Abstract
Although there are clear interactions between circadian rhythms and drug addiction, mechanisms for such interactions remain unknown. Here we establish a role for the Clock gene in regulating the brain's reward circuit. Mice lacking a functional Clock gene display an increase in cocaine reward and in the excitability of dopamine neurons in the midbrain ventral tegmental area, a key brain reward region. These phenotypes are associated with increased expression and phosphorylation of tyrosine hydroxylase (the rate-limiting enzyme in dopamine synthesis), as well as changes in several genes known to regulate dopamine activity in the ventral tegmental area. These findings demonstrate the involvement of a circadian-associated gene, Clock, in regulating dopamine function and cocaine reward.
Keywords: circadian rhythms, dopamine, drug addiction, tyrosine hydroxylase
Drug addiction is associated with disruptions in sleep and circadian rhythmicity (1–3). Moreover, in animal models of addiction, several reward-related behaviors exhibit clear circadian regulation. For example, levels of drug self administration and drug-induced locomotor sensitization vary according to the day/night cycle (4–6). These observations suggest interactions between the brain's circadian and reward systems.
Although many of the genes involved in circadian rhythms are expressed outside the suprachiasmatic nucleus (SCN), the brain's master circadian pacemaker, and are found in limbic regions of the brain, little is known about their function in these other brain regions. The first indication that circadian-associated genes may be involved in drug-related behaviors came from studies in Drosophila, which showed that behavioral sensitization to cocaine depended on expression of Period, Clock, Cycle, and Doubletime (7). More recently, it was reported that locomotor sensitization and conditioned preference for cocaine are abnormal in mice lacking the Period-1 (mPer1) or Period-2 (mPer2) gene (6). These genes are induced as well by cocaine in the dorsal striatum and nucleus accumbens, brain regions important for cocaine's behavioral effects (8, 9). Although these findings support a role for circadian-associated genes in behavioral responses to drugs of abuse, little is known about the mechanisms by which these genes function, or are regulated, within the brain's reward and motor circuits.
Cocaine and other drugs of abuse produce their behavioral effects in part by modulating dopamine neurotransmission in the midbrain ventral tegmental area (VTA), a key component of the brain's reward circuit (10). Several interactions between dopamine and circadian function have been reported. For example, dopamine neurons in the retina regulate adaptations to light (11). Moreover, dopamine D1 receptors in the prenatal SCN are necessary for synchronizing the master circadian clock during development (12). However, a direct link between circadian genes and the VTA dopamine reward system has not been described. CLOCK is a member of the basic helix–loop–helix-PAS (PER-ARNT-SIM) transcription factor family that forms a complex with BMAL1 (brain and muscle ARNT-like protein 1) to become the central transcriptional activator in the brain's circadian clock in the SCN (13). We examined possible interactions among the circadian Clock gene, dopamine transmission, and drug reward by using mice with a point mutation in the gene that results in an inactive protein (14).
Methods
Animals. Homozygous Clock mutant mice (Clock/Clock) and their wild-type (+/+) littermates were used in all experiments and were housed together in groups of two to five per cage on a12/12-h light/dark cycle (lights on at 7:00 a.m., lights off at 7:00 p.m.), with food and water available at all times. All experiments were done in accordance with the policies set out by our institutional animal care and use committee.
Locomotor Activity Assays. Response to novelty. Mice were placed in locomotor activity chambers, and activity was recorded every 5 min over 2 h beginning at Zeitgeber time (ZT) 3.
Twenty-four-hour activity. Mice were placed in chambers at ZT 5, and activity was recorded for 24 h in a 12/12-h light/dark cycle. Beam breaks were recorded and placed into 30-min bins.
Locomotor Responses to Cocaine. A published protocol was used (15). Briefly, mice were habituated to the locomotor activity boxes for 4 days by giving them daily i.p. saline injections and putting them in the boxes for 10 min immediately thereafter. On days 5–9, animals were given 10 mg/kg cocaine or saline i.p., and locomotor activity was measured for 10 min. A challenge cocaine injection was given on day 10 to both groups. All experiments were done between ZT 3 and ZT 5.
