Abstract
Chronic insomnia has the potential to significantly impact physical well-being, occupational performance, and overall quality of life. This review summarizes the clinical and basic research on the central regulatory mechanism of acupuncture in treating primary insomnia (PI), aiming to explore the clinical effectiveness and possible mechanism of acupuncture in treating PI. The currently available drugs for insomnia exhibit notable adverse effects and tend to induce dependence. Empirical evidence from clinical investigations has demonstrated that acupuncture has a favorable safety profile while substantially enhancing the sleep quality of individuals diagnosed with PI. The combination of acupuncture and medication has been shown to augment the therapeutic efficacy of medication while reducing the dosage and mitigating the occurrence of unwanted effects. A review of the current clinical and basic research on the effects of acupuncture on central alterations in PI patients revealed that acupuncture exerts a regulatory influence on the functional activity of brain regions implicated in cognitive and emotional processes. Additionally, acupuncture has been found to impact metabolite levels and circadian clock gene expression and enhance inflammatory responses and energy metabolism. Notably, a single acupuncture intervention had a modulatory effect on functional brain regions similar to that of cumulative acupuncture. The current clinical trials on acupuncture have been limited in scale, and basic research has focused on a single objective. With the continuous progress of brain research, extensive clinical randomized controlled trials of high quality can be combined with various neuroimaging technology modalities. Moreover, different targets and pathways can be explored through basic research. This may serve to enhance the understanding of the fundamental central nervous system mechanisms involved in the efficacy of acupuncture in treating PI.
Keywords: acupuncture, primary insomnia, brain, neural mechanism, neuroimaging
1. Introduction
According to statistical data, approximately 30–36% of adults have reported experiencing at least one symptom associated with nocturnal sleep disturbances, such as difficulties in falling asleep or maintaining sleep or experiencing irregular sleep patterns (1). Primary insomnia (PI) is a specific type of insomnia that persists even after excluding potential secondary factors. PI is characterized by protracted sleep onset, difficulty maintaining sleep, daytime sleepiness, and impaired cognitive functioning (2). These symptoms significantly impact an individual’s quality of life and work performance (3). Inadequate treatment of insomnia may lead to mental health issues, metabolic illnesses, and cognitive impairment (4). Currently, there is a lack of universal agreement regarding pharmacological interventions for the treatment of insomnia. Four primary categories of medications are commonly employed: GABA sedative hypnotic pharmaceuticals, melatonin agonists, orexin receptor antagonists, and sedative antidepressants. However, patients taking these medications are susceptible to developing tolerance and reliance, which can impact daily functioning in the short term and result in long-term dependency (5). In addition to the use of medication, cognitive behavioral therapy (CBT) is a widely accepted and preferred method for managing chronic insomnia (6–8). However, CBT may also cause side effects or adverse reactions, which may lead to increased daytime sleepiness and a predisposition to accidents or injuries (8, 9). Hence, there has been a continuous endeavor to identify a treatment for PI that is more convenient and has fewer adverse effects.
Acupuncture is an important part of complementary and alternative medicine. Multiple investigations have indicated that acupuncture therapy is highly recommended in neurology, musculoskeletal and connective tissue, obstetrics and gynecology, cancer, gastrointestinal illnesses, and other fields (9–11). At present, many clinical or basic have confirmed that acupuncture has benign regulatory effects on sleep quality, especially for primary insomnia, depression-related insomnia, and cancer-related insomnia (12, 13), with few adverse reactions and low costs (14). Clinical studies have shown that acupuncture significantly improves subjective parameters such as sleep quality and duration compared with sham acupuncture or drug therapy (15). The objective outcome indices of acupuncture for improving the severity of insomnia are also better than those of sham acupuncture, which are shown to significantly improve sleep efficiency and total sleep time, as measured by polysomnography and an activity recorder (16, 17). Basic scientific research in animal models shows that acupuncture can stimulate neurons in the brain (18); affect the synthesis, release and role of sleep-related neurotransmitters (such as catecholamine, glutamic acid, and melatonin) (19–22); increase the nitric oxide content in the brain and blood (23); and regulate the neural activity of the autonomic nervous system (24), thus providing a biological rationale for the treatment of insomnia. With the wide application of acupuncture in the treatment of insomnia, many studies have explored their clinical efficacy and potential central mechanism. Recent studies have also clarified the key position of autonomic nerves in the therapeutic role of acupuncture (25). Due to the establishment of the brain plan, the application of central nervous system imaging technology in the clinical research of acupuncture in treating PI patients has increased (26, 27). Neuroimaging technology can be used to visually recognize changes in the central nervous system (28). This approach helps explore the regulatory role of acupuncture in abnormal functional networks. Several studies have used neuroimaging techniques to assess the clinical impact of acupuncture in treating PI patients. Research shows that acupuncture can regulate the function or irregularity of many regions in the brain (29, 30). However, the existing research has not yet explored the exact central mechanism of acupuncture to improve sleep quality, which to some extent limit the clinical application of acupuncture in treating PI, and may cause patients to miss timely and effective treatment.
In view of the increasing literature on the effectiveness of acupuncture in treatment PI and neurobiological pathways, there is still a lack of comprehensive review on the central brain regulation mechanism of acupuncture in treatment PI. Therefore, it is now time to synthesize and review the existing evidence of the mechanism and clinical effectiveness of acupuncture in treatment PI, aiming to consolidate its effectiveness and guide future research, and provide a new perspective on the clinical use of acupuncture for enhancing sleep quality and regulating the sleep–wake cycle in individuals with PI.
2. Methods
2.1. Search strategy
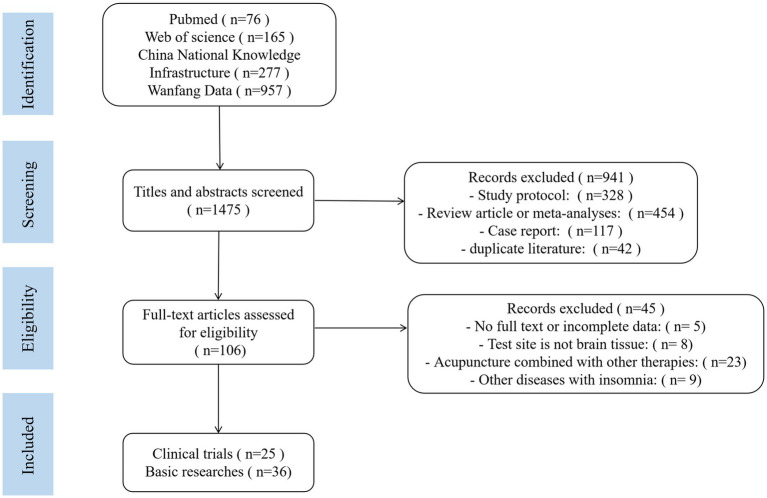
The following six electronic databases have been accessed since the database was created until June 2024 with no restrictions on the state of publications: PubMed, web of science, China National Knowledge Infrastructure and Wanfang Data. Relevant articles from references and reviews of selected publications were also verified. Search terms included “acupuncture” and “insomnia.” After initial screening, a total of 1,475 articles were retrieved. The retrieved studies were filtered using Zotero, and 941 articles were filtered based on title and abstract. Another 45 articles were filtered by reading the full text, and 25 clinical trials and 36 basic research articles were finally included. The flow diagram of literature screening is shown in Figure 1.
