Abstract
Alpinia oxyphylla, a perennial herb belonging to the Zingiberaceae family, has a long history of traditional medicinal use. The present study evaluated the efficacy of different concentrations of Alpinia oxyphylla essential oil (AEO) on the growth performance, serum antioxidation capacities, immune function, apparent digestibility of nutrients, and gut microbiota in fattening pigs. A total of 120 pigs were divided into five treatments, with six replicates each and four pigs per replicate. The pigs were fed a basal diet or basal diet with chlortetracycline (CTC) alone or AEO at 250, 500, and 1,000 mg/kg (referred to as groups AEO1, AEO2, and AEO3, respectively) for 35 days, preceded by a 7-day pre-feed period. The results show that there were no statistically significant differences in growth performance for any dose of AEO supplementation. AEO increased L-DLC content, total protein content and the activity of GSH in serum (p < 0.05). The AEO also exhibited a linear increase in serum IgG content (p < 0.05). Dietary supplementation with AEO improved apparent digestibility of crude ash and calcium (p < 0.05). In gut microbiota, AEO modified the diversity and abundance of bacterial communities in fattening pigs. The abundance of Dorea, Blautia, Butyricicoccus, Bulleidia, and Lactobacillus was higher in the AEO groups compared to the control group, while Clostridium and Turicibacter were lower. The Bifidobacteriales and Pseudomonas were abundant in group AEO1 and AEO3, respectively. In conclusion, dietary supplementation of 1,000 mg/kg AEO has the potential to improve growth performance, immunological, biochemical, and antioxidant statuses. Additionally, AEO can increase the efficiency of nutrient digestion and absorption through the regulation of gut microbiota.
Keywords: Alpinia oxyphylla, essential oil, antioxidation capacities, immune function, gut microbiota, fattening pig
Introduction
Over the past few decades, antibiotics have been widely used in livestock as antibiotic growth promoters (AGPs). Small quantities of antibiotics in animal feed were sufficient to promote growth and prevent diseases. However, the AGPs enter soil and aquatic ecosystems, leading to antibiotic pollution and the emergence of antibiotic resistance eventually (1, 2). After the European Union and the United States implemented a ban on AGPs, China also followed suit in 2020. Currently, the ban on AGPs continues to motivate the livestock industry to explore appropriate antibiotic alternatives that can support animal development and welfare (3), and growth-promoting alternatives to antibiotics in livestock production have become a subject of intense research.
Essential oils (EOs) are mixtures of volatile and hydrophobic compounds extracted from plants (4). They have the ability to enhance animal immunity, ameliorate intestinal function and promote growth, making them a viable alternative to dietary antibiotics in animals. Regularly, multiple concurrent environmental stressors, such as weaning, bacterial, viral infection and heat, continually impose extensive pressures to pigs, and these stress results in oxidative stress, which adversely affects the growth performance and health of pigs (5, 6). EOs contain flavonoids, phenols, aldehydes, and their derivatives, all of which have strong antioxidant and anti-inflammatory activities (7). These properties help cells defend against inflammation and oxidative damage caused by reactive oxygen species (ROS), thereby maintaining homeostasis (8, 9). Moreover, EOs have been reported to maintain the stability of the intestinal environment and improve relative abundance of probiotics, such as Lactobacillus and Bifidobacterium (10, 11). These probiotics positively impact gut health and ultimately contribute to improved growth performance.
Alpinia oxyphylla, a perennial herb belonging to the Zingiberaceae family, is native to the tropical and subtropical regions of China (12). It has a long history of food and traditional medicinal use in China spanning centuries (13), and is widely cultivated in southern China (14). Essential oil extracted from Alpinia oxyphylla is abundant in flavonoids, diarylheptanoids, terpenoids, volatile oils, steroids, and other glycosides (15, 16). These contents in AEO were further confirmed to have anti-hyperuricemic, and other therapeutic mechanisms for disease. In addition, increasing evidence indicates AEO has diverse bioactivities, including antioxidant (17), antitumor (14), anti-inflammatory (18), modulating the gut microbiota (19), protecting intestinal barrier integrity (20), and exhibiting bacteriostasis (21). These characteristics make AEO both cost-effective and easily available, highlighting its potential value as a feed additive. However, few studies have evaluated the effects of AEO on fattening pigs, which limits the use of Alpinia oxyphylla in the animal industry as an alternative to antibiotic growth promoters. In addition, there is a scarcity of data on the effects of AEO on the gut microbiota of pigs. Consequently, the aim of the study is to evaluate the efficacy of different concentrations of AEO in the growth performance, serum antioxidation capacities, immune function, apparent nutrient digestibility, and gut microbiota in fattening pigs, in order to provide data supporting the rational use of AEO.
Materials and methods
Ethical approval
All experimental procedures were reviewed and approved by the animal care committee of Changsha Medical University (No. 2023-01), Changsha, China.
Animals, diets, and treatments
The study employed a single-factor randomized trial design. A sample of 120 male fattening pigs (Landrace × Yorkshire crossbreed pigs (M1) crossed with Duroc (DJ) breed boars), all belonging to the same breed and having similar health and body weight, were randomly assigned to five treatment groups (with six replicates per treatment and four pigs per replicate). Furthermore, pigs were subjected to a pre-fed regimen for 7 days, then the formal test spanned 35 days. The distribution of groups is shown in Table 1.
Table 1.
Experimental design and grouping.
| Group | Diet |
|---|---|
| Con | Basal diet |
| CTC | Basal diet +75 mg/kg chlortetracycline (15%) premix |
| AEO1 | Basal diet +250 mg/kg AEO |
| AEO2 | Basal diet +500 mg/kg AEO |
| AEO3 | Basal diet +1,000 mg/kg AEO |
The corn-soybean meal basal diet was designed based on the National Research Council (NRC, 2012), and the ingredients and nutritional levels are shown in Table 2. Pigs were provided with a pelleted feed throughout the entire period. The AEO was purchased from Hunan Nuoz Biological Technology Co., Ltd.
Table 2.
Composition and nutrition levels of the basal diet (air-dry basis, %).
| Item | Content |
|---|---|
| Ingredients | |
| Corn | 27.50 |
| Rice bran | 10.00 |
| Rice bran meal | 16.00 |
| Broken rice | 30.00 |
| Soybean meal | 12.50 |
| Premix a | 4.00 |
| Total | 100.00 |
| Nutrient level b | |
| DE (Mcal/kg) | 3.20 |
| CP | 15.02 |
| Ca | 0.55 |
| TP | 0.76 |
| AP | 0.20 |
| Lys | 1.15 |
| Thr | 0.60 |
| Met | 0.25 |
| Met Cys | 0.50 |
Premix contained per kg VA, 325 IU; VD, 37.5 IU; VE, 2.75 IU; VK3, 0.013 mg; VB2, 0.63 mg; VB6, 0.25 mg; VB12, 2.5 mg; biotin, 0.013 mg; folic acid, 0.08 mg; D-pantothenic acid, 2.00 mg; hydrochloric acid, 2.5 mg; choline chloride, 0.08 mg; antioxidants, 12.50 mg; FeSO4.H2O, 12.50 mg; CuSO4.H2O, 0.88 mg; ZnO, 15.00 mg; MnSO4.H2O, 0.50 mg; Na2SeO3 0.04 mg; Kl, 0.04 mg.
Digestible energy was a calculated value.
Sampling and collection
In the present study, the methods and parameters collected were according to our previous study (6). Pigs were weighed on an empty stomach at the beginning and the end of the experiment, and average daily gain (ADG) was calculated. Feed intake in each repeated was recorded to calculate average daily feed intake (ADFI) and feed-to-weight ratio (F/G). Moreover, 500 g of feed samples for each treatment were collected, and 1 kg of fresh and uncontaminated fecal samples for each replicate were randomly obtained using disposable sterile gloves. Samples were stored for testing.
