Abstract
Background
The relationship between radiographic disc calcification score and FGF4L2 genotype has been reported in only a small number of dachshunds.
Hypothesis
A genotype with either 0 or 1 FGF4L2 copy will be associated with a lower number of calcified discs (lower K‐n) compared with a genotype with 2 FGF4L2 copies.
Animals
Dachshunds registered with the Norwegian or Finnish Kennel Clubs for which both K‐n and FGF4L2 genotype are known (n = 407).
Methods
Retrospective study (2012‐2024). The frequency and percentage of dachshunds within each K‐n group (K0, K1/2, K3/4, K5+) by FGF4L2 genotype (FGF4L2/FGF4L2, N/FGF4L2, N/N) were reported. The K‐n distribution differences among genotypes were analyzed by the Cochran‐Mantel‐Haenszel test in SAS 9.4. Significance was defined as P < .05.
Results
A difference in K‐n distribution was found between dachshunds that have 0 or 2 FGF4L2 copies. However, no difference was found in the K‐n distribution between dachshunds that have 1 or 2 FGF4L2 copies.
Conclusions and Clinical Importance
Most dachshunds with 1 FGF4L2 copy have radiographic disc calcification scores that are associated with substantially lower risk of symptomatic disc disease. Radiographic disc calcification scores are similar in dachshunds with 0 and 1 FGF4L2 copies. Given the high allele frequency of FGF4L2, breeding to produce progeny with 1 FGF4L2 copy is expected to be a more achievable short‐term goal for dachshund spinal health breeding programs than breeding for 0 copies. It is recommended that both K‐n and FGF4L2 genotype status be considered when choosing dachshund breeding stock.
Keywords: 12‐FGF4, CDDY, chondrodystrophy, disc calcification, K‐number, type 1 intervertebral disc disease
Abbreviations
- CDDY
chondrodystrophy
- FGF4L2
fibroblast growth factor 4 like 2
- IVDD
intervertebral disc disease
- K‐n
K‐number
- NSDTR
Nova Scotia duck tolling retriever
- RDDC
radiographically detectable disc calcifications
- SDD
symptomatic disc disease
1. INTRODUCTION
Hansen's type I intervertebral disc disease (IVDD) is a common disorder affecting the spine of dogs, often resulting in pain and paralysis. 1 The disorder disproportionately affects chondrodystrophic dogs, and the breed with the highest incidence is the dachshund, 2 , 3 with approximately 18% to 25% of dachshunds exhibiting symptomatic disc disease (SDD) over their lifetime. 3 , 4 , 5 , 6 , 7 The estimated worldwide cumulative costs of veterinary care to treat disc disease in chondrodystrophic dogs is in the millions to billions of dollars, making this disorder 1 of the most burdensome to dogs and owners alike. 8
Type I IVDD is characterized by early disc degeneration that causes calcification of some of the intervertebral discs. These degenerated discs may herniate and result in spinal cord compression. 9 Dachshunds with radiographically detectable disc calcifications (RDDC) have been shown to be more likely to develop SDD than those without RDDC. 10 Radiographically detectable disc calcifications also were shown to be heritable. 6 , 10 , 11 , 12 The number of RDDC was shown to increase in young adulthood and then to level off or decrease, with peak number of RDDC occurring between 2 and 4 years of age. 10 , 13 A radiographic disc calcification scoring system was developed in Denmark that assigns a K‐number (K‐n, from the Nordic pronunciation of the word “calcified,” kalki/kalkki) to a dachshund equivalent to the number of RDDC on whole spine radiographs taken between 2 and 4 years of age. 14
Combined reported incidence of SDD in 305 dachshunds across 3 publications showed the relationship of SDD to the dachshund's K‐n as follows: 7% incidence of SDD for K0, 12% incidence of SDD for K1/2, 23% incidence of SDD for K3/4, and 69% incidence of SDD for K5+. 15 , 16 , 17 Based on the reported relationship between SDD and K‐n, Nordic dachshund stakeholders developed breeding schemes to improve spinal health in the breed. The schemes vary in details, but generally discourage the use of high K‐n dachshunds and encourage the use of low K‐n dachshunds for breeding. Spinal health breeding schemes based on K‐n have since been implemented in Nordic countries, the United Kingdom, Germany, South Africa, and Australia.