Figure 1.
The flow diagram of literature screening.
2.2. The inclusion and exclusion criteria
Research types. This review includes all published clinical trials and animal basic researches on acupuncture treatment of primary insomnia. The language is limited to English and Chinese. Exclude meta-analyses, reviews, experimental protocols, cases, conference papers, and dissertations. At the same time, studies without full-text or incomplete data should be excluded.
Research participants. Primary insomnia patients (as diagnosed by a clinician, or using any recognized diagnostic criteria). Exclude participants with other severe mental disorders or physical illnesses, or patients with insomnia due to other illnesses.
Intervention methods. Manual acupuncture, electroacupuncture, transcutaneous auricular vagus nerve stimulation, auricular acupuncture, warm acupuncture or shallow acupuncture. Exclude studies that combine other treatments.
Research results. The study explored the regulation results of acupuncture on the brain center, and excluded studies that did not involve the regulation of the brain center.
2.3. Data extraction
The purpose of the literature search was to analyze the mechanisms of central brain regulation of PI by acupuncture. Validate the final included articles and extract relevant data. Any disagreements were resolved by discussions between authors.
3. Clinical efficacy of acupuncture in treating PI
The number of studies about the efficacy and safety of acupuncture as a treatment modality for insomnia is experiencing notable growth. Multiple systematic reviews and meta-analyses provide empirical evidence supporting the therapeutic efficacy of acupuncture. Table 1 summarizes the results of these systematic reviews. A study conducted by Zhao et al. demonstrated that acupuncture had a significant positive impact on various objective sleep measures, such as an increase in total sleep time and sleep efficiency and a decrease in the number of times waking up after falling asleep and the number of arousals. Additionally, subjective sleep quality was also found to improve in patients with PI. The researchers recommended a minimum threshold of at least 12 acupuncture treatments for optimal results (31). Kim et al. analyzed on the duration of acupuncture treatment cycles. Their findings indicated that patients with PI may experience noteworthy symptom improvement throughout acupuncture treatment lasting longer than 3 weeks (32). A comparative analysis was conducted to assess the efficacy of various acupuncture therapies in enhancing Pittsburgh Sleep Quality Index (PSQI) scores. The findings indicated that acupoint catgut embedding, auricular acupressure, or auricular acupuncture combined with manual acupuncture, electroacupuncture combined with acupoint application, and intradermal needle therapy were effective. However, it is important to note that the certainty of the evidence supporting these conclusions was rated as moderate to low (13). Previous research has examined the effectiveness of singular acupuncture stimulation at the SP6 acupoint for addressing insomnia. These studies demonstrated enhancements in sleep quality as well as increases in the duration of both the deep sleep and rapid eye movement phases. However, the systematic review encompassed a limited number of studies, and the overall quality of the literature was deemed to be moderate (33). Consequently, it is imperative to conduct future investigations to scrutinize the findings of the study. According to a meta-analysis conducted by Cao et al., acupuncture demonstrated greater efficacy than pharmacological interventions in terms of increasing the total sleep time by more than 3 h in patients. However, when considering the average sleep duration, there was no discernible distinction between the effects of acupuncture and medication. The combination of acupuncture and medication yielded superior outcomes compared to the administration of medication alone in terms of total sleep duration (15). A study conducted by Kim et al. demonstrated that the application of syndrome differentiation in traditional Chinese medicine acupuncture led to a notable enhancement in the overall effectiveness of treatment compared to that of Western medicine treatments such as nonbenzodiazepine hypnotics and benzodiazepine receptor agonists. However, the improvement in PSQI score was similar between the two approaches (34). A study of insomnia among senior individuals revealed that various interventions, namely, acupuncture, acupuncture in conjunction with benzodiazepines, behavioral treatment, benzodiazepines alone, benzodiazepines combined with CBT, and CBT, produced favorable outcomes. Significantly, the utilization of combination therapy, such as the combination of benzodiazepines with CBT or benzodiazepines with acupuncture, has been found to exhibit overall superiority compared to other forms of single-therapy treatments (35). Hypnotic medications, namely, benzodiazepine agonists, are associated with notable adverse effects, including but not limited to headaches, nightmares, daytime weariness, aberrant behavior, and gastrointestinal disturbances (36). These side effects impose some constraints on the available treatment choices for patients. Previous research has indicated that hematoma, discomfort, headache, and bleeding are the primary mild adverse effects associated with acupuncture (13). These adverse events have been found to pose a relatively low risk for patients with PI.
Table 1.
Summary of systematic review and meta-analysis results of acupuncture in treating PI.
| Comparison | Outcome measures | Findings | Limitations | Refs. |
|---|---|---|---|---|
| Acupuncture vs. Sham−/placebo-acupuncture or waitlist control | Polysomnography, actigraphy, or micromovement sensitive mattress/pillow sleep monitoring systems |
|
|
(31) |
| Acupuncture vs. Pharmacotherapy or sham-acupuncture | PSQI, ISI | Compared with drug treatment, after more than 3 weeks of acupuncture treatment, the symptoms of insomnia patients may be significantly improved |
|
(32) |
| Acupoint catgut embedding vs. Auricular acupressure or Auricular acupuncture or Electroacupuncture plus Acupoint application. | PSQI, TCM syndrome score, Effective rate | Compared with usual treatment, most types of acupuncture therapy showed the improvementof both subjective and objective sleep indices, especially acupoint catgut embedding, auricular acupressure or auricular acupuncture plus acupuncture, electroacupuncture plus acupoint application |
|
(13) |
| Warm-acupuncture vs. Acupuncture or Acupoint herbal plaster | PSQI, improvement in clinical effect, PSG | Single acupoint stimulation of SP 6 could improve sleep quality, lengthen deep sleep and REM duration of patients with insomnia |
|
(33) |
| Acupuncture vs. Acupuncture plus conventional medication or herbal medicine | PSQI, 12 kinds of scores were used for sleep measurement | Acupuncture appears to be effective in treatment of insomnia. However, further large, rigorous designed trials are warranted | The majority of the included trials were assessed to be of generally fair methodological quality | (15) |
| Manual acupuncture, Electro-acupuncture vs. Medication | PSQI | Acupuncture using pattern identification led to significantly improved total effectiveness rate compared to medication | High risk of bias, not using a standardized pattern-diagnosis-treatment and not comparing with standarized acupuncture without pattern identification | (34) |
| Acupuncture vs. acupuncture combined with benzodiazepines or behavioral therapy or benzodiazepines combined with CBT | PSQI | Combined treatments, including benzodiazepines combined with CBT or with acupuncture, were generally superior to other monotherapies | The risk of bias and imprecision of the meta-analyzed results | (35) |
PSQI, Pittsburgh sleep quality index; ISI, Insomnia Severity Index; TCM, Traditional Chinese Medicine; PSG, polysomnography; CBT, cognitive behavioral therapy.