Serum biochemical parameters
The serum biochemical parameters were measured with an automatic biochemical analyzer (Hitachi automatic biochemical analyzer 7,000, Japan) including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), urea, glucose (GLU), total cholesterol (TC), triglyceride (TG), low-density lipoprotein (LDL), high-density lipoprotein (HDL), total protein (TP), albumin (ALB) and globulin (GLB) (22). Six antioxidant indicators were measured with a microplate reader FluoStar Optima (BMG Labtech, Offenburg, Germany), including superoxide dismutase (SOD), glutathione peroxidase (GPX), catalase (CAT), glutathione (GSH), total antioxidant capacity (T-AOC) and malondialdehyde (MDA) contents, in accordance with our previous method (23). The contents of IgA, IgG, and IgM and complements C3 and C4 in serum were determined by the enzyme-linked immunosorbent assay (ELISA, Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Nutrient digestibility determination
Acid insoluble ash (AIA) was used as a digestibility indicator to determine the apparent total tract digestibility including dietary dry matter (DM), energy, crude protein (CP), crude fat (CF), crude fiber (CF), crude ash, calcium, and phosphorus.
Microbial DNA extraction and PCR amplification
As previously described, the extraction of DNA from fecal specimens and the amplification of 16S ribosomal RNA were conducted in accordance with Wang et al. (24). DNA was isolated from the fecal samples utilizing the E.Z.N.A.® Soil DNA Kit (Omega Biotek, Norcross, GA, United States) following the manufacturer’s protocol. The V3–V4 region of 16S rRNA in bacterial populations was amplified employing universal primers 338F/806R, and the samples were subsequently sequenced on the Illumina Miseq PE300 platform (Illumina, San Diego, CA, United States). Raw sequence data were filtered and denoised initially, and regions of low quality within the sequences were subsequently excised and excluded. The QIIME2 software (version 2019.4) facilitated the creation of a table of amplicon sequence variants (ASVs) and aided in the construction of rarefaction curves along with analyses of both alpha and beta diversity. For the taxonomic classification, the Greengenes database (Version 13.8, available at http://greengenes.secondgenome.com) was employed and appropriate alignments were carried out. Principal coordinate analysis (PCoA) was executed utilizing the Bray–Curtis Dissimilarity metric to analyze the data. A linear discriminant analysis effect size (LEfSe) was conducted to show the maximum difference in the microbial structures between groups (LEfSe version 1.1, https://github.com/SegataLab/Lefse). Sequence reads from the original sequence were uploaded to NCBI’s Sequence Read Archive under accession number PRJNA1090261.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 statistical software. The significant differences among groups were analyzed by one-way ANOVA, and the Duncan’s multiple range test was used when the difference between groups was significant (p < 0.05). In addition, 0.05 < p < 0.1 were considered a trend. The results are reported as means ± standard deviations. Linear and quadratic terms of AOE on various indicators of pigs were also evaluated.
Results
Effect of dietary AEO level on growth performance in pigs
The effects of different dietary contents of AEO on the growth performance of finishing pigs are shown in Table 3. There were no significant differences in the average initial weights between groups. Dietary inclusion of different doses of AEO in finishing pigs had minimal impact on the average final weight, ADG, and ADFI (p > 0.05), compared to the control group. However, the F/G of the 1,000 mg/kg AEO treatment groups decreased by 4% compared to the CTC treatment numerically, although the positive effects did not reach statistical significance.
Table 3.
Effect of dietary AEO levels on growth performance in pigs.
| Items | CTC level, mg/kg | AEO levels, mg/kg | p-value | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| 75 | 0 | 250 | 500 | 1,000 | Linear | Quadratic | ||
| Average initial weight /(kg) | 82.03 ± 2.46 | 82.69 ± 2.84 | 80.22 ± 3.58 | 82.17 ± 4.75 | 81.83 ± 2.98 | 0.773 | 0.772 | 0.397 |
| Average final weight /(kg) | 107.63 ± 2.91 | 107.36 ± 3.32 | 107.47 ± 2.57 | 107.97 ± 4.27 | 108.39 ± 3.11 | 0.983 | 0.591 | 0.823 |
| ADG /(kg) | 0.74 ± 0.06 | 0.72 ± 0.06 | 0.79 ± 0.06 | 0.75 ± 0.08 | 0.77 ± 0.09 | 0.464 | 0.269 | 0.368 |
| ADFI /(kg) | 2.62 ± 0.09 | 2.50 ± 0.16 | 2.69 ± 0.11 | 2.65 ± 0.15 | 2.62 ± 0.19 | 0.246 | 0.160 | 0.114 |
| F/G | 3.55 ± 0.25 | 3.50 ± 0.09 | 3.41 ± 0.14 | 3.56 ± 0.22 | 3.41 ± 0.16 | 0.454 | 0.635 | 0.867 |
In the same row, values with different small letter superscripts mean a significant difference (p < 0.05), the same as in the following.
Effect of dietary AEO level on serum biochemical parameters in pigs
The effects of different AEO levels in diet on serum physiological and biochemical parameters of finishing pigs are presented in Table 4. Dietary supplementation with AEO significantly increased the serum L-DLC content compared to the CTC group (p < 0.05). Supplementation of AEO exhibited a tendency to increase the serum GLB content of pigs (p = 0.088), and there was a linear improvement in the serum total protein with increasing AEO levels (p < 0.05). Dietary supplement with AEO had minimal impact on serum GLU, TG, TCHO, UREA, TP, ALB, H-DLC, ALP, AST and ALT contents compared to the control treatment and CTC addition treatment (p > 0.05).
Table 4.
Effect of dietary AEO levels on serum biochemical indices in pigs.
| Items | CTC level, mg/kg | AEO levels, mg/kg | p-value | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| 75 | 0 | 250 | 500 | 1,000 | Linear | Quadratic | ||
| GLU/(mmol/L) | 2.68 ± 0.67 | 2.46 ± 0.88 | 2.71 ± 0.48 | 2.19 ± 0.31 | 2.38 ± 0.30 | 0.701 | 0.540 | 0.901 |
| TG/(mmol/L) | 0.50 ± 0.20 | 0.50 ± 0.15 | 0.63 ± 0.11 | 0.59 ± 0.25 | 0.56 ± 0.14 | 0.780 | 0.690 | 0.379 |
| TCHO/(mmol/L) | 2.54 ± 0.17 | 2.16 ± 0.71 | 2.31 ± 0.69 | 1.92 ± 0.17 | 2.03 ± 0.67 | 0.554 | 0.585 | 0.935 |
| UREA/(mmol/L) | 4.93 ± 1.31 | 3.50 ± 0.97 | 5.11 ± 1.44 | 5.05 ± 0.55 | 4.91 ± 0.48 | 0.197 | 0.073 | 0.088 |
| TP/(g/L) | 65.11 ± 7.53 | 58.63 ± 10.37 | 63.51 ± 5.79 | 64.85 ± 7.32 | 72.85 ± 5.17 | 0.166 | 0.021 | 0.682 |
| ALB/(g/L) | 24.19 ± 1.88 | 22.19 ± 5.99 | 21.17 ± 1.09 | 20.98 ± 2.96 | 24.11 ± 2.74 | 0.520 | 0.509 | 0.278 |
| GLB/(g/L) | 40.93 ± 5.93 | 36.43 ± 6.44 | 42.34 ± 5.22 | 43.87 ± 4.63 | 48.74 ± 6.01 | 0.088 | 0.010 | 0.857 |
| H-DLC/(mmol/L) | 0.88 ± 0.07 | 0.84 ± 0.10 | 0.89 ± 0.16 | 0.84 ± 0.17 | 0.86 ± 0.11 | 0.970 | 0.940 | 0.725 |
| L-DLC/(mmol/L) | 1.11 ± 0.16a | 0.93 ± 0.22ab | 0.80 ± 0.15b | 0.74 ± 0.13b | 0.82 ± 0.14b | 0.047 | 0.229 | 0.365 |
| ALP/(U/L) | 74.53 ± 5.75 | 79.14 ± 14.86 | 80.65 ± 9.67 | 72.98 ± 7.51 | 82.10 ± 8.08 | 0.627 | 0.959 | 0.481 |
| AST/(U/L) | 20.75 ± 3.75 | 18.93 ± 3.64 | 20.57 ± 7.47 | 21.02 ± 3.45 | 20.25 ± 2.33 | 0.967 | 0.677 | 0.612 |
| ALT/(U/L) | 55.65 ± 10.51 | 42.75 ± 11.37 | 44.28 ± 12.32 | 37.81 ± 6.57 | 43.96 ± 6.97 | 0.186 | 0.898 | 0.641 |
In the same row, values with different small letter superscripts mean a significant difference (p < 0.05), the same as in the following.