Conditioned Place Preference. An unbiased conditioning protocol, based on published methods (9), was used. Briefly, male mice 6–8 weeks old were habituated in the testing room for 30 min to 1 h before testing or conditioning. Mice were tested for 20 min in the place-conditioning apparatus before conditioning on day 1 to ensure there was no bias toward any chamber of the apparatus. Mice that spent >15 min in any one compartment before conditioning were discarded from the study (this accounted for <10% of the total animals). On days 2 and 4, mice were given a saline injection paired with one side chamber of the apparatus, and on days 3 and 5, mice were given a cocaine injection paired with the other side chamber of the apparatus. Each conditioning session lasted 20 min, and sessions were conducted at the same time of day (ZT 3–ZT 5). On day 6, mice were assayed for the time spent in the two side chambers of the apparatus.
Electrophysiology. Methods for extracellular recording were similar to those reported previously (16). For detailed methods, see Supporting Text, which is published as supporting information on the PNAS web site. The coordinates for the VTA were 0.88 mm anterior to λ, 0.6 mm lateral from the midline, and 4.7 mm ventral from the cortical surface (17). Dopamine cells were identified by anatomical location in the VTA according to standard physiological criteria (18, 19). Bursting activity was plotted as percentage of spikes emitted in bursts. Burst events were initiated by a pair of spikes having an interspike interval <80 msec and terminated by interspike intervals >160 msec (18–20).
Immunohystochemistry. For detailed methods, see Supporting Text. Sections were incubated with primary antibodies to tyrosine hydroxylase (TH) (human, Sigma) and CLOCK (human, Santa Cruz Biotechnology). All tissue was taken between ZT 3 and ZT 5.
In Situ Hybridizations. For detailed methods, see Supporting Text. All tissue was taken between ZT 3 and ZT 5.
Real-Time PCR. For detailed methods, see Supporting Text. Real-time PCR was performed by using the Cepheid smart cycler and the Fast start SYBR green PCR master mix (Roche Applied Science, Indianapolis), according to the manufacturer's instructions, with primers for TH and GAPDH. Standard curves were run with whole-brain cDNA dilutions to determine reaction efficiency. Results for TH were normalized to those of GAPDH by using the ΔΔ threshold cycle (CT). All tissue was taken between ZT 3 and ZT 5.
Western Blots. For detailed methods, see Supporting Text. TH (Sigma), phospho-ser31 TH (Zymed), or GAPDH (RDI) antibodies were incubated with the blot at a concentration of 1:10,000 in 5% milk/Tris-buffered saline + 0.1% Tween (TBST) for 1 h at room temperature. Blots were washed in TBST, then the horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch) was incubated in 5% milk TBST at 1:2,000 for 1 h. Blots were washed in TBST and then exposed to film by using the Supersignal West Dura system (Promega). Densitometry was conducted by using nih image software. All tissue was taken between ZT 3 and ZT 5.
Microarray Experiments. Microarray analysis was performed as described (9), with few modifications. Methods for data analysis can be found in Supporting Text. Briefly, total RNA (5 μg per array from tissue taken between ZT 3 and ZT 5) was converted to cDNA, amplified, and labeled according to the Affymetrix protocols (reagents for the single and double-strand cDNA synthesis were from Invitrogen, and the in vitro transcription and biotin labeling were performed by using the ENZO IVT kit from Affymetrix. cRNA was not used if the total RNA recovered after amplification was <30 μg, or if the 260/280 ratio was <1.9. We used the Affymetrix murine genome U74AV2 array (≈12,500 transcripts). RNA fragmentation, hybridization, washing, and scanning were also carried out according to the manufacturer's instructions (Affymetrix).
Results
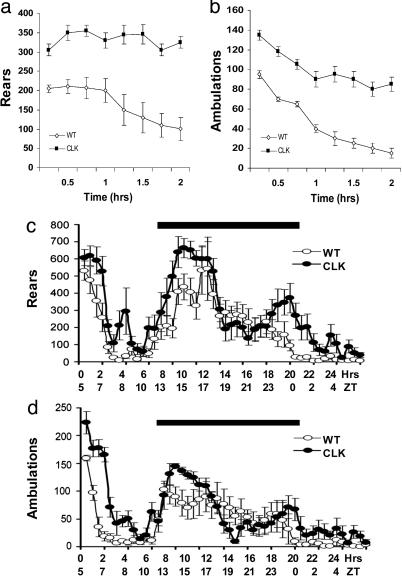
Clock Mutants Show Increased Baseline Activity and Sensitization to Cocaine. We first focused on locomotor activity of the Clock mutant mice in their initial response to novelty and throughout a 24-h light/dark cycle. Clock mutant mice show a pronounced elevation in their locomotor activity in response to a novel context when compared with littermate controls (Fig. 1 a and b). Furthermore, when animals were examined over a 24-h period in a light/dark cycle, the Clock mutants display normal activity rhythms but have elevated activity levels throughout the light/dark cycle, with the most pronounced differences at the beginning of the dark cycle and beginning of the light cycle (Fig. 1 c and d).