In general, acupuncture treatment for PI has produced positive results in many areas, including sleep quality, episodic memory function, daytime drowsiness, attentiveness, stress alleviation, and extended sleep duration, with fewer side effects. However, the quality of the included studies needs to be improved. Therefore, the current evidence suggests that acupuncture alone or combined with drugs for insomnia has a certain therapeutic effect and that the combined treatments can enhance the therapeutic effect of the drug and reduce the required drug dose, thereby reducing adverse reactions.
4. Acupuncture types and acupoint selection
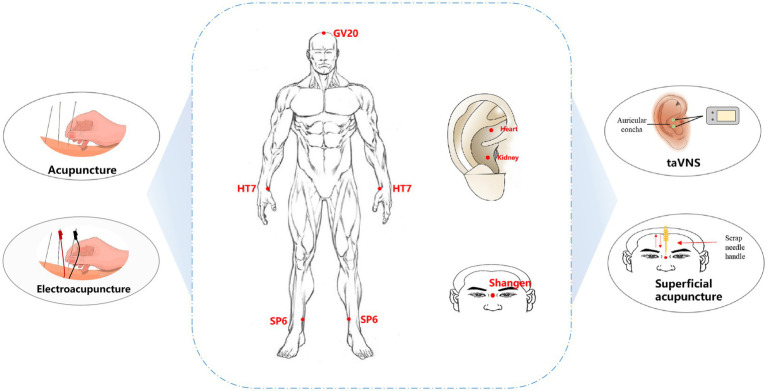
The growing public fascination with complementary and alternative medicine has led to a heightened focus on the utilization of acupuncture as a potential treatment for insomnia. A comprehensive examination of alternative medicine therapies for insomnia revealed that acupuncture and acupoint massage exhibit promising potential as treatment options for insomnia. These therapies appear to have an impact on the modulation of serotonin, dopamine, and endogenous opioids, which may contribute to their efficacy (37, 38). The acupuncture techniques examined in this study included manual acupuncture, electroacupuncture, transcutaneous auricular vagus nerve stimulation (taVNS), superficial acupuncture, and warm acupuncture (Figure 2), and these approaches elicited therapeutic outcomes by targeting acupoints located on the body’s surface.
Figure 2.
Acupuncture types and acupoint selection for PI treatment. taVNS: transcutaneous auricular vagus nerve stimulation; GV20, Baihui; HT7, Shenmen; SP6, Sanyinjiao.
Manual acupuncture or electroacupuncture are the most commonly used intervention approaches for PI, and most of the acupuncture manipulations involve mild reinforcing and reducing manipulations. The most frequently used acupoints are HT7 (Shenmen), SP6 (Sanyinjiao), and GV20 (Baihui). These three acupoints are the main recommended points in the Evidence-Based Guideline of Chinese Medicine for Insomnia (Chinese Academy of Chinese Medical Sciences.,2016). HT7 is located at the radial wrist joint of the ulnar flexor muscle at the concave edge of the bean-shaped bone and is considered “the foremost acupoint to calm and regulate the spirit” (39). Multiple meta-analyses have shown that acupuncture is the first choice for treating insomnia and can adjust the imbalance of the core of sleep dysfunction (40, 41). SP6 is the intersection of three Yin meridians (spleen, liver, and kidney meridians from foot to head) and is located three inches above the inner ankle in the depression behind the tibia. A study revealed that stimulating SP6 can increase the content of GABA in the brain, which is similar to benzodiazepine drugs (41). A study on the application patterns of SP6 revealed that it has a wide range of clinical applications and is most commonly used to treat insomnia (42). GV20 is the intersection of the ruling vessel and three yang channels of the hand and foot. It is commonly used in traditional Chinese medicine for the therapeutic management of conditions related to the head, facial features, and mental health disorders (33, 43). TaVNS is also commonly used as a therapeutic approach for PI and involves the combination of traditional auricular acupuncture and vagus nerve stimulation to treat PI by stimulating auricular points located in the vagus nerve region of the ear to modulate the activity of the brainstem, thalamus and cerebral cortex (44). Currently, taVNS is widely used to treat PI, irritable bowel syndrome, depression, and posttraumatic stress disorder (45–47). In the treatment of PI, auricular points are mostly chosen for the heart and kidney regions. According to traditional acupuncture theory, PI is related to heart and kidney disorders, and stimulation of heart and kidney auricular points can regulate the balance of yin-yang and qi-blood in the heart and kidney meridians and improve the clinical symptoms of PI (48). In addition, superficial acupuncture via the Shangen acupoint has been used to treat PI (49). By employing a rhythmic and persistent scraping and pressing motion on the needle handle, subtle and uninterrupted physical vibration is produced, exerting an influence on the Shangen acupoint and eliciting stimulation of the meridian Qi. This stimulation is intended to facilitate sleep and promote a tranquil state of mind.
Notably, in the field of clinical acupuncture, the exact amount of acupuncture stimulation must be mastered to maximize the therapeutic effect. The therapeutic efficacy of acupuncture can be influenced by various factors, including the direction, size, duration, and interval of the needling force. During acupuncture treatment, the patient must provide acupuncture sensory feedback, and any adverse reactions and incidents associated with acupuncture should be documented. Furthermore, comprehensive training must be administered to acupuncturists prior to commencing official experiments to enhance the standardization and uniformity of operational procedures. There were differences in acupuncture parameters among the included studies, indicating the need for large-scale clinical trials to establish standardized operation procedures and acupuncture parameters.
5. Effects of acupuncture on brain functional activity in PI patients
The primary pathophysiology of PI is hyperarousal, which leads to alterations in brain regions within the central nervous system that are intimately associated with emotional and cognitive activities in individuals with PI. These alterations are linked to the development, progression, and severity of PI. For instance, sensations of wakefulness can be attributed to alterations in thalamic metabolism (50) and the brainstem reticular arousal system (51, 52).Additionally, impaired subjective sleep quality has been linked to disruptions in hippocampal memory function (53, 54).Currently, various neuroimaging techniques, including functional magnetic resonance imaging (fMRI), electroencephalography (EEG), functional near-infrared spectroscopy (fNIRS), magnetic resonance spectroscopy (MRS), and positron emission tomography (PET), are employed to investigate the underlying central pathogenesis of PI. These techniques offer valuable empirical evidence for studying the pathogenesis and mechanisms of treatment for PI. Recent clinical studies have shown that fMRI, EEG and fNIRS have been used to investigate the effects of acupuncture on functional brain activity in patients with PI, and Table 2 summarizes the characteristics and outcomes of these studies.