Effect of dietary AEO levels on serum antioxidant parameters in pigs
As shown in Table 5, the supplementation of AEO at doses of 250 and 500 mg/kg AEO had a tendency to increase the serum total antioxidant capacity levels in pigs (p = 0.060). Furthermore, the activity of glutathione (GSH) in the serum increased linearly and quadratically (p < 0.05) with increasing dietary concentrations of AEO from 0 to 1,000 mg/kg. The SOD and the content of GSH-Px, CAT and MDA in serum of pigs were not significantly affected (p > 0.05).
Table 5.
Effect of dietary AEO levels on serum antioxidant indices in pigs.
| Items | CTC level, mg/kg | AEO levels, mg/kg | p-value | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| 75 | 0 | 250 | 500 | 1,000 | Linear | Quadratic | ||
| SOD/(U/ml) | 35.19 ± 16.78 | 41.69 ± 10.11 | 40.01 ± 7.94 | 38.68 ± 8.73 | 40.78 ± 6.42 | 0.918 | 0.833 | 0.662 |
| GPX/(U/ml) | 1751.23 ± 343.41 | 1553.69 ± 239.55 | 1708.31 ± 220.69 | 1781.56 ± 222.59 | 1750.18 ± 272.26 | 0.755 | 0.204 | 0.613 |
| CAT/(U/ml) | 4.19 ± 1.07 | 4.65 ± 1.35 | 5.23 ± 0.83 | 5.65 ± 0.63 | 5.13 ± 0.55 | 0.265 | 0.296 | 0.361 |
| GSH/(μmol/L) | 43.37 ± 11.71 | 37.70 ± 9.95 | 49.02 ± 4.61 | 52.60 ± 4.98 | 44.90 ± 3.90 | 0.123 | 0.045 | 0.026 |
| T-AOC/(mmol/L) | 1.17 ± 0.13 | 1.31 ± 0.05 | 1.35 ± 0.07 | 1.32 ± 0.08 | 1.28 ± 0.05 | 0.060 | 0.662 | 0.180 |
| MDA/(nmol/ml) | 2.55 ± 0.86 | 2.58 ± 0.58 | 2.30 ± 0.62 | 2.35 ± 0.65 | 2.33 ± 0.68 | 0.958 | 0.576 | 0.712 |
In the same row, values with different small letter superscripts mean a significant difference (p < 0.05), the same as in the following.
Effect of dietary AEO levels on serum immune index and in pigs
The effects of AEO on serum complement C3 and C4 parameters and immunoglobulins in finishing pigs are presented in Table 6. Dietary supplementation with AEO and CTC had a tendency to increase the serum IgG levels (p = 0.083), and the IgG concentration in serum increased linearly as the dietary AEO concentrations increased from 0 to 1,000 mg/kg (p < 0.05). Additionally, the IgA, IgM, C3 and C4 concentration showed no significant differences among all treatments (p > 0.05).
Table 6.
Effect of dietary AEO levels on serum immune indices in pigs.
| Items | CTC level, mg/kg | AEO levels, mg/kg | p-value | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| 75 | 0 | 250 | 500 | 1,000 | Linear | Quadratic | ||
| IgA (μg/mL) | 641.90 ± 36.78 | 634.34 ± 17.11 | 639.10 ± 25.03 | 642.82 ± 26.42 | 638.49 ± 55.82 | 0.997 | 0.811 | 0.844 |
| IgG (mg/mL) | 19.60 ± 1.73 | 17.24 ± 1.63 | 19.14 ± 0.92 | 19.22 ± 0.92 | 19.90 ± 1.07 | 0.083 | 0.007 | 0.555 |
| IgM (mg/mL) | 19.70 ± 0.72 | 19.93 ± 1.39 | 19.54 ± 1.55 | 19.01 ± 1.35 | 19.02 ± 1.82 | 0.846 | 0.356 | 0.963 |
| C3 (μg/mL) | 107.26 ± 12.24 | 103.37 ± 7.09 | 103.29 ± 1.89 | 100.52 ± 6.97 | 102.56 ± 2.90 | 0.764 | 0.658 | 0.850 |
| C4 (μg/mL) | 7.62 ± 0.85 | 7.62 ± 0.70 | 7.35 ± 0.46 | 7.41 ± 0.78 | 7.53 ± 1.59 | 0.991 | 0.869 | 0.709 |
In the same row, values with different small letter superscripts mean a significant difference (p < 0.05), the same as in the following.
Effect of dietary AEO levels on nutrient apparent digestibility in pigs
Both AEO and CTC tended to increase dry matter digestibility compared to the control group (p = 0.086, Table 7). The dose of AEO showed a quadratic relationship with the apparent digestibility of crude ash and calcium (p < 0.05). Additionally, CTC tend to improve the apparent digestibility of crude fiber (p = 0.065), and significantly increased the apparent digestible energy of the feed (p < 0.05). Neither AEO nor CTC had an effect on the digestibility of CP, EE, GE and phosphorus (p > 0.05).
Table 7.
Effect of dietary AEO levels on nutrient apparent digestibility in pigs.
| Items | CTC level, mg/kg | AEO levels, mg/kg | p-value | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| 75 | 0 | 250 | 500 | 1,000 | Linear | Quadratic | ||
| DM | 84.09 ± 0.57 | 82.18 ± 1.14 | 83.53 ± 0.96 | 83.37 ± 1.82 | 83.33 ± 0.78 | 0.086 | 0.108 | 0.324 |
| CP | 82.03 ± 1.08 | 80.49 ± 1.22 | 81.88 ± 1.65 | 80.31 ± 4.59 | 81.08 ± 1.95 | 0.663 | 0.816 | 0.679 |
| EE | 87.29 ± 1.02 | 87.84 ± 1.34 | 86.94 ± 1.49 | 86.85 ± 1.93 | 87.86 ± 0.26 | 0.662 | 0.688 | 0.201 |
| Ash | 34.95 ± 2.05ab | 30.51 ± 1.88c | 34.62 ± 4.52abc | 37.08 ± 1.27a | 31.65 ± 4.04bc | 0.021 | 0.157 | 0.020 |
| CF | 44.95 ± 5.51 | 38.20 ± 6.48 | 37.69 ± 2.21 | 36.15 ± 2.98 | 37.69 ± 2.72 | 0.065 | 0.690 | 0.745 |
| Ca | 39.99 ± 2.76a | 34.33 ± 3.47b | 43.45 ± 5.31a | 41.38 ± 4.03a | 38.99 ± 1.60ab | 0.014 | 0.038 | 0.011 |
| P | 48.41 ± 3.81 | 46.89 ± 2.84 | 45.32 ± 5.26 | 45.33 ± 3.11 | 44.38 ± 9.17 | 0.730 | 0.472 | 0.967 |
| GE | 86.38 ± 0.68 | 85.46 ± 1.23 | 85.86 ± 0.71 | 85.62 ± 1.97 | 85.44 ± 0.66 | 0.630 | 0.962 | 0.606 |
| AME (Mcal/kg) | 3.27 ± 0.03a | 3.17 ± 0.06b | 3.16 ± 0.03b | 3.17 ± 0.09b | 3.15 ± 0.02b | 0.006 | 0.734 | 0.851 |
In the same row, values with different small letter superscripts mean a significant difference (p < 0.05), the same as in the following.
