12). Table S13 shows the results of the four-way effect decomposition analysis. No significant interaction was found for MDD with the mediating metabolites (all p for interaction >0.05). Therefore, excess relative risks of MDD on vascular complications compared to non-MDD were attributable to the controlled direct and pure indirect effects (mediation only).
Discussion
In this prospective cohort study including 13,706 UK adults with T2DM, we found that people with comorbid MDD or depressive symptoms had a higher risk of multiple adverse vascular complications, including HF, total ASCVD, CAD, total microvascular complications, DKD, and DN, with up to 7.8% of the incident adverse complications attributable to MDD and 3.8% to depressive symptoms. Moreover, 7.29%–26.87% of the associations between MDD and vascular complications were jointly explained by a series of biomarkers involving lipid profile, renal function, and inflammation.
The positive associations of MDD and depressive symptoms with incident macrovascular events in this study were generally consistent with previous studies and demonstrated equally substantial increased risks of vascular complications among individuals with T2DM.6,7,36 In a cohort study involving 259,875 Scottish patients with T2DM, the individuals with a history of MDD had a 1.59-fold higher risk of developing composite CVD events.6 Similar results were observed in two cohort studies but with a slightly lower magnitude of the associations.7,36 However, these previous studies mainly focused on CVD events, and the evidence for specific vascular events is still limited, which may not be conducive to tailored prevention strategies. In this study, we find that the associations of MDD and depressive symptoms with the composite ASCVD events were significant only for CAD but not for IS and PAD, inconsistent with the reported 19% elevated risk of IS in patients with depression relevant to those without any mental disorders in South Korea.36 Due to the different control settings, further research is needed to evaluate the associations and elucidate the potential biological mechanisms. However, given the wide confidence intervals in our analysis, the non-significant associations of MDD or depressive symptoms with PAD and IS may be due to insufficient statistical power. Longer follow-up of the study participants is warranted to reveal the associations. Regarding the risk of HF, we provide the first prospective evidence for the association of MDD with the development of the disease among adults with T2DM, which may be used to screen high-risk populations.
Prior research on MDD or depressive symptoms and microvascular complications in people with diabetes has produced inconsistent findings. In line with our results, a longitudinal study including 4623 patients with T2DM demonstrated a significant association of depression symptoms assessed by the PHQ-9 with the risk of total microvascular complications.7 In a matched cohort study in Taiwan covering 192,685 diabetes adults, however, no significant association was observed between MDD and microvascular complications,36 which may be explained by the inclusion of patients with type 1 diabetes and the broad definitions of the outcomes. In this study, we evaluated the relationship of MDD and depressive symptoms with individual microvascular complications and observed significant associations of MDD and depressive symptoms with DN and DKD. Of note, the associations of MDD and depressive symptoms with DN were stronger than those with other microvascular complications, suggesting the pivotal role of depression in nerve damage in the population with T2DM.37 In addition, the association with DKD was only observed for MDD but not for depressive symptoms, indicating the importance of containing the progression of depressive symptoms to MDD in the prevention of DKD. The null association of MDD and depressive symptoms with DR may be partly explained by reduced retinopathy screening in people concurrently with T2DM and psychiatric disorders (including depression).38
Identifying mediators, especially those modifiable, might gain better insight into the underlying mechanisms and facilitate the development of optimal interventions in clinical and public health practice.39 Our data show that the MDD-related risk of CAD was mainly explained by the lipid profile, i.e. the levels of triglycerides, LDL-C, HDL-C apolipoprotein A, apolipoprotein B, and TC. The results were supported by the significantly attenuated associations observed among the users of lipid-lowering agents. Biologically, MDD is characterised by an overall increase in oxidative damage to lipids and significantly reduced reverse cholesterol transport, which could promote atherogenicity, lipid peroxidation, and inflammation.17,40 Moreover, cystatin C possessed relatively high mediation proportions in the relationship between MDD and DKD (17.0%) and HF (7.8%), which is biologically plausible. Observational studies showed that elevated cystatin C is an independent risk factor for depression and continues to worsen in patients.18,41 In addition to being a direct marker of glomerular filtration rate,42 cystatin C is significantly associated with heart failure.43 Of note, despite the strong association of MDD with DN observed, the extent of explanation by the identified mediators is relatively limited (7.3%). Although the exact pathophysiological mechanisms of DN remain unclear, hyperlipidemia may be one of the key factors.44 This is supported by the significant mediating effect of triglycerides (4.6%) in this study.