Table 2.
Clinical study on the regulation of brain function activity in primary insomnia by acupuncture.
| Neuroimaging technologies | Therapy | Acupoints | Neuroimaging results | Refs |
|---|---|---|---|---|
| rs-fMRI | MA | EX-HN1, HT7, GV20, SP6 | PI (post) vs. HC: decreased DC value in MTG, HIP, PUT, PHG, CUN. | (30) |
| MA | EX-HN1, GV20, GV16, GB20, GV18, MS13, Anmian, EX-HN13, GV14 | PI (post vs. pre): increased FC in dorsal CAU to LING, cerebellum; ventral CAU to frontal lobe, INS, ACG; ventral CAU to PreCG, PCG. | (9) | |
| MA | LI11, ST40, LR3 | PI (post vs. pre): increased ALFF value in PUT, PAL, CAU; decreased ALFF value in CUN, SFG, MFG. | (29) | |
| MA | GV20, GV24, EX-HN1, HT7, GB13, PC6, SP6 | PI (post vs. pre): decreased FC in AMYG and THA. | (55) | |
| EA | Single acupoints group (HT7); multi-acupoints group (HT7, SP6, GV20); Non-acupoint group (the junction point between the biceps brachii muscle and deltoid muscle) | Single acupoints group (post vs. pre): increased fALFF value in ITG, MTG, STG, PHG, PCUN, SFG; Multi-acupoints group (post vs. pre): increased fALFF value in PHG, MTG, STG, MFG, IFG, PreCG, PoCG, IPL, SPL, ANG, SMG, PCUN, CUN; Non-acupoint group (post vs. pre): SFG, MFG, IFG, ACG, INS. |
(26) | |
| EA | HT7 | Male PI (post vs. pre): increased fALFF value in MTG, SFG, MFG, PCUN, PHG, SPL, PoCG. Female PI (post vs. pre): increased fALFF value in MT, PCG, IFG, MFG, STG, SMG. |
(56) | |
| SA | Shangen | PI (post vs. pre): increased FC in AMYG to INS, brain stem, CAU, cingulate; decreased FC in AMYG to MFG, PCUN. | (57) | |
| taVNS | Auricular cavum concha | PI (post vs. pre): increased ReHo value in SFGmed, ACG; decreased ReHo value in ITG, MTG, THA. | (58) | |
| taVNS | Auricular cavum concha | PI (post vs. pre): decreased FC in mPFC to dorsal ACG; decreased FC in mPFC to PCUN, CUN, CAL, SOG, LING. | (59) | |
| taVNS | Auricular cavum concha | Group A (post vs. pre): decreased fALFF value in cerebellum, SFGmed, SMA. | (46) | |
| taVNS | Auricular cavum concha | PI (post vs. pre): decreased FC in PCG to PCUN, ANG, SFG, MFG, MTG, ORBsupmed; increased FC in PCG to LING, CAL. | (60) | |
| taVNS | Auricular points, heart and kidney areas | Correlation analysis: ALFF in SFG, MFG, ACGdor, fALFF in IPL, SMG, ANG, ReHo in SFG, SMA, ACGdor, PCGdor. | (61) | |
| taVNS | Auricular points, heart and kidney areas | PI (post vs. pre): increased FC in preoptic area to PCUN, PCG, CUN; decreased FC in medial THA to PreCG, auxiliary motor area; increased FC in medial hypothalamus to PCUN, PCG; increased FC in anterior THA to PreCG, SFG, MFG; increased FC in anterior beak cingulate gyrus to LING, IOG; decreased FC in anterior beak cingulate gyrus to ITG, MTG, IPL, SMG, ANG. | (62) | |
| taVNS | Auricular points, heart and kidney areas | PI (post vs. pre): increased FC in PCG to MFG, PCG to MFG and medial prefrontal lobe. | (59) | |
| taVNS | Auricular points, heart and kidney areas | PI (post vs. pre): decreased ALFF in PCUN, increased ALFF in MOG; increased FC in PCUN to ANG, SFG, and MFG. | (63) | |
| taVNS | Auricular points, heart and kidney areas | PI (post vs. pre): decreased FC in THA to ANG, ACG to PCUN. | (64) | |
| task-fMRI | MA | HT7, SP6 | PI (post) vs. HC: activated areas included ventral anterior nucleus of THA, putamen, medial globus pallidus, PreCG, CAU. | (65) |
| MA | HT7, GV20, SP6 | There were differences in brain response areas among the three groups, and the most activated areas included cerebellum, IFG, IPL and MTG. | (66) | |
| MA | SP6 | NOR group: activated areas included STG, IPL, PoCG; SD group: activated areas included ACG, INS, basal ganglia, THA; Sham group: activated areas included THA and cerebellum. |
(67) | |
| fNIRS | MA | GV20, PC6, HT7, SP6, KI5 | PI (post vs. HC): decreased HBO concentrations in frontal primary motor cortex, prefrontal orbitofrontal area, parietal somatosensory association cortex, postero-lateral prefrontal area and premotor cortex. | (68) |
| EEG | SA | Shangen | PI (post vs. rest): decreased ApEn in frontal and occipital lobes, increased CD in frontal lobe. | (69) |
| SA | Shangen | PI (rest→intervention→post): ApEn results for prefrontal lobe →posterior temporal lobe →occipital lobe PI (post vs. rest): CD results for frontal, anterior temporal and frontal lobes. |
(49) | |
| MA | BL44, BL47, BL49, BL42, BL52 | Observation group (post vs. pre): decreased in non-rapid eye movement NREM time, total sleep time; increased in sleep efficiency (ratio of actual sleep to total sleep). | (70) | |
| MA | EX-HN22, PC6, HT7, LI4, ST36, KI6, BL62, LR3 | AA (post vs. rest): the brain electrical potential changes in the frontal lobe, right temporal lobe, and occipital lobe. | (71) | |
| taVNS | auricular points, heart, liver and kidney areas | PI (post vs. rest): increased in SE, NREM 3, power spectrum of NREM 1-delta1; decreased in SL and power spectrum of NREM 1-delta1. | (72) |
rs-fMRI, resting-state functional magnetic resonance imaging; task-fMRI, task-based functional magnetic resonance imaging; PI, primary insomnia; HC, healthy control; MA, manual acupuncture; EA, electroacupuncture; taVNS, transcutaneous auricular vagus nerve stimulation; SA, superficial acupuncture; DC, degree centrality; ReHo, regional homogeneity; FC, functional connectivity; ALFF, amplitude of low frequency fluctuation; fALFF, fractional amplitude of low frequency fluctuations; fNIRS, functional near-infrared spectroscopy; EEG, electroencephalogram; EX-HN1, Sishencong; HT7, Shenmen; GV20, Baihui; SP6, Sanyinjiao; GV16, Fengfu; GB20, Fengchi; GV18, Qiangjian; MS13, Dingshangpangxian; EX-HN13, Yuye; GV14, Dazhui; LI11, Quchi; ST40, Fenglong; LR3, Taichong; PC6, Neiguan; KI5, Shuiquan; BL42, Pohu; BL44, Shentang; BL47, Hunmen; BL49, Yishe; BL52, Fuxi; EX-HN22, Jingbi; LI4, Hegu; ST36, Zusanli; KI6, Zhaohai; BL62, Shenmai; MTG, middle temporal gyrus; HIP, hippocampus; PUT, lenticular nucleus, putamen; PHG, parahippocampal; CUN, cuneus; MOG, middle occipital gyrus; SOG, superior occipital gyrus; PCUN, precuneus; PoCG, postcentral gyrus; MFG, middle frontal gyrus; CAU, caudate nucleus; LING, Lingual gyrus; INS, insula; DMN, default mode network; ACG, anterior cingulate and paracingulate gyri; PreCG, precental gyrus; PCG, posterior cingulate gyrus; PAL, lenticular nucleus, pallidum; SFG, superior frontal gyrus; ITG, inferior temporal gyrus; STG, superior temporal gyrus; IFG, inferior frontal gyrus; IPL, inferior parietal, but supramarginal and angular gyri; SPL, superior parietal gyrus; ANG, angular gyrus; SMG, supramarginal gyrus; AMYG, amygdala; SFGmed, superior frontal gyrus, medial; THA, thalamus; mPFC, medial prefrontal cortex; CAL, calcarine fissure and surrounding cortex; SMA, supplementary motor area; ORBsupmed, superior frontal gyrus, medial orbital; ACGdor, anterior cingulate and paracingulate gyri, dorsolateral; PCGdor, posterior cingulate gyrus, dorsolateral; ESS, epworth sleepiness scale; IOG, inferior occipital gyrus; HBO, oxyhemoglobin; ApEn, approximate entropy; CD, correlation dimension; NREM, non-rapid eye movement; SE, sleep efficiency; SL, sleep latency.