Fig. 1.
Clock mutant mice are hyperactive. Rearing behavior (a) and locomotor activity (b) were measured in a novel environment over 2 hours with the number of beam breaks measured every 15 min. (c and d) Activity throughout the light/dark cycle was measured for 24 h, with activity recorded every 30 min. The solid dark bars indicate the dark cycle. All data points are significant (a and b) (P < 0.05 by ANOVA, n = 6). The total activity over 24 h is also significantly different for both rears and ambulations (P < 0.05 by ANOVA, n = 6). Clk, Clock mutants; WT, wild-type mice.
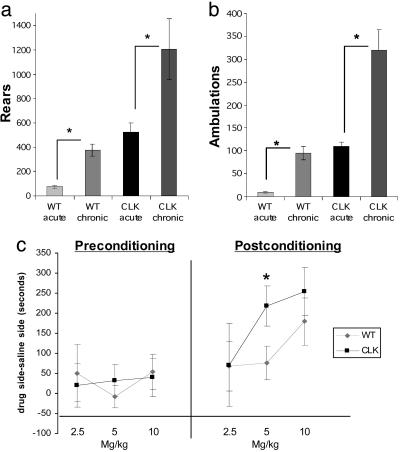
We next determined whether Clock mutants would develop behavioral sensitization to cocaine with repeated administration. In this experiment, we again observed consistent hyperactivity by the Clock mutants, compared with wild-type littermate controls, over 4 days of saline treatment when the animals were habituated to the test chambers (data not shown). Despite this greatly elevated baseline activity, Clock mutants develop high levels of behavioral sensitization to repeated cocaine (Fig. 2 a and b). These results suggest that a functional Clock gene is not necessary for cocaine-induced locomotor activation, and in fact normal Clock expression may dampen the levels of cocaine sensitization.
Fig. 2.
Clock mutant mice sensitize to cocaine. (a and b) Clock mutants (Clk) and wild-type (WT) controls were habituated to the locomotor activity boxes for 4 days with saline injections. On days 5–9, animals were given 10 mg/kg cocaine or saline i.p., and locomotor activity was measured for 10 min. Plotted are the results from a challenge cocaine injection on day 10. *, P < 0.05 by ANOVA, n = 6–8. (c) Clock mutants have an increased preference for cocaine. Clock mutants and WT controls were tested for bias on day 1, conditioned on days 2–4, and tested for preference for cocaine on day 5 by using an unbiased protocol at 2.5, 5, and 10 mg/kg. *, P < 0.05 by ANOVA, n = 11–14.
Clock Mutants Find Cocaine More Rewarding. Increased locomotor responses to novelty and robust cocaine sensitization are phenotypes correlated with increased vulnerability to cocaine's rewarding effects (22). We therefore studied Clock mutant mice in the cocaine place-preference paradigm, a more direct measure of cocaine's rewarding properties. Compared with wild-type littermate mice, Clock mutant mice developed greater degrees of place conditioning to a lower dose of cocaine (Fig. 2c). These results suggest that the Clock mutant mice are more sensitive to the rewarding effects of cocaine.
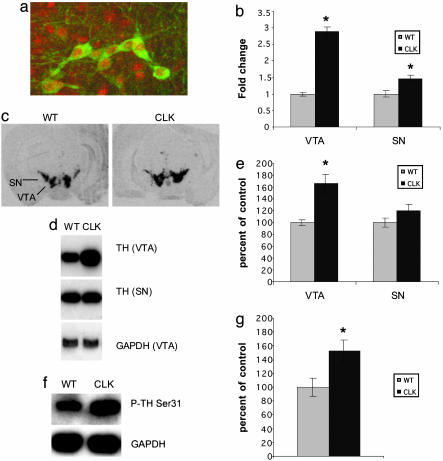
TH Expression in the VTA Is Increased in Clock Mutants. To better understand the mechanism underlying Clock's regulation of cocaine reward, we focused on the VTA dopamine system. First, we determined whether CLOCK protein is expressed in VTA dopamine neurons through the use of double-labeling immunohistochemistry with antibodies specific for CLOCK and TH, the rate-limiting enzyme in dopamine synthesis. We found CLOCK protein expression throughout the anterior–posterior axis of the VTA, including robust expression in all dopamine (TH+) neurons. Analysis of multiple sections from each of five mice revealed no TH+ cell that was CLOCK-. Representative TH+ CLOCK+ neurons are shown in Fig. 3a.