Research into the genetic basis of disc calcification led to the discovery of a causal variant associated with RDDC in dachshunds on chromosome 12, 18 later determined to be a fibroblast growth factor‐4 retrogene insertion (originally 12‐FGF4, now FGF4L2) dominant for both chondrodystrophy and type I IVDD in dogs. 19 However, results of a computed tomography‐based study in young asymptomatic Nova Scotia duck tolling retrievers (NSDTR) suggested that there may be a gene dose effect of FGF4L2 in relation to early disc degeneration in that breed. 20 The NSDTR study showed that all but 1 FGF4L2 heterozygote had calcified discs but approximately half as many calcified discs were seen in FGF4L2 heterozygotes compared with homozygotes. In dachshunds, the FGF4L2 insertion has a very high allele frequency (0.92). 21 The FGF4L2 insertion became colloquially known as CDDY (for “chondrodystrophy”) and was added to many direct‐to‐consumer genetic panels used by dachshund breeders without specific guidance as to how to use FGF4L2 status to inform breeding decisions. 20 , 22
Our aim was to describe the relationship between radiographic disc calcification score (K‐n) and FGF4L2 genotype in dachshunds, and to provide guidance to dachshund breed clubs as to how to incorporate FGF4L2 genotype status into breeding schemes. Our hypothesis was that a genotype with either 0 or 1 FGF4L2 copies will be associated with a lower number of calcified discs (lower K‐n) compared with a genotype with 2 FGF4L2 copies.
2. MATERIALS AND METHODS
2.1. Study design
Ours was a retrospective study in dachshunds (n = 407) from January 2012 until July 2024.
2.2. Inclusion criteria
Eligible dachshunds were those that had been registered with the Finnish or Norwegian Kennel Clubs and for which both K‐n and FGF4L2 genotype data were available. To be included in the study, the dachshund must have both: (1) a K‐n assigned according to standard kennel club procedures in their country of registration, and the results recorded to that country's national public dachshund pedigree database (a requirement of the scoring process), and (2) an FGF4L2 genotype of the dachshund voluntarily recorded to the relevant country's national public dachshund pedigree database (Finland, n = 234; Norway, n = 173).
2.3. Data acquisition
Live animals were not used in this study. The data were acquired from Nordic dachshund breed club representatives as part of the process of building a combined Nordic public dachshund health database. Because the data used in our study was voluntarily contributed by the dog owners to a public dachshund pedigree database and because no information relating to the dog owners was collected, owner consent was not needed. The data acquired included: country of registration, K‐n (radiographic disc calcification score), and FGF4L2 genotype (FGF4L2/FGF4L2, N/FGF4L2, or N/N). The data from the dogs then were placed into K‐n groups that correlate with risk categories of SDD: K0 (very low risk), K1/2 (approximately half the breed average risk), K3/4 (approximately breed average risk), and K5+ (very high risk).
2.4. Statistical analysis
Allele frequency of FGF4L2 was calculated by summing the total number of FGF4L2 insertions in the study population and dividing by the total number of alleles in the study population. The frequency and percentage of dachshunds within each K‐n group (K0, K1/2, K3/4, K5+) by FGF4L2 genotype (FGF4L2/FGF4L2, N/FGF4L2, N/N) was summarized and the distribution of K‐n by genotype graphed. Because of the ordinal nature of the variable K‐n, K‐n distribution differences among genotypes were tested by the Cochran‐Mantel‐Haenszel test. Using a Bonferroni corrected Type I error rate of 0.0167, pair‐wise comparisons were made to identify which genotypes produced different K‐n distributions. All analyses were conducted by SAS 9.4 (programming code available upon request). Significance was determined as P < .05.