5.1. Changes in brain functional activity based on neuroimaging technology
In the studies employing fMRI techniques, task-based functional magnetic resonance imaging (task-fMRI) was commonly employed to evaluate the immediate impact of acupuncture, while resting-state functional magnetic resonance imaging (rs-fMRI) was utilized to analyze the cumulative effects of acupuncture. Studies utilizing task-fMRI demonstrated that the administration of acupuncture immediately following treatment can potentially improve insomnia symptoms to a certain degree (55–57). Notably, there are similarities between cumulative acupuncture and immediate acupuncture about the enhancement of brain areas involved in PI. Moreover, the majority of the brain regions that exhibit coordinated activity are associated with cognitive and emotional processes. The investigation of the immediate impact of acupuncture suggested that it may offer valuable clinical guidance for the utilization of acupuncture in patients with PI who have a shorter duration of illness or exhibit minimal symptoms.
Numerous studies have examined the specificity and commonality of acupuncture in the therapeutic application of regularly utilized acupoints for the treatment of PI. In the included studies using rs-fMRI technology, HT7 was the most frequently used acupoint for treating PI. Electroacupuncture of HT7 as a treatment for PI increased the fractional amplitude of low frequency fluctuation (fALFF) values in the parahippocampal gyrus (PHG), superior temporal gyrus (STG), middle temporal gyrus (MTG), superior frontal gyrus (SFG), middle frontal gyrus (MFG), and precuneus (PCUN). The PHG is related to the maintenance of working memory and long-term memory encoding (Schon et al.,2016). The STG and MTG play important roles in attention networks involving emotional regulation and cognitive behavioral processing (58). Abnormal temporal lobe function can cause emotional and cognitive disorders, impulsiveness, poor decision-making, and cognitive regulation disorders (59). The SFG and MFG are believed to be involved in alertness, attention, and higher-order cognitive processes, all of which are disrupted in insomnia patients (60). The function of the PCUN is bidirectional to sleep status. People with increased blood oxygen level-dependent signals in the bilateral anterior cuneiform lobe exhibit greater subjective and objective differences in sleep quality (61), and the functional connectivity density of the anterior cuneiform lobe decreases after sleep deprivation (62). Most of these areas belong to the default mode network (DMN), and the change in fALFF values in these areas after electroacupuncture of HT7 may indicate that PI is treated through the relevant areas of the default network. In a further investigation, acupuncture at the SP6 point elicited more robust and extensive activation of specific brain regions within the saliency network, namely, the anterior cingulate cortex, bilateral insula, left basal ganglia, and thalamus, than sham acupuncture (57). An interesting study compared the effects of single acupuncture (HT7), multiacupuncture combinations (HT7, SP6, GV20), and pseudoacupuncture (junctions between the biceps and deltoids) on PI. These findings indicate that acupuncture is effective at treating PI and that the utilization of a combination of multiple acupoints can enhance its effectiveness. The imaging results showed that the relationship between multipoint combination and brain activity was significantly more regulated than that of single or false acupuncture spots (26). A study conducted by the same research team also investigated potential sex-related variations in the treatment of insomnia with electroacupuncture at HT7. The findings indicated that the PSQI score was significantly lower in women than in men and that disparities related to sex in the effects of electroacupuncture were primarily observed in the posterior cingulate gyrus and supramarginal gyrus (63). These findings offer a neuroimaging-based quantitative foundation for enhancing the understanding of the efficacy of acupoints, as well as their general and unique therapeutic effects.
Research based on fMRI data indicates that acupuncture in treating PI can trigger a series of brain responses. The brain areas primarily implicated in this study included the SFG, MFG, MTG, PHG, PCUN, postcentral gyrus (PoCG), supramarginal gyrus (SMG), and anterior cingulate gyrus (ACG). Among them, the SFG, MTG, PHG, PCUN, SMG, and ACG are important brain regions of the DMN. The relationships between the DMN and emotional processing and episodic memory function have been extensively studied (64). A noteworthy study revealed that individuals diagnosed with PI exhibit disparities in the anterior and posterior subregions of the DMN, impacting cognitive and emotional processing (65, 66). Alterations in functional connectivity within the DMN have an impact on the functioning of both the network itself and the central executive network. Consequently, individuals with PI have symptoms of heightened neural activity, such as excessive daytime drowsiness, weariness, cognitive impairment, and reduced efficiency. The SFG and MFG are located within the frontal lobe and are closely linked to processes related to self-perception and emotional regulation (67). Research has revealed that individuals diagnosed with PI exhibit fewer connections within the right frontoparietal network, specifically in the superior parietal lobule and SFG. These findings suggest a potential association between connectivity reductions and the presence of mood problems in patients suffering from insomnia (68). The PoCG is in the somatosensory cortex, the primary area responsible for receiving tactile input and playing a crucial role in emotional regulation (69). A recent study examined the correlation between sleep quality and functional connectivity within the somatomotor network in a sample of young adult males. The results indicated that individuals with poorer sleep quality, as indicated by higher scores on the PSQI, exhibited heightened functional connectivity within specific regions of the somatomotor network, including the PoCG, left paracentral lobule, bilateral precentral gyrus, and supplementary motor areas. These findings suggest a potential association between sleep quality and the strength of functional connectivity within the somatomotor network in young men (70). Furthermore, a study utilizing fNIRS technology examined the immediate impact of acupuncture on cerebral oxygen saturation in individuals with PI. The findings revealed that acupuncture exerted a positive influence on the functionality of brain regions associated with the frontal and orbitofrontal cortex, as well as the parietal lobe. This effect was achieved through the regulation of oxygenated hemoglobin levels in the cerebral cortex, leading to enhanced control over the involuntary activation of irrelevant stimuli and the modulation of hyperarousal activity. Consequently, acupuncture demonstrated potential benefits in ameliorating insomnia symptoms.