Alteration of dietary AEO levels on gut microbiota in pigs
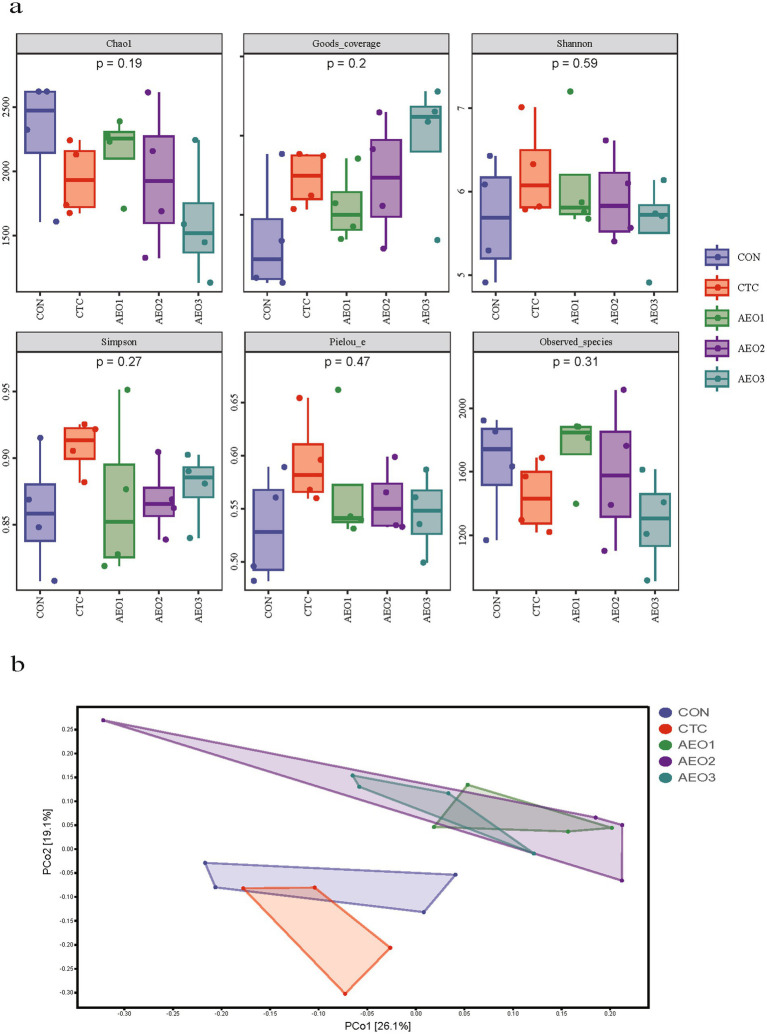
The rarefaction curves nearly reached the saturation plateau (Supplementary Figure S1), indicating that the sequencing captured most of the bacterial diversity. The gut microbiota community did not exhibit significant differences for the α-diversity index (Figure 1a), but β-diversity index revealed that AEO administration differed significantly from both the CON and CTC groups (Figure 1b).
Figure 1.
(a) Alpha diversity indices of the fecal bacterial communities in pigs. Con represents the group fed a basal diet, and CTC represents the group fed a basal diet with antibiotics, AEO1, AEO2, and AEO3 combined with 250 mg/kg, 500 mg/kg, and 1,000 mg/kg, respectively. (b) Principal coordinates analysis (PCoA) of fecal contents bacterial community of pigs. Con represents the group fed a basal diet, and CTC represents the group fed a basal diet with antibiotics, AEO1, AEO2, and AEO3 combined with 250 mg/kg, 500 mg/kg, and 1,000 mg/kg, respectively.
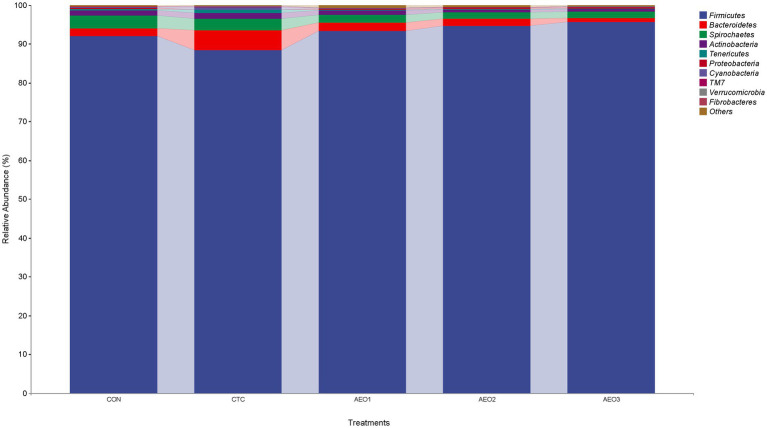
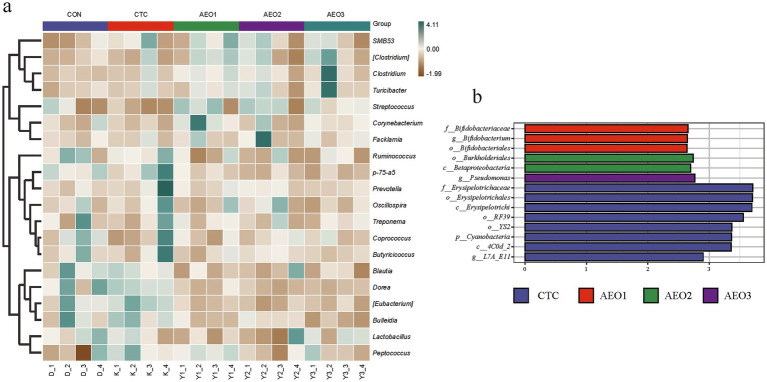
In the pig gut microbiota, microorganisms at the phylum level mainly consist of Firmicutes, Bacteroidetes, Spirochaetes, and Actinobacteria (Figure 2). The heatmap illustrates differences in microbial composition among the five groups at the genus level (Figure 3a). The AEO groups exhibited higher relative abundances of Dorea, Blautia, Butyricicoccus, Bulleidia, and Lactobacillus compared to the control and CTC groups. On the other hand, the relative abundances of Clostridium, Streptococcus, and Turicibacter were lower. Within the Bifidobacteriales group, which includes Bifidobacteriaceae and Bifidobacterium, significantly higher abundances were observed in the AEO1 group (Figure 3b). Pseudomonas were significantly more abundant in the AEO3 group, while Burkholderiales and Betaproteobacteria were significantly more abundant in the AEO2 group (Figure 3b).
Figure 2.
Distribution of fecal bacteria at the phylum level in pigs. Con represents the group fed a basal diet, and CTC represents the group fed a basal diet with antibiotics, AEO1, AEO2, and AEO3 combined with 250 mg/kg, 500 mg/kg, and 1,000 mg/kg, respectively.
Figure 3.
Effects of different concentrations AEO on fecal bacteria at the genus level in pigs. (a) Distribution of fecal top 30 bacteria at the genus level in pigs. (b) Differential microbe composition between the study groups determined by LEfSe. Con represents the group fed a basal diet, and CTC represents the group fed a basal diet with antibiotics, AEO1, AEO2, and AEO3 combined with 250 mg/kg, 500 mg/kg, and 1,000 mg/kg, respectively.
Discussion
Generally, various stressors impact pig health, including pathogens, oxidative stress, inflammation, and reduced growth performance (9). Dietary essential oils have been shown to support pig health through several biological mechanisms, such as anti-inflammatory, antioxidant, antimicrobial, feed palatability enhancement, gut microbiome alteration, and immunomodulatory activities (6, 25–28). The current study aims to evaluate the effectiveness of different concentrations of AEO in improving growth performance, serum antioxidant capacities, immune function, apparent digestibility of nutrients, and gut microbiota in fattening pigs. The results show that different concentrations of AEO had no statistical effects on growth performance and feed efficiency in pigs. However, other studies have corroborated the positive effects of essential oils on pig health and growth performance, as varied compounds present in essential oils contributed to the fragrance of the oil, increasing feed palatability and improving feed intake eventually (29, 30). In this study, the supplementation of 1,000 mg/kg AEO in the diet might result in better growth performance in finishing pigs.
Serum biochemical parameters usually serve as indicators of nutritional metabolism and organ functions in animals (31). The levels of TP, ALB, and GLB in serum are commonly used to assess liver function and the nutritional status of animals (32). Serum TP concentration is closely associated with nitrogen digestibility and protein synthesis capacity (33, 34). Therefore, improved nitrogen digestibility and protein synthesis capacity in animals can lead to a reduction in fecal noxious gas concentration and subsequent decrease in fecal gas emission. Furthermore, GLB, comprising the alpha, beta, and gamma globulin fractions based on protein electrophoretic fractionation, can also reflect immune response and serve as an evaluation point in routine toxicity studies (35, 36). In the present study, when supplemented with AEO in dietary, pigs had higher serum TP, relatively higher GLB and lower L-DLC. This suggests that AEO contributes to better serum biochemical parameters in pigs, and the enhanced capacity for protein and energy metabolism may result in a higher lean meat percentage and better health in lean pigs. These results are consistent with previous studies reported by Su. et al. (6, 37). Besides, when supplemented with AEO, a trend of improved serum IgG concentration has been observed in this research, suggesting enhanced immune function in pigs.