3. RESULTS
The allelic frequency for FGF4L2 was 0.88. The relationship between K‐n and FGF4L2 genotype is summarized in Table 1 and the distribution of K‐n by genotype is presented in Figure 1.
TABLE 1.
K‐n and FGF4L2 genotype in Nordic dachshunds.
| K‐number | FGF4L2/FGF4L2 | % | N/FGF4L2 | % | N/N | % | Total | % |
|---|---|---|---|---|---|---|---|---|
| K0 | 84 | 26% | 60 | 75% | 6 | 86% | 150 | 37% |
| K1/2 | 119 | 37% | 18 | 23% | 1 | 14% | 138 | 34% |
| K3/4 | 67 | 21% | 1 | 1% | 0 | 0% | 68 | 17% |
| K5+ | 50 | 16% | 1 | 1% | 0 | 0% | 51 | 13% |
| Total | 320 | 100% | 80 | 100% | 7 | 100% | 407 | 100% |
Note: Numbers in columns under genotypes are the number of dogs with that genotype for a given K‐n.
FIGURE 1.
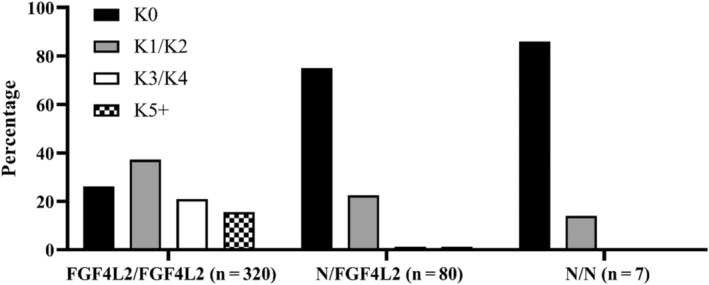
The distribution of K‐n across genotypes.
When comparing the distribution of K‐n (K0, K1/2, K3/4, K5+) across the 3 genotypes (FGF4L2/FGF4L2, N/FGF4L2, N/N), significant differences were found across genotypes (χ 2 = 63.9358, degrees of freedom = 2, P < .0001). Conducting pair‐wise comparisons among the genotypes, a significant difference in K‐n distribution was found between FGF4L2/FGF4L2 and N/FGF4L2 genotypes (χ 2 = 58.2168, degrees of freedom = 1, P < .0001). Also, a significant difference in K‐n distribution was found between the FGF4L2/FGF4L2 and N/N genotypes (χ 2 = 8.2152, degrees of freedom = 1, P = .004). However, the K‐n distribution between N/FGF4L2 and N/N (χ 2 = 0.4563, degrees of freedom = 1, P = .5) was not different.
4. DISCUSSION
Our data show that regarding FGF4L2 genotype, the presence of 1 N allele is associated with the best radiographic disc calcification scores (K0, K1, K2) in dachshunds and that rarely does a dachshund with 1 N allele have the worst radiographic disc calcification score (K5+). Additionally, the data show that although dachshunds with 2 N alleles have numerically lower radiographic disc calcification scores than dachshunds with 1 N allele, this difference was not significant. Furthermore, nearly all dogs with 1 or 2 N alleles have spinal scores (K0, K1, K2) that are associated with a risk of SDD that is substantially lower than average risk for the breed.
The radiographic disc calcification score (K‐n) was developed as an indicator of the degree of early disc degeneration. It is well established that young asymptomatic dachshunds with fewer disc calcifications (equivalent to a lower radiographic disc calcification score) have a lower risk of SDD. 10 , 15 , 16 , 17 The K0 radiographic score is associated with an approximately 7% risk of SDD in dachshunds, 15 , 16 , 17 which is much less than the breed average risk of 18% to 25%. 3 , 4 , 5 , 6 , 7 Our data show that 75% of dachshunds with 1 N allele and 86% of dachshunds with 2 N alleles have a radiographic disc calcification score of K0. Therefore, most dachshunds with ≥1 N allele have substantially decreased risk of SDD when compared with breed average.