This exploratory study is among the first to investigate the relationship between MDD or depressive symptoms and individual vascular complications of diabetes. The strengths of this study included the prospective study design, the large sample size (tens of thousands), and the long-term follow-up (median follow-up of 13 years), which enabled us not only to examine the associations of MDD and depressive symptoms with the risks of composite vascular complications in UK adults with T2DM, but also the risks of specific outcomes, including HF, CAD, IS, PAD, DKD, DN, and DR. The extensive data collection on circulating metabolites allows us to identify potential mediators from a series of metabolic biomarkers, and comprehensively evaluate the potential mechanisms underlying the observed associations. Finally, we applied multiple statistical methods to evaluate the associations of MDD and depressive symptoms with vascular complications, estimated the mediation effects of circulating metabolites in the associations, and performed various sensitivity analyses to test the robustness of our results, all of which ensured the accuracy of our results.
However, this study should be interpreted in light of its potential limitations. First, as the incident vascular complications were identified through hospital inpatient records, part of primary care data (about half of the study population), and death registries, there may be underreporting of the outcomes. Full linkage to primary care data is warranted for its huge advantages in improving the ascertainment of diabetes complications at baseline and during follow-up. Second, depressive symptoms were assessed using one-time PHQ-2 at recruitment, which might change over the follow-up period; therefore, the exposure data might be prone to measurement error, leading to non-differential misclassification bias and attenuating the associations. However, MDD in the UK Biobank was diagnosed by medical professionals, minimizing the potential measurement error. Similarly, we used the biomarkers measured at baseline only, which may lead to an underestimation of the magnitude of the associations between the biomarkers and outcomes. However, we did not find a significant difference between the measurements at baseline (2006–2010) and those at the first repeat assessment (2012–2013) (data not shown), partly mitigating our concern about the possible information bias. In addition, the assessment of depressive symptoms using the simplified PHQ-2 may introduce information bias. However, the results for depressive symptoms measured by the PHQ-9 remained unchanged (data not shown), mitigating our concern about the potential misclassification bias. Third, the potential false negatives may have led to differential or non-differential misclassification bias. If false negatives occurred in both exposed and unexposed groups independent of depressive status, the induced non-differential misclassification bias may have resulted in underestimated associations; if false negatives occurred less in the exposed group due to more opportunities to detect diabetic complications, the differential misclassification bias may have led to underestimation or overestimation of the associations. Fourth, mediation analyses assume the causality of MDD with the biomarkers and vascular complications among individuals with T2DM, which largely remain unclear. Fortunately, MDD was determined before the baseline survey, guaranteeing the temporality of diagnosis of MDD, measurements of circulating metabolites, and incident vascular outcomes in mediation analysis. Furthermore, since not all eigenvalues of the principal component analysis (only >95%) were used for the mediation analysis, the mediation effect of the biomarkers may be underestimated. Fifth, although we have made great efforts to adjust for potential confounders, residual or unknown confounders might exist due to the observational design of the study, which may have biased our results. Finally, as a majority of the participants of the UK Biobank were of British or Irish ancestry, our findings may not be fully generalised to other racial and ethnic populations. Further studies are warranted to test our results in other ethnic populations.
In conclusion, this prospective study contributes to the literature regarding the potential effect of MDD or depressive symptoms on the risks of vascular complications among individuals with T2DM. Once the causality of the associations is confirmed, it may be a cost-effective measure to prevent advanced complications by prompt treatment of depression in patients with T2DM, which should be investigated in future work. Furthermore, the mediation effect of biomarkers involving lipid profile, renal function, and systemic inflammation in the associations indicates the great potential in reducing the risk of advanced complications through metabolic modification interventions.
Contributors
Concept and design: W.H.X.
Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: G.C.L.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: G.C.L., Y.F.Y. and S.C.Z.
Obtained funding: W.H.X., and Y.F.Y.