Cellular redox homeostasis is intricately maintained through the generation and elimination of reactive oxygen species (ROS). These ROS not only have the potential to induce cellular damage through the oxidation of proteins, lipids, and DNA, but also serve as signaling molecules that regulate transcription factors and epigenetic pathways, ultimately influencing cell survival and apoptosis (38). Cells in a healthy body usually maintain a redox balance between the generation of reactive oxygen species (ROS) and ROS elimination (39). Many environmental stressors, such as weaning, bacterial and viral infections, and heat, continually impose extensive antioxidant pressures on pigs. These stressors result in ROS accumulation and oxidative stress, putting animals under metabolic stress (40). Under metabolic stress, the redox balance becomes vulnerable and easily damaged (41). Therefore, these findings highlight the importance of antioxidant capacity. In this study, when supplemented with AEO, the pigs exhibited higher enzyme activity in T-AOC and GSH. Total antioxidant capacity is a primary measurement to evaluate the state and potential of oxidative stress, and GSH has been reported to efficiently maintains cellular redox balance (42, 43). These antioxidant capacities, which are involved in the NF-E2-related factor (Nrf2) pathway, could also have hepatic protective effects under pathological conditions (44). Furthermore, meat quality is closely related to the body’s antioxidant status (45), which is also a major factor affecting the consumers’ assessment. Hence, dietary supplementation with AEO has the potential to enhance the body’s antioxidant capacity, effectively protecting pigs from oxidative stress and alleviate metabolic stress.
Digestive enzymes in the intestinal tract play a crucial role in the digestion and absorption of nutrients. Previous studies have demonstrated that essential oils can improve the activity of digestive enzymes and improved nutrient digestibility eventually, which aligns with the findings of this study (46). For instance, Peng et al. (47) reported that dietary supplement with essential oil increased the ratio of villus height to crypt depth in duodenum and sucrase activity in the jejunum mucosa. Furthermore, essential oils were found to up-regulate claudin-1 and IGF-2 mRNA levels and down-regulate TRAF-6, TNFSF15, and TOLLIP mRNA levels in the jejunum of broilers, exhibiting anti-apoptotic and anti-inflammatory effects (48). Essential oils consist of phenolics and aromatic compounds, which enhance feed palatability and intake due to their better flavor and odor (49). these compounds stimulate digestive enzyme secretion and oronasal sensing, specifically for appetite regulation (50–52). Moreover, essential oils have been shown to improve intestinal morphology in pigs, potentially leading to enhanced nutritional absorption and digestion (53). In our study, both CTC and 250 mg/kg of essential oils improved nutrient digestibility, including dry matter, crude ash, calcium, and digestible energy.
The diversity and stability of the gut microbiota are intimately linked to a balanced and stable microecological barrier. A healthy digestive system contributes to improved growth performance (45). The PCoA plots of beta diversity demonstrated that both dietary CTC and AEO altered the composition of the gut microbiota, with AEO showing a greater significant impact. At the phylum level, treatment with CTC and AEO led to a decrease in the relative abundance of Proteobacteria, which includes various pathogens and opportunistic pathogens such as Escherichia, Salmonella and Vibrio (54). Research has also shown a positive correlation between Proteobacteria and gut inflammation in different mouse models of colitis (55, 56). The reduced abundance of Proteobacteria may decrease susceptibility to inflammatory conditions in the intestinal barrier of animals. Additionally, the gut microbiota of pigs are dominated by Firmicutes and Bacteroidetes, similar to humans (57). CTC also reduced the Firmicutes to Bacteroidetes ratio, a relevant marker of gut dysbiosis and obesity (58, 59).
A balanced gut microbiota forms a natural barrier on the surface of the intestinal mucosa and participates in normal digestion and absorption in animals. It also regulates immune function and prevents the invasion of pathogenic bacteria and opportunistic pathogen (60, 61). The Blautia is a genus of anaerobic bacteria exhibiting probiotic characteristics, widely found in the feces and intestines of mammals (62). It primarily provides beneficial anti-inflammatory effects (63). Pigs that were fed with AEO also had a high abundance of Lactobacillus in gut microbiota, which has a long history as an exogenous probiotic (64). Similarly, Butyricicoccus, a kind of butyrate producer with probiotic potential and abundant in AEO group, has been reported to have a positive impact on gut health. These gut microbes play a crucial role in digesting dietary crude fiber and producing short-chain fatty acids (SCFAs), which offer various health benefits to the host (65, 66). SCFAs can bind and activate G protein-coupled receptors (GPRs) such as GPR41 and GPR43, which are expressed in the gastrointestinal tract (67). These receptors have several health benefits, including regulating glucose metabolism, improving insulin sensitivity, and reducing inflammation (68). Specifically, butyrate, a type of SCFA, can initiate a epigenetic program in macrophages, resulting in a reduction in glycolysis and mTOR signaling while promoting the maturation of autophagosome-lysosome (69). In summary, the metabolite products of probiotics have a positive impact on maintaining gut homeostasis. Furthermore, the LEfSe analysis also revealed that some probiotics were abundant in AEO groups. The Bifidobacterium, for instance, positively influences gut health by aiding digestion, producing essential compounds such as B vitamins and healthy fatty acids, and occupying a niche to prevent infections by pathogens (70, 71). The Pseudomonas is known to play a role in breaking down complex carbohydrates and producing short-chain fatty acids in pigs, contributing to gut health maintenance (72). However, research suggests that some microbes in AEO groups may have both beneficial and potentially harmful effects on health, which are not yet well understood, including Dorea, Bulleidia, Burkholderiales and Betaproteobacteria. Dorea may prevent food allergies and sensitivities in infants (73), and prevent obesity and insulin resistance (74). A recent study has also shown that high bacterial abundances of Dorea in the gut microbiome are linked to expansion, immune checkpoint expression, and efficacy of CD19-directed CAR T-cells in patients with relapsed/refractory Diffuse-Large B-Cell Lymphoma (75). Among the decreased relative abundance in the gut microbiota of pigs influenced by AEO, Streptococcus is a commonly found bacterial pathogen in pigs (76). For Clostridium, research in pigs is limited, and its impact varies depending on the specific species. For example, a research reported that Clostridium perfringens PLC could the trigger ERK1/2 pathway to cause cytotoxicity (77). Conversely, the enrichment of Clostridium subgroups may contribute to the improvement of T1D and associated immune imbalance (78). In addition, it has been found that Turicibacter is considered a pro-inflammatory bacteria, and its abundance increases during an enteritis episode (79). Overall, these results demonstrate that AEO modulated the gut microbiota of fattening pigs by increasing the abundance of probiotics and decreasing the abundance of opportunistic pathogens.
Conclusion
Dietary supplementation of 1,000 mg/kg AEO in the pigs’ diet has the potential to improve growth performance and various physiological and biochemical indicators. AEO can also enhance antioxidant levels and increase the efficiency of nutrient digestion and absorption through the regulation of gut microbiota. These findings suggest that AEO may be a viable alternative to CTC.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This research was funded by the National Natural Science Foundation of China (32102594), the Science and Technology Innovation Program of Hunan Province (2022RC1160), Natural Science Foundation of Hunan Province (2023JJ50332 and 2023JJ40085), Science Research Project of Hunan Provincial Department of Education (22B0894, 22C0674 and 22A0661), The Hunan Provincial University Key Laboratory of the Regional Characteristic Traditional Chinese Medicine Resources and Ecological Agriculture, and the ESI Discipline Special Project of Changsha Medical University (2022CYY023 and 2022CYY018).
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA1090261.
Ethics statement
All experimental procedures were reviewed and approved by the animal care committee of Changsha Medical University, Changsha, China (No.2023-01). The study was conducted in accordance with the local legislation and institutional requirements.