Fig. 3.
CLOCK is expressed in dopamine neurons, and TH levels and phosphorylation are increased in Clock mutants. (a) Sections containing the VTA were labeled with antibodies against CLOCK (red) and TH (green). Fluorescence was integrated by using confocal microscopy. Results are representative of multiple sections obtained throughout the anterior–posterior axis of the VTA of five mice (data not shown). (b–g) Clock mutants have increased TH protein and mRNA levels in the VTA. mRNA levels were determined from VTA and substantia nigra (SN) tissue punches from Clock mutants (Clk) and wild-type (WT) controls by real-time PCR (n = 5) (b) and in situ hybridization (n = 8). (c) Protein levels and phosphorylation (d–g) were determined by Western blot analysis (representative blots are shown in d and f, n = 5). In all cases, GAPDH was used as a control. *, P < 0.05 by ANOVA.
Previous studies have shown that CLOCK functions as a transcription factor. Therefore, we wanted to characterize changes in gene expression in the Clock mutants, which could underlie the increased locomotor activity and cocaine preference seen in these mice. We first examined levels of expression of TH. Clock mutant mice exhibit substantially increased levels (≈2- to 3-fold) of TH mRNA and protein compared with littermate controls (Fig. 3 b–e). Also, there is an equivalent increase in phosphorylated TH at Ser-31 (Fig. 3 f and g), showing that the additional TH protein produced in the Clock mutants is phosphorylated and thus active. Expression levels of TH have long been thought to relate to the activity level of the cell and, more recently, it has been shown in retinal dopamine cells that phosphorylation of TH at Ser-31 is particularly sensitive to the neuron's activity level (23). Thus, the observed increase in TH expression and phosphorylation in the VTA is consistent with elevated dopaminergic transmission in the reward circuit of Clock mutant mice and supports a possible suppressant effect of CLOCK on dopamine function. Interestingly, although CLOCK is also expressed in dopamine neurons in the nearby substantia nigra (SN, data not shown), these neurons showed a much smaller increase in TH mRNA levels and no significant increase in protein levels in the Clock mutants (Fig. 3 b–d). This suggests that CLOCK exerts greater modulatory control over dopamine neurotransmission in the VTA compared with the SN (24).
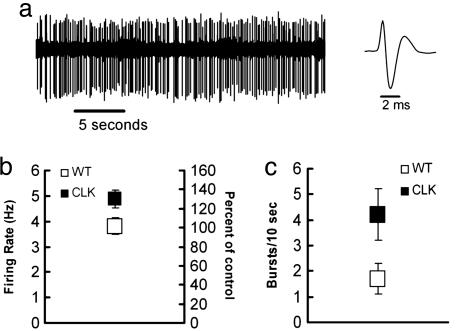
Dopamine Cell Firing and Bursting Are Enhanced in Clock Mutants. Previous work has linked increased excitability of VTA dopamine neurons to increased levels of cocaine sensitization and reward (22, 25). To determine whether the loss of functional CLOCK protein affects dopamine neuron excitability, we measured impulse activity of VTA dopamine neurons in Clock mutant mice. As shown in Fig. 4, VTA dopamine neurons display elevated impulse activity (bursting and firing rate) in the Clock mutants compared with wild-type controls. The degree of elevated impulse activity is similar to that seen in other animal models of increased sensitivity to cocaine, such as high novelty locomotor responding rats (22).
Fig. 4.