Dachshunds with radiographic disc calcification scores of K1/2 have an approximately 12% risk of SDD 15 , 16 , 17 and when combined with K0 dachshunds, K0/1/2 dogs comprise a group with <50% of the breed average risk of SDD. Our results show that 98% of dachshunds with at least 1 N allele have a radiographic disc calcification score of K0, 1 or 2. Therefore, nearly all dachshunds with ≥1 N allele have a risk of SDD that is less than half of the breed average.
Dachshunds with a radiographic disc calcification score of K5 or higher have a 69% risk of SDD, and many of these dogs eventually are euthanized because of repeated disc herniation. 15 , 16 , 17 Our results show that only 1% of dachshunds with 1 N allele and 0% of dachshunds with 2 N alleles have a radiographic disc calcification score of K5+. Therefore, almost no dachshunds with ≥1 N allele are at high risk of SDD.
These findings may seem surprising given the earlier report that the FGF4L2 insertion acts in a dominant manner, 23 which suggested that 1 or 2 FGF4L2 copies result in equally poor spinal phenotype in dogs. However, our results are consistent with those of the NSDTR study 20 and suggest that a gene dose effect for disc calcification may be the rule across dog breeds, rather than an exception seen in the NSDTR breed. Furthermore, although FGF4L2 may act in a dominant manner for the presence or absence of early disc degeneration (calcification), the degree of early disc degeneration as seen on radiographs (K‐n) is less with 0 or 1 FGF4L2 gene copies.
Our results are important to breed clubs that wish to develop spinal health breeding schemes, and particularly important in breeds such as dachshunds that are nearly fixed for the FGF4L2 gene. Rather than advising breeders to avoid breeding dachshunds that have the FGF4L2 insertion, our results suggest that dachshund clubs initially could recommend that breeders prioritize breeding dachshunds with at least 1 N allele, with the goal of increasing the frequency of the N allele in the population. However, in our study population of dachshunds with a relatively low FGF4L2 allele frequency of 0.88, only 22.5% of dachshunds carry at least 1 N allele. This means a substantial number of dachshunds with 2 FGF4L2 copies must be kept in the breeding pool to avoid a genetic bottleneck, and that other criteria, such as K‐n, might be useful is selecting which FGF4L2 homozygotes to breed. This approach would have the added benefit of gradually increasing the number of N/N dachshunds, a genotype that is currently too rare to assess for overall health beyond the absence of early disc degeneration. The current position of many dachshund stakeholders is that FGF4L2 genotyping is not useful when making breeding decisions in dachshunds. 24 , 25 Our results suggest that FGF4L2 genotyping will be useful in spinal health breeding schemes and that FGF4L2 status should be a consideration when making breeding decisions in dachshunds.
An important limitation of our study is related to the study population. Because the study population consisted of dachshunds that had been both radiographically scored for disc calcification and genotyped, it is likely that the population represents a group of dachshunds being considered for breeding, and that they are owned by individuals particularly concerned about spinal health. This population may have resulted from multiple generations of dachshunds with a lower K‐n compared with a randomly selected group of dachshunds. Because 26% of dogs with 2 FGF4L2 copies in our study had the K0 phenotype, it is probable that FGF4L2 is not the only determinant of K‐n in dachshunds, and the study population may be comprised of dogs that are the product of selection pressure related to multiple determinants of K‐n.
In conclusion, our research hypothesis that a genotype with either 0 or 1 FGF4L2 copies will be associated with a lower number of calcified discs (lower K‐n) compared with a genotype with 2 FGF4L2 copies was confirmed. The analysis of the current data sets does not provide statistical evidence for a difference in K‐n distribution between the FGF4L2/N and N/N genotypes, but it is possible that this evidence is lacking because of the small number of N/N dogs (n = 7). Radiographic disc calcification scores do not differ between dachshunds with 1 or 0 FGF4L2 copies. Most FGF4L2/N dachshunds have K‐n values that are associated with substantially decreased risk of SDD compared with the reported breed average risk. Given the high allele frequency of FGF4L2, breeding to produce progeny with the FGF4L2/N genotype is expected to be a more achievable short‐term goal for dachshund spinal health breeding programs than breeding for 0 copies. It is recommended that both K‐n and genotype be considered when choosing dachshund breeding stock.