Author contributions
FC: Conceptualization, Methodology, Writing – original draft, Writing – review & editing. ZhiL: Data curation, Writing – original draft, Writing – review & editing. CX: Data curation, Writing – review & editing. JieH: Data curation, Formal analysis, Writing – review & editing. JC: Methodology, Validation, Writing – review & editing. KP: Methodology, Validation, Writing – review & editing. XC: Methodology, Validation, Writing – review & editing. JiaH: Investigation, Resources, Writing – review & editing. ZheL: Investigation, Resources, Software, Writing – review & editing. HY: Investigation, Resources, Writing – review & editing. KK: Project administration, Supervision, Writing – review & editing. BH: Funding acquisition, Project administration, Supervision, Writing – review & editing. QL: Funding acquisition, Investigation, Supervision, Writing – review & editing.
Conflict of interest
ZhiL was employed by Hunan Nuoz Biological Technology Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer HZ declared a shared affiliation with the author CX to the handling editor at the time of review.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2024.1468520/full#supplementary-material
Rarefaction curves of observed bacterial sequences in the fecal contents of pigs. Con represents the group fed a basal diet, and CTC represents the group fed a basal diet with antibiotics, AEO1, AEO2, and AEO3 combined with 250 mg/kg, 500 mg/kg, and 1000 mg/kg, respectively.
References
- 1.Kuang Y, Guo X, Hu J, Li S, Zhang R, Gao Q, et al. Occurrence and risks of antibiotics in an urban river in northeastern tibetan plateau. Sci Rep. (2020) 10:20054. doi: 10.1038/s41598-020-77152-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kivits T, Broers HP, Beeltje H, van Vliet M, Griffioen J. Presence and fate of veterinary antibiotics in age-dated groundwater in areas with intensive livestock farming. Environ Pollut. (2018) 241:988–98. doi: 10.1016/j.envpol.2018.05.085 [DOI] [PubMed] [Google Scholar]
- 3.Heydarian M, Ebrahimnezhad Y, Meimandipour A, Hosseini SA, Banabazi MH. Effects of dietary inclusion of the encapsulated thyme and oregano essential oils mixture and probiotic on growth performance, immune response and intestinal morphology of broiler chickens. Poult Sci J. (2020) 8:17–25. doi: 10.22069/psj.2020.17101.1497 [DOI] [Google Scholar]
- 4.Ge C, Luo X, Wu L, Lv Y, Hu Z, Yu D, et al. Plant essential oils improve growth performance by increasing antioxidative capacity, enhancing intestinal barrier function, and modulating gut microbiota in muscovy ducks. Poult Sci. (2023) 102:102813. doi: 10.1016/j.psj.2023.102813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hyun Y, Ellis M, Riskowski G, Johnson RW. Growth performance of pigs subjected to multiple concurrent environmental stressors. J Anim Sci. (1998) 76:721–7. doi: 10.2527/1998.763721x, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Chen F, Wang Y, Wang K, Chen J, Jin K, Peng K, et al. Effects of litsea cubeba essential oil on growth performance, blood antioxidation, immune function, apparent digestibility of nutrients, and fecal microflora of pigs. Front Pharmacol. (2023) 14:1166022. doi: 10.3389/fphar.2023.1166022, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarfaraz D, Rahimmalek M, Sabzalian MR, Gharibi S, Matkowski A, Szumny A. Essential oil composition and antioxidant activity of oregano and marjoram as affected by different light-emitting diodes. Molecules. (2023) 28:3714. doi: 10.3390/molecules28093714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miguel MG. Antioxidant and anti-inflammatory activities of essential oils: a short review. Molecules. (2010) 15:9252–87. doi: 10.3390/molecules15129252, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Omonijo FA, Ni L, Gong J, Wang Q, Lahaye L, Yang C. Essential oils as alternatives to antibiotics in swine production. Anim Nutr. (2018) 4:126–36. doi: 10.1016/j.aninu.2017.09.001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aslan I, Tarhan Celebi L, Kayhan H, Kizilay E, Gulbahar MY, Kurt H, et al. Probiotic formulations containing fixed and essential oils ameliorates SIBO-induced gut dysbiosis in rats. Pharmaceuticals (Basel). (2023) 16:1041. doi: 10.3390/ph16071041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mo K, Li J, Liu F, Xu Y, Huang X, Ni H. Superiority of microencapsulated essential oils compared with common essential oils and antibiotics: effects on the intestinal health and gut microbiota of weaning piglet. Front Nutr. (2021) 8:808106. doi: 10.3389/fnut.2021.808106, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li C, Lin X, Cai Q, Zhang W, Wu X, Tan Z, et al. Essential oil composition anti-inflammatory activities and distribution of fruits of alpinia oxyphylla miq. J Essent Oil Bear Plants. (2022) 25:482–94. doi: 10.1080/0972060X.2022.2091954 [DOI] [Google Scholar]
- 13.Zhao X, Wei J, Shu X, Kong W, Yang M. Multi-elements determination in medical and edible alpinia oxyphylla and morinda officinalis and their decoctions by icp-ms. Chemosphere. (2016) 164:430–5. doi: 10.1016/j.chemosphere.2016.08.122, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Zhang Q, Zheng Y, Hu X, Hu X, Lv W, Lv D, et al. Ethnopharmacological uses, phytochemistry, biological activities, and therapeutic applications of alpinia oxyphylla miquel: a review. J Ethnopharmacol. (2018) 224:149–68. doi: 10.1016/j.jep.2018.05.002, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Yuan L, Pan K, Li Y, Yi B, Gao B. Comparative transcriptome analysis of alpinia oxyphylla miq. Reveals tissue-specific expression of flavonoid biosynthesis genes. BMC Genom Data. (2021) 22:19. doi: 10.1186/s12863-021-00973-4, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Z, Kong X, Zuo L, Kang J, Hou L, Zhang X. Rapid extraction and determination of 25 bioactive constituents in alpinia oxyphylla using microwave extraction with ultra high performance liquid chromatography with tandem mass spectrometry. J Sep Sci. (2016) 39:603–10. doi: 10.1002/jssc.201501056, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Wang C, Yuan H, Bao X, Lan M. In vitro antioxidant and cytotoxic properties of ethanol extract of alpinia oxyphylla fruits. Pharm Biol. (2013) 51:1419–25. doi: 10.3109/13880209.2013.794844, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Lee YM, Son E, Kim S, Kim D. Effect of alpinia oxyphylla extract in vitro and in a monosodium iodoacetate-induced osteoarthritis rat model. Phytomedicine. (2019) 65:153095. doi: 10.1016/j.phymed.2019.153095, PMID: [DOI] [PubMed] [Google Scholar]
- 19.Xie Y, Xiao M, Ni Y, Jiang S, Feng G, Sang S, et al. Alpinia oxyphylla miq. Extract prevents diabetes in mice by modulating gut microbiota. J Diabetes Res. (2018) 2018:4230590. doi: 10.1155/2018/4230590, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ji F, Gu L, Rong G, Hu C, Sun W, Wang D, et al. Using extract from the stems and leaves of yizhi (alpiniae oxyphyllae) as feed additive increases meat quality and intestinal health in ducks. Front Vet Sci. (2021) 8:793698. doi: 10.3389/fvets.2021.793698, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Busayo FK, Oluwatosin O, Toba OA, Esther OD, Chen H. Antibacterial activity of organic compounds from the leaves of alpinia oxyphylla on multi-drug resistant bacteria isolated from patients with urinary tract infections. Int J Sci Res Arch. (2023) 8:351–6. doi: 10.30574/ijsra.2023.8.2.0266 [DOI] [Google Scholar]