Dopamine cell-firing rates and bursting are increased in Clock mutant mice. (a)(Left) Representative recording of a mouse dopamine neuron. (Right) Example of an averaged dopamine triphasic waveform. (b) Clock mutant mice (Clk) (n = 24) exhibited higher basal dopamine firing rates compared with wild-type (WT) (n = 26) mice (P < 0.03). Squares represent the mean ± SEM of each group. (c) Clock mutant mice exhibited more burst events per 10 sec compared with wild-type controls (P < 0.01). Each vertical bar represents the mean ± SEM of each group. The percentage of bursting activity, i.e., spikes emitted in bursts, was greater compared with wild-type mice (Clk, 23.5 ± 4.3 vs. 10.6 ± 3.6 in WT; P < 0.03). Clock mutant mice had larger burst sizes (number of spikes/burst) compared with controls (2.8 ± 0.1 vs. 2.0 ± 0.1; P < 0.008).
Genes That Influence Dopaminergic Transmission Are Regulated by CLOCK. To further examine the molecular mechanisms of the increased dopamine neurotransmission and cocaine reward observed in Clock mutant mice, we carried out DNA microarray analysis of the VTA. Importantly, the Clock mutants show decreased expression of several genes known to be targets for CLOCK, or involved in circadian rhythms, in the SCN (26). These include Period 1, Period 2, Cryptochrome 2, Casein kinase 1 ε, and D site albumin promoter-binding protein (Table 1). These results support this approach for identifying CLOCK target genes and suggest that these various circadian genes are targets for CLOCK outside the SCN. Interestingly, there is an increase in Clock mRNA expression in the VTA of Clock mutant mice. Presumably, this increase represents an attempt by these neurons to compensate for the loss of CLOCK protein activity due to the point mutation in the gene, which results in an inactive protein (14).
Table 1. Genes that are regulated in the VTA of Clock mutant mice vs. wild-type littermate controls that may directly affect dopaminergic transmission or are known to be involved in circadian rhythms.
| Up-regulated | Down-regulated |
|---|---|
| Genes that may directly regulate dopaminergic transmission | |
| TH | Cholecystokinin |
| Synaptobrevin-like 1 | Potassium channel Q2 |
| NMDA receptor-regulated gene 1 | Preproenkephalin 1 |
| Glutamate receptor 1 | GABAA receptor β 1 |
| Glutamate receptor ionotropic kainate 5 γ 2 | |
| Glutamate receptor NMDA ζ 1 | |
| Synaptotagmin 5 | |
| Genes known to be involved in circadian rhythms | |
| Clock | Period 1 |
| Period 2 | |
| D site albumin promoter-binding protein | |
| Cryptochrome 2 | |
| Casein kinase 1 ε |
Among the genes that are differentially regulated in Clock mutant mice (a complete list of genes is available in Table 2, which is published as supporting information on the PNAS web site), we find several that are known to influence dopaminergic transmission. Regulation of these genes, in addition to TH, could contribute to the increased dopaminergic function observed in these mice (Table 1).
Discussion
Results of the present study establish that CLOCK protein is involved in regulating dopaminergic transmission in the brain's reward circuit. Increased midbrain dopamine transmission and burst activity are associated with increased locomotor activity and drug reward (27). Indeed, Clock mutants display hyperactive behavior, high levels of cocaine-induced locomotor sensitization, and increased drug reward compared with wild-type controls. It may be somewhat surprising that Clock mutants sensitize to cocaine, given that Drosophila that harbor a mutation in the Clock gene fail to do so (7). However, mice that lack mPer1 or mPer2, two target genes of CLOCK, have opposite responses to chronic cocaine, with mPer1 knock-out mice showing little to no sensitization and mPer2 knock-out mice showing hypersensitized responses, suggesting this process in mammals is complex (6).
The regulation of the VTA dopamine system by CLOCK is presumably achieved through its actions as a transcription factor. We show that several genes in the VTA known to control dopaminergic activity, including the rate-limiting enzyme in dopamine synthesis, TH, are differentially regulated in Clock mutants. The mechanism underlying CLOCK regulation of TH has yet to be determined. A recent study found that TH mRNA expression in the VTA exhibits a circadian pattern, suggesting that its transcription may be under the control of circadian genes (28). Furthermore, there is an enhancer element that lies just upstream of the TH gene that contains an E-box, the known binding site for several transcription factors including CLOCK (29). Thus, it is conceivable that CLOCK, acting as a transcriptional repressor, may directly regulate TH gene transcription. Alternatively, increased TH expression may be secondary to the increased firing of the VTA cells, because TH transcription is tied to dopaminergic activity, as stated earlier.