CONFLICT OF INTEREST DECLARATION
The database was compiled by including all public spinal score and genotype data available for dachshunds in Finland and Norway. These countries were chosen because they are the only countries with large databases that contain both data points. To minimize bias, no data was excluded from the study.
OFF‐LABEL ANTIMICROBIAL DECLARATION
Authors declare no off‐label use of antimicrobials.
INSTITUTIONAL ANIMAL CARE AND USE COMMITTEE (IACUC) OR OTHER APPROVAL DECLARATION
No live animals were used in this study, and the Auburn University IACUC advised that approval was not needed.
HUMAN ETHICS APPROVAL DECLARATION
All data used is available as part of public databases, no data was collected that could identify a human, and Auburn University Institutional Review Board advised that approval was not needed.
ACKNOWLEDGMENT
No funding was received for this study. The authors thank Ida Sorensen and Niklas Karlsson for their efforts in compiling the dataset used in this study, and to the Norwegian Federation of Dachshund Clubs, Finnish Dachshund Club, Norwegian Kennel Club, and Finnish Kennel Clubs for building, maintaining and encouraging the use of the public dachshund pedigree and health databases.
Sullivan S, Redden D, Hardeng F, Sundqvist M, Kutzler M. The relationship between radiographic disc calcification score and FGF4L2 genotype in dachshunds. J Vet Intern Med. 2025;39(1):e17281. doi: 10.1111/jvim.17281
REFERENCES
- 1. Olby NJ, Moore SA, Brisson B, et al. ACVIM consensus statement on diagnosis and management of acute canine thoracolumbar intervertebral disc extrusion. J Vet Intern Med. 2022;36(5):1570‐1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bergknut N, Egenvall A, Hagman R, et al. Incidence of intervertebral disk degeneration‐related diseases and associated mortality rates in dogs. J Am Vet Med Assoc. 2012;240(11):1300‐1309. [DOI] [PubMed] [Google Scholar]
- 3. Simpson ST. Intervertebral disc disease. Vet Clin North Am Small Anim Pract. 1992;22(4):889‐897. [DOI] [PubMed] [Google Scholar]
- 4. Ball MU, McGuire J, Swaim SF, Hoerlein BF. Patterns of occurrence of disk disease among registered dachshunds. J Am Vet Med Assoc. 1982;180(5):519‐522. [PubMed] [Google Scholar]
- 5. Goggin JE, Li AS, Franti CE. Canine intervertebral disk disease: characterization by age, sex, breed, and anatomic site of involvement. Am J Vet Res. 1970;31(9):1687‐1692. [PubMed] [Google Scholar]
- 6. Stigen O. Calcification of intervertebral discs in the dachshund: a radiographic study of 115 dogs at 1 and 5 years of age. Acta Vet Scand. 1996;37(3):229‐237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lappalainen A, Norrgård M, Alm K, Snellman M, Laitinen O. Calcification of the intervertebral discs and curvature of the radius and ulna: a radiographic survey of Finnish miniature dachshunds. Acta Vet Scand. 2001;42(2):229‐236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dickinson PJ, Bannasch DL. Current understanding of the genetics of intervertebral disc degeneration. Front Vet Sci. 2020;7:431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hansen HJ. A pathologic‐anatomical study on disc degeneration in dog, with special reference to the so‐called enchondrosis intervertebralis. Acta Orthop Scand Suppl. 1952;11:1‐117. [DOI] [PubMed] [Google Scholar]
- 10. Havranek‐Balzaretti B. Beitrag zur Aetiologie der Dackellähme und Vorschlag zur züchterischen Selektion [The Etiology of Intervertebral Disc Disease in the Dachshund and Proposal of an Eradication Programme]. Zurich: Veterinary Surgery Clinic and Institute of Veterinary Pathology, University of Zurich; 1980:66. [Google Scholar]