- 22.Wang K, Zhou M, Gong X, Zhou Y, Chen J, Ma J, et al. Starch-protein interaction effects on lipid metabolism and gut microbes in host. Front Nutr. (2022) 9:1018026. doi: 10.3389/fnut.2022.1018026, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen F, He J, Wang X, Lv T, Liu C, Liao L, et al. Effect of dietary ramie powder at various levels on the growth performance, meat quality, serum biochemical indices and antioxidative capacity of yanling white geese. Animals. (2022) 12:2045. doi: 10.3390/ani12162045, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang K, Ma J, Li Y, Han Q, Yin Z, Zhou M, et al. Effects of essential oil extracted from artemisia argyi leaf on lipid metabolism and gut microbiota in high-fat diet-fed mice. Front Nutr. (2022) 9:1024722. doi: 10.3389/fnut.2022.1024722, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shao Y, Peng Q, Wu Y, Peng C, Wang S, Zou L, et al. The effect of an essential oil blend on growth performance, intestinal health, and microbiota in early-weaned piglets. Nutrients. (2023) 15:450. doi: 10.3390/nu15020450, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen J, Chen F, Peng S, Ou Y, He B, Li Y, et al. Effects of artemisia argyi powder on egg quality, antioxidant capacity, and intestinal development of roman laying hens. Front Physiol. (2022) 13:902568. doi: 10.3389/fphys.2022.902568, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dosoky NS, Kirpotina LN, Schepetkin IA, Khlebnikov AI, Lisonbee BL, Black JL, et al. Volatile composition, antimicrobial activity, and in vitro innate immunomodulatory activity of echinacea purpurea (l.) Moench essential oils. Molecules. (2023) 28:7330. doi: 10.3390/molecules28217330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pan J, Zhu Y, Abdel-Samie MA, Li C, Cui H, Lin L. Biological properties of essential oil emphasized on the feasibility as antibiotic substitute in feedstuff. Grain Oil Sci Technol. (2023) 6:10–23. doi: 10.1016/j.gaost.2022.11.001 [DOI] [Google Scholar]
- 29.Ornaghi MG, Passetti RA, Torrecilhas JA, Mottin C, Vital ACP, Guerrero A, et al. Essential oils in the diet of young bulls: effect on animal performance, digestibility, temperament, feeding behaviour and carcass characteristics. Anim Feed Sci Technol. (2017) 234:274–83. doi: 10.1016/j.anifeedsci.2017.10.008 [DOI] [Google Scholar]
- 30.Mary Mawumenyo Mamattah K, Kusiwaa Adomako A, Nketia Mensah C, Borquaye LS. Chemical characterization, antioxidant, antimicrobial, and antibiofilm activities of essential oils of plumeria alba (forget-me-not). Biochem Res Int. (2023) 2023:1040478. doi: 10.1155/2023/1040478, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng X, Li X, Liu Y, Ma Y, Zhang R, Zhang Y, et al. Dna methylome and transcriptome identified key genes and pathways involved in speckled eggshell formation in aged laying hens. BMC Genomics. (2023) 24:31. doi: 10.1186/s12864-022-09100-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tan N, Gao Y, Wang Y, Deng S, Yuan P, Jiang T, et al. The influence of hypothermia hibernation combined with co (2) anesthesia on life and storage quality of large yellow croaker (pseudosciaena crocea). Foods. (2022) 11:514. doi: 10.3390/foods11040514, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho JH, Chen YJ, Min BJ, Kim HJ, Kwon OS, Shon KS, et al. Effects of essential oils supplementation on growth performance, igg concentration and fecal noxious gas concentration of weaned pigs. Asian Australas J Anim Sci. (2005) 19:80–5. doi: 10.5713/ajas.2006.80 [DOI] [Google Scholar]
- 34.Sun X, Su Y, Hao Y, Zhang J, Yue X, Wang W, et al. Novel process methods for the whole cottonseed: effect on the digestibility, productivity, fat profile, and milk gossypol levels in lactating dairy cows. Front Nutr. (2022) 9:801712. doi: 10.3389/fnut.2022.801712, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nagyová V, Arfuso F, Rizzo M, Zanghì E, Nagy O, Piccione G. Stability of total proteins and their electrophoretic fractions in goat serum (capra hircus), maintained under different condition. Small Rumin Res. (2016) 144:145–8. doi: 10.1016/j.smallrumres.2016.09.016 [DOI] [Google Scholar]
- 36.Kaminski NE, Kaplan BLF, Holsapple MP. Toxic responses of the immune system. In: C. Klaassen editor, Casarett and Doull’s Toxicology:The Basic Science of Poisons. 7th ed, New York: McGraw-Hill., vol. 12 (2008). 485–555. [Google Scholar]
- 37.Su G, Zhou X, Wang Y, Chen D, Chen G, Li Y, et al. Effects of plant essential oil supplementation on growth performance, immune function and antioxidant activities in weaned pigs. Lipids Health Dis. (2018) 17:139. doi: 10.1186/s12944-018-0788-3, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang H, Gong W, Wu S, Perrett S. Hsp 70 in redox homeostasis. Cells. (2022) 11:829. doi: 10.3390/cells11050829, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun D, Wang Z, Zhang P, Yin C, Wang J, Sun Y, et al. Ruthenium-loaded mesoporous silica as tumor microenvironment-response nano-Fenton reactors for precise cancer therapy. J Nanobiotechnology. (2021) 19:98. doi: 10.1186/s12951-021-00848-x, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J, Chen D, Yu B, Luo Y, Zheng P, Mao X, et al. Chlorogenic acid attenuates oxidative stress-induced intestinal mucosa disruption in weaned pigs. Front Vet Sci. (2022) 9:806253. doi: 10.3389/fvets.2022.806253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao Y, Hu X, Liu Y, Dong S, Wen Z, He W, et al. Ros signaling under metabolic stress: cross-talk between ampk and akt pathway. Mol Cancer. (2017) 16:79. doi: 10.1186/s12943-017-0648-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang S, Othman A, Koshkin A, Fischer S, Fischer D, Zamboni N, et al. Astrocyte glutathione maintains endothelial barrier stability. Redox Biol. (2020) 34:101576. doi: 10.1016/j.redox.2020.101576, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Teleanu DM, Niculescu A, Lungu II, Radu CI, Vladâcenco O, Roza E, et al. An overview of oxidative stress, neuroinflammation, and neurodegenerative diseases. Int J Mol Sci. (2022) 23:5938. doi: 10.3390/ijms23115938, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang Q, Hu X, Hui F, Song Q, Cui C, Wang C, et al. Ethanol extract and its dichloromethane fraction of alpinia oxyphylla miquel exhibited hepatoprotective effects against ccl (4)-induced oxidative damage in vitro and in vivo with the involvement of nrf2. Biomed Pharmacother. (2017) 91:812–22. doi: 10.1016/j.biopha.2017.04.131 [DOI] [PubMed] [Google Scholar]
- 45.Kang K, Zhou N, Peng W, Peng F, Ma M, Li L, et al. Multi-omics analysis of the microbiome and metabolome reveals the relationship between the gut microbiota and wooden breast myopathy in broilers. Front Vet Sci. (2022) 9:922516. doi: 10.3389/fvets.2022.922516, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen J, Chen L. Effects of chitosan-supplemented diets on the growth performance, nonspecific immunity and health of loach fish (misgurnus anguillicadatus). Carbohydr Polym. (2019) 225:115227. doi: 10.1016/j.carbpol.2019.115227, PMID: [DOI] [PubMed] [Google Scholar]
- 47.Peng X, Zhou Q, Wu C, Zhao J, Tan Q, He Y, et al. Effects of dietary supplementation with essential oils and protease on growth performance, antioxidation, inflammation and intestinal function of weaned pigs. Anim Nutr. (2022) 9:39–48. doi: 10.1016/j.aninu.2021.12.003, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pham VH, Kan L, Huang J, Geng Y, Zhen W, Guo Y, et al. Dietary encapsulated essential oils and organic acids mixture improves gut health in broiler chickens challenged with necrotic enteritis. J Anim Sci Biotechnol. (2020) 11:18. doi: 10.1186/s40104-019-0421-y, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kroismayr A, Steiner T, Zhang C. Influence of a phytogenic feed additive on performance of weaner piglets. J Anim Sci. (2006) 84:329. doi: 10.3389/fanim.2021.767034 [DOI] [Google Scholar]
- 50.Windisch W, Schedle K, Plitzner C, Kroismayr A. Use of phytogenic products as feed additives for swine and poultry. J Anim Sci. (2008) 86:E140–8. doi: 10.2527/jas.2007-0459 [DOI] [PubMed] [Google Scholar]
- 51.Flachowsky G, Chesson A, Aulrich K. Animal nutrition with feeds from genetically modified plants. Arch Anim Nutr. (2005) 59:1–40. doi: 10.1080/17450390512331342368 [DOI] [PubMed] [Google Scholar]