In addition to the regulation of TH, we observed decreased expression of the β1 subunit of the GABAA receptor. It has been shown that activation of the GABAA receptor inhibits burst firing of VTA dopamine neurons (30). The β1 subunit seems to be the most abundant of all GABAA receptor β subunits expressed in VTA dopamine neurons (31). This subunit is also reduced in response to chronic GABA treatment, and reductions in the subunit are seen in models of epilepsy (32). GABAA receptor function is also regulated in a circadian pattern in the SCN, which suggests that its expression may be regulated by circadian genes (33). Thus, reduced expression of the GABAA receptor β1 subunit could be one mechanism underlying the increased excitability of these neurons. In addition, Clock mutants display down-regulation of a voltage-gated potassium channel (KcnQ2) in the VTA, which could also contribute to the observed increase in VTA neuronal excitability (34). Additionally, Clock mutants have increased levels of the GluR1 subunit of the AMPA glutamate receptor. Increased levels of GluR1 in the VTA are associated with the development of cocaine sensitization and increased drug reward (35, 36). These and other interesting potential target genes of CLOCK may influence dopamine neuronal excitability and now warrant future study.
Conclusion
Taken together, these findings establish an important role for CLOCK as a key regulator of the brain's reward circuitry. They also suggest a more widespread influence of CLOCK on complex behavior, well beyond the gene's classic role in the entrainment of circadian rhythms by light mediated via the SCN.
Supplementary Material
Acknowledgments
We thank Cathy Steffen, Olivier Berton, and Michel Barrot for technical assistance and advice. This work was funded by grants from the National Institute on Drug Abuse, the National Institute of Mental Health, and the Onassis Public Benefit Foundation.
Author contributions: C.A.M., J.S.T., F.J.W., D.C.C., and E.J.N. designed research; C.A.M., K.S., M.V., and D.C.C. performed research; C.A.M. and D.C.C. analyzed data; and C.A.M. and E.J.N. wrote the paper.
Abbreviations: VTA, ventral tegmental area; SCN, suprachiasmatic nucleus; ZT, Zeitgeber time; TH, tyrosine hydroxylase.
References
- 1.Kowatch, R. A., Schnoll, S. S., Knisely, J. S., Green, D. & Elswick, R. K. (1992) J. Addict. Dis. 11, 21-45. [DOI] [PubMed] [Google Scholar]
- 2.Watson, R., Bakos, L., Compton, P. & Gawin, F. (1992) Am. J. Drug Alcohol Abuse 18, 21-28. [DOI] [PubMed] [Google Scholar]
- 3.Johanson, C.E., Roehrs, T., Schuh, K. & Warbasse, L. (1999) Exp. Clin. Psychopharmacol. 7, 338-346. [DOI] [PubMed] [Google Scholar]
- 4.Baird, T. J. & Gauvin, D. (2000) Pharmacol. Biochem. Behav. 65, 289-299. [DOI] [PubMed] [Google Scholar]
- 5.Roberts, D. C., Brebner, K., Vincler, M. & Lynch, W. J. (2002) Drug Alcohol Depend. 67, 291-299. [DOI] [PubMed] [Google Scholar]
- 6.Abarca, C., Albrecht, U. & Spanagel, R. (2002) Proc. Natl. Acad. Sci. USA 99, 9026-9030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Andretic, R., Chaney, S. & Hirsh, J. (1999) Science 285, 1066-1068. [DOI] [PubMed] [Google Scholar]
- 8.Yuferov, V., Kroslak, T., Laforge, K. S., Zhou, Y., Ho, A. & Kreek, M. J. (2003) Synapse 48, 157-169. [DOI] [PubMed] [Google Scholar]
- 9.McClung, C. A. & Nestler, E. J. (2003) Nat. Neurosci. 6, 1208-1215. [DOI] [PubMed] [Google Scholar]
- 10.Wise, R. A. (1987) Pharmacol. Ther. 35, 227-263. [DOI] [PubMed] [Google Scholar]
- 11.Witkovsky, P. (2004) Doc. Ophthalmol. 108, 17-40. [DOI] [PubMed] [Google Scholar]
- 12.Ferguson, S. A., Rowe, S. A., Krupa, M. & Kennaway, D. J. (2000) Brain Res. 858, 284-289. [DOI] [PubMed] [Google Scholar]
- 13.Gekakis, N., Staknis, D., Nguyen, H. B., Davis, F. C., Wilsbacher, L. D., King, D. P., Takahashi, J. S. & Weitz, C. J. (1998) Science 280, 1564-1569. [DOI] [PubMed] [Google Scholar]
- 14.King, D. P., Zhao, Y., Sangoram, A. M., Wilsbacher, L. D., Tanaka, M., Antoch, M. P., Steeves, T. D., Vitaterna, M. H., Kornhauser, J. M. & Lowrey, P. L. (1997) Cell 89, 641-653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman, Z., Schwarz, J., Gold, S. J., Zachariou, V., Wein, M. N., Choi, K. H., Kovoor, A., Chen, C. K., DiLeone, R. J., Schwarz, S. C., et al. (2003) Neuron 38, 941-952. [DOI] [PubMed] [Google Scholar]
- 16.Henry D. J., Greene M. A. & White F. J. (1989) Pharmacol. Exp. Ther. 251, 833-839. [PubMed] [Google Scholar]
- 17.Paxinos, G. & Franklin, K. B. J. (2001) Mouse Brain in Stereotaxic Coordinates (Academic, San Diego).