- 11. Jensen VF, Christensen KA. Inheritance of disc calcification in the dachshund. J Vet Med A Physiol Pathol Clin Med. 2000;47(6):331‐340. [DOI] [PubMed] [Google Scholar]
- 12. Stigen O, Christensen K. Calcification of intervertebral discs in the dachshund: an estimation of heritability. Acta Vet Scand. 1993;34(4):357‐361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Jensen VF, Arnbjerg J. Development of intervertebral disk calcification in the dachshund: a prospective longitudinal radiographic study. J Am Anim Hosp Assoc. 2001;37(3):274‐282. [DOI] [PubMed] [Google Scholar]
- 14. Mogensen MS, Karlskov‐Mortensen P, Proschowsky HF, et al. Genome‐wide association study in dachshund: identification of a major locus affecting intervertebral disc calcification. J Hered. 2011;102(Suppl 1):S81‐S86. [DOI] [PubMed] [Google Scholar]
- 15. Bruun CS, Bruun C, Marx T, Proschowsky HF, Fredholm M. Breeding schemes for intervertebral disc disease in dachshunds: is disc calcification score preferable to genotyping of the FGF4 retrogene insertion on CFA12? Canine Med Genet. 2020;7(1):18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jensen VF, Beck S, Christensen KA, Arnbjerg J. Quantification of the association between intervertebral disk calcification and disk herniation in dachshunds. J Am Vet Med Assoc. 2008;233(7):1090‐1095. [DOI] [PubMed] [Google Scholar]
- 17. Lappalainen AK, Vaittinen E, Junnila J, Laitinen‐Vapaavuori O. Intervertebral disc disease in dachshunds radiographically screened for intervertebral disc calcifications. Acta Vet Scand. 2014;56(1):89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mogensen M, Scheibye‐Alsing K, Karlskov‐Mortensen P, et al. Validation of genome‐wide intervertebral disk calcification associations in dachshund and further investigation of the chromosome 12 susceptibility locus. Front Genet. 2012;3:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brown EA, Dickinson PJ, Mansour T, et al. FGF4 retrogene on CFA12 is responsible for chondrodystrophy and intervertebral disc disease in dogs. Proc Natl Acad Sci U S A. 2017;114(43):11476‐11481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bianchi CA, Marcellin‐Little DJ, Dickinson PJ, et al. FGF4L2 retrogene copy number is associated with intervertebral disc calcification and vertebral geometry in Nova Scotia duck tolling retrievers. Am J Vet Res. 2023;84(3):1‐10. [DOI] [PubMed] [Google Scholar]
- 21. Chondrodystrophy (CDDY and IVDD) and Chondrodysplasia (CDPA). 2024. Accessed November 14, 2024. https://vgl.ucdavis.edu/test/cddy-cdpa
- 22. Chondrodystrophy and Intervertebral Disc Disease. 2024. Accessed November 14, 2024. https://embarkvet.com/products/dog-health/health-conditions/chondrodystrophy-and-intervertebral-disc-disease-cddy-ivdd-type-i-ivdd/
- 23. Batcher K, Dickinson P, Giuffrida M, et al. Phenotypic effects of FGF4 retrogenes on intervertebral disc disease in dogs. Genes (Basel). 2019;10(6). 10.3390/genes10060435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Proschowsky H, Fredholm M. Ingen brugbar DNA‐test for diskusprolaps I denne omgang [There is No Useful DNA Test for Disc Disease at This Time]. Gravhunden; 2018:12‐14. [Google Scholar]
- 25. Dachshund Club of America Health Statement. 2022. Accessed November 14, 2024. https://www.akc.org/wp-content/uploads/2022/08/Dachshunds-Health-Statemnt-rev-May-2022.pdf