Administrative, technical, or material support: G.C.L., C.Q.L.
Supervision: W.H.X., C.Q.L., and H.T.
G.C.L., Y.F.Y., and W.H.X. have accessed and verified the underlying study data.
Data sharing statement
The data supporting the findings of this study are available from the UK Biobank (http://www.ukbiobank.ac.uk/), but there are restrictions on the availability of these data, which have been used under the license for the current study and are therefore not publicly available. This manuscript was not pre-registered.
Declaration of interests
None of the authors reported a conflict of interest related to the study.
Acknowledgements
This study was supported by the Three-Year Public Health Action Plan of Shanghai (GWVI-11.1-22 and GWVI-11.2-XD10), and conducted using the UK Biobank Resource under Application Number 98410. The UK Biobank has received ethical approval from the North West Multi-Centre Research Ethics Committee, and informed consent was given by all participants on a touch screen using a signature capture device. The data that support the findings of this study are available on application to the UK Biobank team at http://www.ukbiobank.ac.uk/. We gratefully thank all the staff and participants of the UK Biobank for their contributions to this research.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.eclinm.2024.102982.
Appendix A. Supplementary data
References
- 1.Sun H., Saeedi P., Karuranga S., et al. IDF diabetes atlas: global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183 doi: 10.1016/j.diabres.2021.109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Forbes J.M., Cooper M.E. Mechanisms of diabetic complications. Physiol Rev. 2013;93(1):137–188. doi: 10.1152/physrev.00045.2011. [DOI] [PubMed] [Google Scholar]
- 3.Roy T., Lloyd C.E. Epidemiology of depression and diabetes: a systematic review. J Affect Disord. 2012;142(Suppl):S8–S21. doi: 10.1016/S0165-0327(12)70004-6. [DOI] [PubMed] [Google Scholar]
- 4.Whooley M.A., de Jonge P., Vittinghoff E., et al. Depressive symptoms, health behaviors, and risk of cardiovascular events in patients with coronary heart disease. JAMA. 2008;300(20):2379–2388. doi: 10.1001/jama.2008.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walker R.J., Gebregziabher M., Martin-Harris B., Egede L.E. Understanding the influence of psychological and socioeconomic factors on diabetes self-care using structured equation modeling. Patient Educ Couns. 2015;98(1):34–40. doi: 10.1016/j.pec.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleetwood K.J., Wild S.H., Licence K.A.M., et al. Severe mental illness and type 2 diabetes outcomes and complications: a nationwide cohort study. Diabetes Care. 2023;46(7):1363–1371. doi: 10.2337/dc23-0177. [DOI] [PubMed] [Google Scholar]
- 7.Lin E.H., Rutter C.M., Katon W., et al. Depression and advanced complications of diabetes: a prospective cohort study. Diabetes Care. 2010;33(2):264–269. doi: 10.2337/dc09-1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ting R.Z., Lau E.S., Ozaki R., et al. High risk for cardiovascular disease in Chinese type 2 diabetic patients with major depression--a 7-year prospective analysis of the Hong Kong diabetes registry. J Affect Disord. 2013;149(1–3):129–135. doi: 10.1016/j.jad.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y., Long C., Xing Z. Depression is associated with heart failure in patients with type 2 diabetes mellitus. Front Public Health. 2023;11 doi: 10.3389/fpubh.2023.1181336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nouwen A., Adriaanse M.C., van Dam K., et al. Longitudinal associations between depression and diabetes complications: a systematic review and meta-analysis. Diabet Med. 2019;36(12):1562–1572. doi: 10.1111/dme.14054. [DOI] [PubMed] [Google Scholar]
- 11.Wu C.S., Hsu L.Y., Wang S.H. Association of depression and diabetes complications and mortality: a population-based cohort study. Epidemiol Psychiatr Sci. 2020;29 doi: 10.1017/S2045796020000049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vreeburg S.A., Hoogendijk W.J., van Pelt J., et al. Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry. 2009;66(6):617–626. doi: 10.1001/archgenpsychiatry.2009.50. [DOI] [PubMed] [Google Scholar]