The regulation of the aforementioned brain regions through acupuncture may contribute to the amelioration of difficulties associated with sleep onset, early awakening, excessive daytime sleepiness, fatigue, memory impairment, and reduced cognitive efficiency. This effect is believed to be achieved through the modulation of brain regions involved in cognitive processes, memory formation, emotional regulation, and physical motor functions. The utilization of neuroimaging quantifications, as opposed to relying only on subjective observations, aids in the objective evaluation of the efficacy of acupuncture in treating PI.
5.2. Changes in brain functional activity based on EEG technology
The results of studies utilizing EEG techniques have shown that the Shangen acupoint is the most commonly used acupoint, and shallow stimulation of the Shangen acupoint can increase the CD value of the frontal lobe. The frontal cortex is a key component of the pathway for positive and negative emotions (71) while integrating signals from other brain regions that are highly involved in understanding past and current experiences and planning future actions (72). Acupuncture at the Shangen acupoint can enhance frontal lobe brain activity in PI patients, which may help alleviate subjective insomnia and environmental discomfort, thereby reducing bedtime anxiety and contemplation (73). Additionally, acupuncture has the potential to decrease the complexity of EEG signals and exert a significant influence on the electrical activity of the frontal, temporal and occipital lobes (49, 74). The frontal lobe of the brain serves as the primary hub for higher cognitive functions in humans. Additionally, it maintains a significant connection with the regulation of autonomic processes and contributes significantly to autonomous behavior (75). The medial temporal lobe is widely recognized as the “medial temporal lobe memory system,” which specializes in memory function in mammalian brains. This brain region plays a crucial role in the regulation of memory and mood (76). The occipital lobe plays a crucial role in visual processing, making it a significant region for the integration of sensory functions and visual pathways (77). The decreases in approximate entropy and increases in correlation dimension values within the aforementioned brain regions indicate that acupuncture reduces the complexity of PI; makes brain signals more synchronized, stable and orderly; and helps improve difficulty with falling asleep in PI patients. Acupuncture has been found to have the ability to modify the sleep continuity of individuals with PI, manage their sleep structure by decreasing non-rapid eye movement (REM) time and total sleep time, enhance sleep efficiency by increasing the ratio of actual sleep to total sleep, and alleviate the cortical hyperarousal state (78). The findings from these investigations utilizing EEG-based methods established a foundation for elucidating the efficacy and potential mechanisms of acupuncture in treating PI.
6. Effects of acupuncture on brain metabolites in PI
Insomnia is strongly correlated with the sleep–wake cycle, which is governed by a diverse range of neurotransmitters and neuromodulators (79). Neurotransmitters can be divided into two primary categories: excitatory and inhibitory neurotransmitters. Acupuncture treatment for PI has been demonstrated to modulate alterations in central neurotransmitter levels that are intimately associated with sleep. This therapeutic approach effectively regulates sleep architecture, enhances sleep promotion, and ultimately improves the overall quality of sleep. Table 3 comprehensively summarizes the key features and results of relevant fundamental research investigating the effect of acupuncture on brain metabolites in experimental animals with PI. Several studies have shown that acupuncture at HT7 and SP6 can increase the 5-HT content in the hypothalamus. Acupuncture at GV20, HT7, and SP6 can increase the 5-HT and MT levels in the hypothalamus, hippocampus, pineal body, and brain while decreasing the NE and DA levels.
Table 3.
Basic researches on the regulation of brain metabolites in PI by acupuncture.
| Detecting brain regions | Experimental animals | Therapy | Acupoints | Biochemical measurements | Refs |
|---|---|---|---|---|---|
| Hypothalamus | SD rats | EA | BL13, BL15, BL18, BL20, BL23 | 5-HT↑, 5-HIAA↑ | (92) |
| SD rats | EA | HT7, SP6 | 5-HT↑, Glu↓, GABA↓, Glu/GABA↓ | (93) | |
| Wistar rats | EA | GV20, EX-HN3 | Glu↓, Glu/ GABA↓, GABA↑, GABAA↑ | (94) | |
| SD rats | EA | GV20, ST36, SP6 | GABA↑ | (95) | |
| Wistar rats | EA | BL13, BL15, BL18, BL20, BL23 | 5-HT↑, 5-HIAA↑ | (96) | |
| Wistar rats | EA, MA | GV20, EX-HN1 | Glu↓, GABA↑ | (97) | |
| SD rats | EA | HT7, SP6, PC6 | 5-HT↑, GABA↑, Glu↓ | (98) | |
| SD rats | MA | BL62, KI6 | Glu↓, GABA↑, Glu /GABA↓, GAD↑, GS↓ | (99) | |
| SD rats | MA | GV20, HT7, SP6 | MT↑, MT1, MT2↑ | (100) | |
| SD rats | MA | GV20, HT7, SP6 | MT↑, MT1, MT2↑ | (101) | |
| SD rats | MA | GV20, HT7, SP6 | 5-HT1A↑, 5-HT2A↓ | (102) | |
| SD rats | MA | GV20, HT7, SP6 | MT↑ | (103) | |
| Wistar rats | EA, MA | GV20, EX-HN1 | 5-HT↑, 5-HIAA↑ | (104) | |
| SD rats | MA | HT7, SP6, ST36, PC6, BL62, KI6 | GABA↑, GABAR↑ | (105) | |
| SD rats | WA | Dinghui, Heyi, Xin | DA↓ | (106) | |
| Wistar rats | EA | HT7, SP6 | 5-HT↑, 5-HIAA↑, NE↓ | (107) | |
| C57BL/6 J mice | EA | auricular branch of the vagus nerve | GABA↑ | (108) | |
| Hippocampus | Wistar rats | MA | HT7, SP6, ST36, SJ6 | 5-HT1A↑, 5-HT2A↓ | (109) |
| SD rats | WA | Dinghui, Heyi, Xin | NE↓ | (110) | |
| Wistar rats | MA | HT7, SJ6、ST36, SP6 | 5-HT↑, 5-HIAA↑ | (111) | |
| Hypothalamus, hippocampus | SD rats | MA | GV20, HT7, SP6 | 5-HT↑, NE↓, DA↓ | (112) |
| Brainstem | Wistar rats | EA | GB20, Gongxue | 5-HT↑, NE↓, DA↓ | (113) |
| Brainstem | SD rats | WA | GV20, HT7, SP6 | 5-HT↑, NE↓, DA↓ | (114) |
| Right brain | Wistar rats | MA | HT7, PC6, SP6, ST36, BL62, KI6 | 5-HT↑ | (115) |
MA, manual acupuncture; WA, warm acupuncture; EA, electroacupuncture; GV20, Baihui; HT7, Shenmen; SP6, Sanyinjiao; BL13, Feishu; BL15, Xinshu; BL18, Ganshu; BL20, Pishu; BL23, Shenshu; ST36, Zusanli; PC6, Neiguan; BL62, Shenmai; KI6, Zhaohai; EX-HN3, Yintang; EX-HN1, Sishencong; 5-HT, 5-hydroxytryptamine; 5-HIAA, 5-hydroxyindole acetic acid; NE, noradrenaline; DA, dopamine; MT, melatonin; Glu, glutamic acid; GABA, γ-aminobutyric acid; GABAR, γ-aminobutyric acid receptor; GAD, glutamic acid decarboxylase; GS, glutamine synthetase.