- 18.Grace, A. A. & Bunney, B. S. (1983) Neuroscience 10, 333-348. [DOI] [PubMed] [Google Scholar]
- 19.White, F. J. (1996) Annu. Rev. Neurosci. 19, 405-436. [DOI] [PubMed] [Google Scholar]
- 20.Grace, A. A. & Bunney, B. S. (1984) J. Neurosci. 4, 2877-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gold, S. J., Han, M. H., Herman, A. E., Ni, Y. G., Pudiak, C. M., Aghajanian, G. K., Liu, R. J., Potts, B. W., Mumby, S. M. & Nestler, E. J. (2003) Eur. J. Neurosci. 17, 971-980. [DOI] [PubMed] [Google Scholar]
- 22.Marinelli, M. & White, F. J. (2000) J. Neurosci. 20, 8876-8885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witkovsky, P., Veisenberger, E., Haycock, J. W., Akopian, A., Garcia-Espana, A. & Meller, E. (2004) J. Neurosci. 24, 4242-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wise, R. A. & Hoffman, D. C. (1992) Synapse 10, 247-263. [DOI] [PubMed] [Google Scholar]
- 25.Saal, D., Dong, Y., Bonci, A. & Malenka, R. C. (2003) Neuron 37, 577-582. [DOI] [PubMed] [Google Scholar]
- 26.Reppert, S. M. & Weaver, D. R. (2001) Annu. Rev. Physiol. 63, 647-676. [DOI] [PubMed] [Google Scholar]
- 27.Cooper, D. C. (2002) Neurochem. Int. 41, 333-340. [DOI] [PubMed] [Google Scholar]
- 28.Weber, M., Lauterburg. T., Tobler, I. & Burgunder, J. M. (2004) Neurosci. Lett. 358, 17-20. [DOI] [PubMed] [Google Scholar]
- 29.Yoon, S. O. & Chikaraishi, D. M. (1994) J. Biol. Chem. 269, 18453-18462. [PubMed] [Google Scholar]
- 30.Komendantov, A. O., Komendantova, O. G., Johnson. S. W. & Canavier, C. C. (2004) J. Neurophysiol. 91, 346-357. [DOI] [PubMed] [Google Scholar]
- 31.Okada, H., Matsushita, N., Kobayashi, K. & Kobayashi, K. (2004) J. Neurochem. 89, 7-14. [DOI] [PubMed] [Google Scholar]
- 32.Steiger, J. L. & Russek, S. J. (2004) Pharmacol. Ther. 101, 259-281. [DOI] [PubMed] [Google Scholar]
- 33.Ning, K., Li, L., Liao, M., Liu, B., Mielke, J. G., Chen, Y., Duan, Y., El-Hayek, Y. H. & Wan, Q. (2004) Nat. Neurosci. 7, 489-490. [DOI] [PubMed] [Google Scholar]
- 34.Robbins, J. (2001) Pharmacol. Ther. 90, 1-19. [DOI] [PubMed] [Google Scholar]
- 35.Carlezon, W. A., Jr., & Nestler, E. J. (2002) Trends Neurosci. 25, 610-615. [DOI] [PubMed] [Google Scholar]
- 36.Thomas, M. J. & Malenka, R. C. (2003) Philos. Trans. R. Soc. London B 358, 815-819. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.