- 52.Dehghani N, Afsharmanesh M, Salarmoini M, Ebrahimnejad H. In vitro and in vivo evaluation of thyme (thymus vulgaris) essential oil as an alternative for antibiotic in quail diet1. J Anim Sci. (2019) 97:2901–13. doi: 10.1093/jas/skz179, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang W, Wang Y, Lu Y, Tian X, Chen S, Wu B, et al. Inositol hexaphosphate promotes intestinal adaptation in short bowel syndrome via an hdac3-mediated epigenetic pathway. Food Nutr Res. (2023) 67:10–29219. doi: 10.29219/fnr.v67.8694, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oh JK, Pajarillo EAB, Chae JP, Kim IH, Kang D. Protective effects of bacillus subtilis against salmonella infection in the microbiome of hy-line brown layers. Asian Australas J Anim Sci. (2017) 30:1332–9. doi: 10.5713/ajas.17.0063, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Selvanantham T, Lin Q, Guo CX, Surendra A, Fieve S, Escalante NK, et al. Nkt cell-deficient mice harbor an altered microbiota that fuels intestinal inflammation during chemically induced colitis. J Immunol. (2016) 197:4464–72. doi: 10.4049/jimmunol.1601410, PMID: [DOI] [PubMed] [Google Scholar]
- 56.Maharshak N, Packey CD, Ellermann M, Manick S, Siddle JP, Huh EY, et al. Altered enteric microbiota ecology in interleukin 10-deficient mice during development and progression of intestinal inflammation. Gut Microbes. (2013) 4:316–24. doi: 10.4161/gmic.25486, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Adams S, Che D, Hailong J, Zhao B, Rui H, Danquah K, et al. Effects of pulverized oyster mushroom (pleurotus ostreatus) on diarrhea incidence, growth performance, immunity, and microbial composition in piglets. J Sci Food Agric. (2019) 99:3616–27. doi: 10.1002/jsfa.9582, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stojanov S, Berlec A, Štrukelj B. The influence of probiotics on the firmicutes/bacteroidetes ratio in the treatment of obesity and inflammatory bowel disease. Microorganisms. (2020) 8:1715. doi: 10.3390/microorganisms8111715, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Magne F, Gotteland M, Gauthier L, Zazueta A, Pesoa S, Navarrete P, et al. The firmicutes/bacteroidetes ratio: a relevant marker of gut dysbiosis in obese patients? Nutrients. (2020) 12:1474. doi: 10.3390/nu12051474, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bäckhed F, Ding H, Wang T, Hooper LV, Koh GY, Nagy A, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. (2004) 101:15718–23. doi: 10.1073/pnas.0407076101, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cui H, Hu Y, Li J, Yuan K. Hypoglycemic mechanism of the berberine organic acid salt under the synergistic effect of intestinal flora and oxidative stress. Oxidative Med Cell Longev. (2018) 2018:8930374. doi: 10.1155/2018/8930374, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu X, Mao B, Gu J, Wu J, Cui S, Wang G, et al. Blautia-a new functional genus with potential probiotic properties? Gut Microbes. (2021) 13:1–21. doi: 10.1080/19490976.2021.1875796, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Chiu C, Chan Y, Tsai M, Wang C, Chiang M, Chiu C, et al. Cross-talk between airway and gut microbiome links to ige responses to house dust mites in childhood airway allergies. Sci Rep. (2020) 10:13449. doi: 10.1038/s41598-020-70528-7, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang G, Chen Y, Fei S, Xie C, Xia Y, Ai L. Colonisation with endogenous lactobacillus reuteri r28 and exogenous lactobacillus plantarum ar17-1 and the effects on intestinal inflammation in mice. Food Funct. (2021) 12:2481–8. doi: 10.1039/d0fo02624g [DOI] [PubMed] [Google Scholar]
- 65.Yousi F, Kainan C, Junnan Z, Chuanxing X, Lina F, Bangzhou Z, et al. Evaluation of the effects of four media on human intestinal microbiota culture in vitro. AMB Express. (2019) 9:69. doi: 10.1186/s13568-019-0790-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kang K, Hu Y, Wu S, Shi S. Comparative metagenomic analysis of chicken gut microbial community, function, and resistome to evaluate noninvasive and cecal sampling resources. Animals. (2021) 11:1718. doi: 10.3390/ani11061718, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silva YP, Bernardi A, Frozza RL. The role of short-chain fatty acids from gut microbiota in gut-brain communication. Front Endocrinol (Lausanne). (2020) 11:25. doi: 10.3389/fendo.2020.00025, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang D, Jian Y, Zhang Y, Li Y, Gu L, Sun H, et al. Short-chain fatty acids in diseases. Cell Commun Signal. (2023) 21:212. doi: 10.1186/s12964-023-01219-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Møller SH, Wang L, Ho P. Metabolic programming in dendritic cells tailors immune responses and homeostasis. Cell Mol Immunol. (2022) 19:370–83. doi: 10.1038/s41423-021-00753-1, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Callaway TR, Edrington TS, Anderson RC, Harvey RB, Genovese KJ, Kennedy CN, et al. Probiotics, prebiotics and competitive exclusion for prophylaxis against bacterial disease. Anim Health Res Rev. (2008) 9:217–25. doi: 10.1017/S1466252308001540, PMID: [DOI] [PubMed] [Google Scholar]
- 71.O'Callaghan A, van Sinderen D. Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol. (2016) 7:925. doi: 10.3389/fmicb.2016.00925, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang S, Zhang H, Zhang C, Wang G, Shi C, Li Z, et al. Composition and evolutionary characterization of the gut microbiota in pigs. Int Microbiol. (2023) 27:993–1008. doi: 10.1007/s10123-023-00449-8, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Savage JH, Lee-Sarwar KA, Sordillo J, Bunyavanich S, Zhou Y, O'Connor G, et al. A prospective microbiome-wide association study of food sensitization and food allergy in early childhood. Allergy. (2018) 73:145–52. doi: 10.1111/all.13232, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao J, Guo X, Wei W, Li R, Hu K, Liu X, et al. The association of fried meat consumption with the gut microbiota and fecal metabolites and its impact on glucose homoeostasis, intestinal endotoxin levels, and systemic inflammation: a randomized controlled-feeding trial. Diabetes Care. (2021) 44:1970–9. doi: 10.2337/dc21-0099, PMID: [DOI] [PubMed] [Google Scholar]
- 75.Blumenberg V, Busch G, Baumann S, Schmidt S, Schuhmacher H, Winkelmann M, et al. High bacterial abundances of dorea and pediococcus in the gut microbiome linked to expansion, immune checkpoint expression and efficacy of cd19-directed car t-cells in patients with r/r DLBCL. Blood. (2021) 138:2792. doi: 10.1182/blood-2021-153117 [DOI] [Google Scholar]
- 76.He W, Gao Y, Guo Z, Yang Z, Wang X, Liu H, et al. Effects of fermented wheat bran and yeast culture on growth performance, immunity, and intestinal microflora in growing-finishing pigs. J Anim Sci. (2021) 99:skab308. doi: 10.1093/jas/skab308, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Srinon V, Withatanung P, Chaiwattanarungruengpaisan S, Thongdee M, Meethai C, Stevens JM, et al. Functional redundancy of burkholderia pseudomallei phospholipase c enzymes and their role in virulence. Sci Rep. (2020) 10:19242. doi: 10.1038/s41598-020-76186-z, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jia L, Shan K, Pan L, Feng N, Lv Z, Sun Y, et al. Clostridium butyricum cgmcc0313.1 protects against autoimmune diabetes by modulating intestinal immune homeostasis and inducing pancreatic regulatory t cells. Front Immunol. (2017) 8:1345. doi: 10.3389/fimmu.2017.01345, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yu X, Fu C, Cui Z, Chen G, Xu Y, Yang C. Inulin and isomalto-oligosaccharide alleviate constipation and improve reproductive performance by modulating motility-related hormones, short-chain fatty acids, and feces microflora in pregnant sows. J Anim Sci. (2021) 99:skab257. doi: 10.1093/jas/skab257, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rarefaction curves of observed bacterial sequences in the fecal contents of pigs. Con represents the group fed a basal diet, and CTC represents the group fed a basal diet with antibiotics, AEO1, AEO2, and AEO3 combined with 250 mg/kg, 500 mg/kg, and 1000 mg/kg, respectively.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA1090261.