In randomized controlled clinical research, the therapeutic efficacy of warm acupuncture combined with auricular acupressure and oral eszolam pills was investigated in patients with PI. The findings of the study demonstrated that both interventions helped enhance sleep quality, with success rates of 83.1 and 87.7%, respectively. However, the use of the encephalofluctuography technique for monitoring alterations in neurotransmitter levels revealed that warm acupuncture combined with auricular acupressure increased the serotonin (5-HT) ratio and the γ-aminobutyric acid/glutamate (GABA/Glu) ratio. Conversely, there was a decrease in norepinephrine (NE) levels. Notably, the administration of oral eszolam tablets did not elicit any changes in neurotransmitter expression within the brain (80).
Previous studies have demonstrated that acupuncture can modulate the expression levels of neurotransmitters in the hypothalamus, hippocampus, and brainstem tissues of rats with chlorophenylalanine-induced insomnia. Specifically, acupuncture has been found to enhance the expression of 5-HT, melatonin (MT), and GABA while reducing the expression of dopamine (DA), NE, Glu, and Glu/GABA. Prior research has indicated that the neurotransmitters 5-HT, DA, and Glu are classified as excitatory neurotransmitters, whereas GABA and NE are classified as inhibitory neurotransmitters. 5-HT is a neurotransmitter that is involved in the sleep–wake mechanism. It plays an important role in the occurrence and maintenance of slow-wave sleep and is considered a “hypnogenic factor” in the brain (81). DA is involved in the regulation of arousal and the modulation of behavioral excitement (82). Glu serves as the primary excitatory neurotransmitter in the central nervous system, facilitating neuronal communication, while GABA neurons function as inhibitory agents, regulating neural activity. The Glu/GABA ratio is considered a reliable measure for assessing the balance between excitation and inhibition in nerve cells, providing valuable insights into the therapeutic efficacy of insomnia treatment (83). NE primarily influences the regulation of REM sleep and wakefulness, exhibiting both excitatory and inhibitory impacts on central neurons. Notably, NE plays distinct roles in particular regions (84). Furthermore, MT is a prominent hormone that is actively secreted by the pineal gland in mammals. The secretion of MT is intricately linked to light exposure, and it plays a crucial role in regulating the circadian rhythm of sleep and influencing the overall quality of sleep (85). Acupuncture modulates these neurotransmitters to enhance the sleep–wake cycle and ameliorate the quality of sleep.
Simultaneously, the researchers conducted basic experiments to investigate the optimal electroacupuncture stimulation parameters for treating insomnia in rats. Their findings indicated that the sparse wave (10 Hz) had a superior effect compared to the dense wave (50 Hz) and the combined sparse wave (10/50 Hz) (86, 87). The efficacy of a stimulation frequency of 2 Hz was found to be superior to that of frequencies of 50 Hz and 100 Hz (88). Furthermore, a stimulation intensity of 2 V was more effective than 1 V (87), and the immediate acupuncture effect lasted more than 12 h. The results of this study could lead to the development of clinical electroacupuncture interventions. Low-frequency electroacupuncture stimulation may be more suited for modulating the sleep–wake cycle to induce relaxing and hypnotic impacts.
7. Other effects of acupuncture in the brain on PI
The sleep–wake cycle can be influenced by various mechanisms in the brain, such as circadian clock genes, immunological components, energy metabolism, and apoptosis. Several researchers have also examined the potential of acupuncture to enhance sleep quality through the regulation of these parameters. Table 4 presents a comprehensive overview of the key attributes and outcomes derived from existing fundamental investigations about the neurological impacts of acupuncture in treating PI.
Table 4.
Basic researches on acupuncture regulating other effects of PI in the brain.
| Detecting brain regions | Experimental animals | Therapy | Acupoints | Biochemical measurements | Refs |
|---|---|---|---|---|---|
| Hypothalamus | SD rats | WA | Dinghui, Heyi, Xin | PRELP, NSMF, TMEM41B, MAP1B | (123) |
| SD rats | EA | HT7, SP6 | AMPK↓, Ac-CoA↑, NA+-K+-ATP↑ | (124) | |
| SD rats | EA | BL13, BL15, BL18, BL20, BL23 | TNF-α↑, IL-1β↑ | (92) | |
| SD rats | MA | BL62, KI6 | Per1↑, Per2↑ | (125) | |
| SD rats | MA | GV20, HT7, SP6 | Bmal1↑, Clock↑ | (126) | |
| Wistar rats | MA, EA | GV20, EX-HN1 | Bmal1↑, Clock↑, Per1↑ | (97) | |
| SD rats | MA | BL62, KI6 | Clock↑, Per2↑ | (99) | |
| SD rats | MA | HT7, LR3, BL15, BL18 | P38 MAPK↓ | (127) | |
| Hippocampus, brainstem | SD rats | MA | GV20, HT7, SP6 | Bcl-2↑, Bax↓, Bad↓, Caspase-3↓, TrkB↑, p-TrkB↓, PI3K↑, p-Akt↑ | (112) |
| Hippocampus | SD rats | EA | GV20, HT7, SP6 | PKA-Cβ↑, p-CREB↑, BDNF↑, TrkB↑ | (128) |
| Brain | SD rats | MA | BL15, BL18, BL20, BLl3, BL23 | PI3K↑, Akt | (129) |
| Wistar rats | MA | GV20, PC6, HT7, LR3 | blood oxygen metabolism in the prefrontal and occipital lobes of the cerebral cortex↑ | (130) |
MA, manual acupuncture; WA, warm acupuncture; EA, electroacupuncture; GV20, Baihui; HT7, Shenmen; SP6, Sanyinjiao; BL13, Feihu; BL15, Xinshu; BL18,Ganshu; BL20, Pishu; BL23, Shenshu; BL62, Shenmai; KI6, Zhaohai; EX-HN1, Sishencong; PRELP, prolargin; NSMF, NMDA receptor synaptonuclear-signaling and neuronal migration factor; TMEM41B, transmembrane protein 41B; MAP1B, microtubule-associated protein 1B; PBR, peripheral benzodiazepine receptor; TrkB, tropomysin related kinase B; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; AMPK, adenosine 5′-monophosphate (AMP)-activated protein kinase; Ac-CoA, acetyl-coenzyme A; ATP, adenosine triphosphate; TNF-α, tumour necrosis factor alpha; IL-1β, interleukin-1beta; Per1, period1; Bmal1, bain and muscle arnt-like protein-1; Clock, circadian locomotor output cycles kaput; PKA-Cβ, catalytic subunit of protein kinase A; p-CREB, phosphorylated cAMP-responsive element-binding protein; BDNF, brain-derived neurotrophic factor.