- 13.Okamura F., Tashiro A., Utumi A., et al. Insulin resistance in patients with depression and its changes during the clinical course of depression: minimal model analysis. Metabolism. 2000;49(10):1255–1260. doi: 10.1053/meta.2000.9515. [DOI] [PubMed] [Google Scholar]
- 14.Qiao Y., Ding Y., Li G., Lu Y., Li S., Ke C. Role of depression in the development of cardiometabolic multimorbidity: findings from the UK Biobank study. J Affect Disord. 2022;319:260–266. doi: 10.1016/j.jad.2022.09.084. [DOI] [PubMed] [Google Scholar]
- 15.Martinac M., Pehar D., Karlovic D., Babic D., Marcinko D., Jakovljevic M. Metabolic syndrome, activity of the hypothalamic-pituitary-adrenal axis and inflammatory mediators in depressive disorder. Acta Clin Croat. 2014;53(1):55–71. [PubMed] [Google Scholar]
- 16.Marano C.M., Workman C.I., Lyman C.H., et al. The relationship between fasting serum glucose and cerebral glucose metabolism in late-life depression and normal aging. Psychiatry Res. 2014;222(1-2):84–90. doi: 10.1016/j.pscychresns.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tkachev A., Stekolshchikova E., Vanyushkina A., et al. Lipid alteration signature in the blood plasma of individuals with schizophrenia, depression, and bipolar disorder. JAMA Psychiatry. 2023;80(3):250–259. doi: 10.1001/jamapsychiatry.2022.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han T., Zhang L., Jiang W., Wang L. Persistent depressive symptoms and the changes in serum cystatin C levels in the elderly: a longitudinal cohort study. Front Psychiatry. 2022;13 doi: 10.3389/fpsyt.2022.917082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Beurel E., Toups M., Nemeroff C.B. The bidirectional relationship of depression and inflammation: double trouble. Neuron. 2020;107(2):234–256. doi: 10.1016/j.neuron.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Geng T., Zhu K., Lu Q., et al. Healthy lifestyle behaviors, mediating biomarkers, and risk of microvascular complications among individuals with type 2 diabetes: a cohort study. PLoS Med. 2023;20(1) doi: 10.1371/journal.pmed.1004135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alruwaili N.S., Al-Kuraishy H.M., Al-Gareeb A.I., et al. Antidepressants and type 2 diabetes: highways to knowns and unknowns. Diabetol Metab Syndr. 2023;15(1):179. doi: 10.1186/s13098-023-01149-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sudlow C., Gallacher J., Allen N., et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3) doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Collins R. What makes UK Biobank special? Lancet. 2012;379(9822):1173–1174. doi: 10.1016/S0140-6736(12)60404-8. [DOI] [PubMed] [Google Scholar]
- 24.Eastwood S.V., Mathur R., Atkinson M., et al. Algorithms for the capture and adjudication of prevalent and incident diabetes in UK biobank. PLoS One. 2016;11(9) doi: 10.1371/journal.pone.0162388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gao X., Geng T., Jiang M., et al. Accelerated biological aging and risk of depression and anxiety: evidence from 424,299 UK Biobank participants. Nat Commun. 2023;14(1):2277. doi: 10.1038/s41467-023-38013-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Han X., Hou C., Yang H., et al. Disease trajectories and mortality among individuals diagnosed with depression: a community-based cohort study in UK Biobank. Mol Psychiatry. 2021;26(11):6736–6746. doi: 10.1038/s41380-021-01170-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lowe B., Wahl I., Rose M., et al. A 4-item measure of depression and anxiety: validation and standardization of the Patient Health Questionnaire-4 (PHQ-4) in the general population. J Affect Disord. 2010;122(1–2):86–95. doi: 10.1016/j.jad.2009.06.019. [DOI] [PubMed] [Google Scholar]
- 28.Dregan A., Rayner L., Davis K.A.S., et al. Associations between depression, arterial stiffness, and metabolic syndrome among adults in the UK biobank population study: a mediation analysis. JAMA Psychiatr. 2020;77(6):598–606. doi: 10.1001/jamapsychiatry.2019.4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harshfield E.L., Pennells L., Schwartz J.E., et al. Association between depressive symptoms and incident cardiovascular diseases. JAMA. 2020;324(23):2396–2405. doi: 10.1001/jama.2020.23068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semenkovich K., Brown M.E., Svrakic D.M., Lustman P.J. Depression in type 2 diabetes mellitus: prevalence, impact, and treatment. Drugs. 2015;75(6):577–587. doi: 10.1007/s40265-015-0347-4. [DOI] [PubMed] [Google Scholar]
- 31.Alexopoulos G.S. Depression in the elderly. Lancet. 2005;365(9475):1961–1970. doi: 10.1016/S0140-6736(05)66665-2. [DOI] [PubMed] [Google Scholar]
- 32.Lee I.M., Shiroma E.J., Lobelo F., et al. Effect of physical inactivity on major non-communicable diseases worldwide: an analysis of burden of disease and life expectancy. Lancet. 2012;380(9838):219–229. doi: 10.1016/S0140-6736(12)61031-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crouse W.L., Keele G.R., Gastonguay M.S., Churchill G.A., Valdar W. A Bayesian model selection approach to mediation analysis. PLoS Genet. 2022;18(5) doi: 10.1371/journal.pgen.1010184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Si J., Li J., Yu C., et al. Improved lipidomic profile mediates the effects of adherence to healthy lifestyles on coronary heart disease. Elife. 2021;10 doi: 10.7554/eLife.60999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.VanderWeele T.J. A unification of mediation and interaction: a 4-way decomposition. Epidemiology. 2014;25(5):749–761. doi: 10.1097/EDE.0000000000000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim H., Lee K.N., Shin D.W., Han K., Jeon H.J. Association of comorbid mental disorders with cardiovascular disease risk in patients with type 2 diabetes: a nationwide cohort study. Gen Hosp Psychiatry. 2022;79:33–41. doi: 10.1016/j.genhosppsych.2022.10.005. [DOI] [PubMed] [Google Scholar]
- 37.Song Y., Cao H., Zuo C., et al. Mitochondrial dysfunction: a fatal blow in depression. Biomed Pharmacother. 2023;167 doi: 10.1016/j.biopha.2023.115652. [DOI] [PubMed] [Google Scholar]
- 38.Petersen G.B., Byberg S., Vistisen D., et al. Factors associated with nonattendance in a nationwide screening program for diabetic retinopathy: a register-based cohort study. Diabetes Care. 2022;45(2):303–310. doi: 10.2337/dc21-1380. [DOI] [PubMed] [Google Scholar]
- 39.Li G., Jankowich M.D., Lu Y., Wu L., Shao L., Ke C. Preserved ratio impaired spirometry, metabolomics, and the risk of type 2 diabetes. J Clin Endocrinol Metab. 2023;108(9):e769–e778. doi: 10.1210/clinem/dgad140. [DOI] [PubMed] [Google Scholar]
- 40.Almulla A.F., Thipakorn Y., Algon A.A.A., Tunvirachaisakul C., Al-Hakeim H.K., Maes M. Reverse cholesterol transport and lipid peroxidation biomarkers in major depression and bipolar disorder: a systematic review and meta-analysis. Brain Behav Immun. 2023;113:374–388. doi: 10.1016/j.bbi.2023.08.007. [DOI] [PubMed] [Google Scholar]
- 41.Tanifuji T., Okazaki S., Otsuka I., et al. Epigenetic clock analysis reveals increased plasma cystatin C levels based on DNA methylation in major depressive disorder. Psychiatry Res. 2023;322 doi: 10.1016/j.psychres.2023.115103. [DOI] [PubMed] [Google Scholar]
- 42.Shlipak M.G., Inker L.A., Coresh J. Serum cystatin C for estimation of GFR. JAMA. 2022;328(9):883–884. doi: 10.1001/jama.2022.12407. [DOI] [PubMed] [Google Scholar]
- 43.van der Laan S.W., Fall T., Soumare A., et al. Cystatin C and cardiovascular disease: a mendelian randomization study. J Am Coll Cardiol. 2016;68(9):934–945. doi: 10.1016/j.jacc.2016.05.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feldman E.L., Callaghan B.C., Pop-Busui R., et al. Diabetic neuropathy. Nat Rev Dis Primers. 2019;5(1):42. doi: 10.1038/s41572-019-0097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.