Insufficient sleep or dysrhythmia can have a direct impact on the circadian clock genes responsible for regulating the operation of circadian rhythms in the brain. Disruption of the circadian rhythm motor output cycle failure (clock)–brain and muscle aromatic hydrocarbon receptor nuclear transport-like 1 (bmal1)-related pathway (89) leads to noticeable inflammatory effects characterized by an imbalance in the ratio of proinflammatory factors to anti-inflammatory factors (90, 91). Circadian rhythm problems have been found to potentially induce apoptosis of neuronal cells in the brain through the involvement of the Bcl-2 family and caspase family (92). Additionally, these disorders can impact mitochondrial energy metabolism via the AMPK signaling pathway (93). Furthermore, alterations in the mitochondrial membrane potential might further contribute to the exacerbation of apoptosis. The hypothalamus is a crucial brain region involved in the regulation of sleep, energy metabolism, and the immune response. Within this region, the paraventricular nucleus plays a significant role in the regulation of arousal, and the suprachiasmatic nucleus serves as the primary circadian “pacemaker” in the mammalian brain (94, 95). Consequently, the hypothalamus is predominantly utilized by researchers for observation.
In studies conducted by Wei, Guo, Xing, and Liu, acupuncture was shown to increase the expression levels of circadian clock genes (Clock, Bmal1, and recombinant period circadian protein (Per)) in the hypothalamus of rats with insomnia. This upregulation of gene expression was found to enhance rhythms of spontaneous activity and facilitate the restoration of the disrupted sleep–wake cycle (96–98). Tang’s study demonstrated that the application of electroacupuncture at the Wushu point effectively modulated the release of neurotransmitters, specifically 5-HT and 5-HIAA, through the upregulation of the proinflammatory factors TNF-α, L-1β and P38 MAPK in the hypothalamus of insomnia rats. This mechanism ultimately led to the amelioration of pathological damage in hippocampal tissues and the improvement of insomnia symptoms in the experimental rats (99). In a study conducted by Zheng, acupuncture administered at the HT7 and SP6 acupoints in rats with insomnia resulted in a notable decrease in the expression of AMPK, a receptor involved in cellular energy regulation within the hypothalamus. Conversely, there was a large increase in the levels of Ac-CoA and ATP generated through energy metabolism. These findings suggest that acupuncture has the potential to exert a positive regulatory influence on energy metabolism, leading to notable improvements in insomnia and feelings of exhaustion after experiencing insomnia (100). In the study conducted by Cao, the focus was on investigating the regulatory mechanism of acupuncture on insomnia in rats. Specifically, this study examined the impact of acupuncture on apoptosis and identified the effects of acupuncture at specific acupoints, namely, GV20, HT7, and SP6. Acupuncture at these acupoints can increase the expression of Bcl-2; reduce the expression of Bax, Bad and Caspase-3; and reduce the apoptosis of cells in the brain by regulating the expression of proteins related to the PI3K/AKT signaling pathway (101). Furthermore, Xu employed proteomics technology to investigate the proteomic alterations induced by acupuncture treatment in the hypothalamus of rats with insomnia. The findings revealed that the therapeutic impact of acupuncture primarily involved the modulation of four distinct proteins associated with the repair of nerve injuries (PRELP, NSMF, TMEM41B, and MAP1B). A new study has explored the potential mechanism of acupuncture in improving spatial learning and memory deficits in a rat model of PI. The research results found that electroacupuncture with GV20, HT7, and SP6 can upregulate PKA/CREB and BDNF/TrkB signaling in the hippocampus of PI rats, regulate hippocampal neural plasticity, inhibit neuronal apoptosis, and exert a synergistic effect on improving spatial learning and memory impairment (102). These results provide additional evidence suggesting that acupuncture can restore neurodevelopment and may serve as a crucial therapeutic approach for effectively alleviating symptoms of insomnia (103). In addition, basic research has also found similar results to clinical research, suggesting that acupuncture may improve abnormal behavior in insomnia rats by regulating blood oxygen metabolism in the prefrontal and occipital lobes of the cerebral cortex (104).
8. Limitations and perspectives for future studies
This study has certain limitations. The included studies were all in Chinese and English, so there may be a language bias. In terms of clinical research, this review explored the role of acupuncture and the factors that affect acupuncture efficacy from multiple perspectives. However, several clinical trials had small sample sizes, which might have contributed to larger random errors and limited statistical power. Some existing trials lack unified quantitative indicators of acupuncture treatment (acupuncture depth, stimulation intensity, acupuncture angle, treatment cycle, stimulation duration, etc.). To improve the repeatability and comparability of research findings, future studies should strive to develop standardized acupuncture operation procedures (105). “Deqi” is a key factor in the effectiveness of acupuncture treatment, but most of the included studies lack a description of the sensation and intensity of “Deqi.” Future studies should combine the standardized assessment of the “Deqi” sensation, which will also provide valuable insights into its therapeutic role. To further study the complex mechanism of acupuncture and improve the quality of experimental results, future research can use multimodal neuroimaging technology and diversified analysis methods (106), which will help to more comprehensively and carefully understand the neural changes caused by acupuncture. In addition, given the scattered entry points of current basic research and poor correlation, most studies mainly focus on the level of neurotransmitters, and the effect of acupuncture is the result of the joint action of multiple systems. Therefore, a systematic research plan integrating multiple systems, organs and targets is needed, which will provide an overall perspective on the effect of acupuncture in treating insomnia, and help deepen the understanding of the underlying central nervous system mechanism of acupuncture in treating insomnia.
9. Conclusion
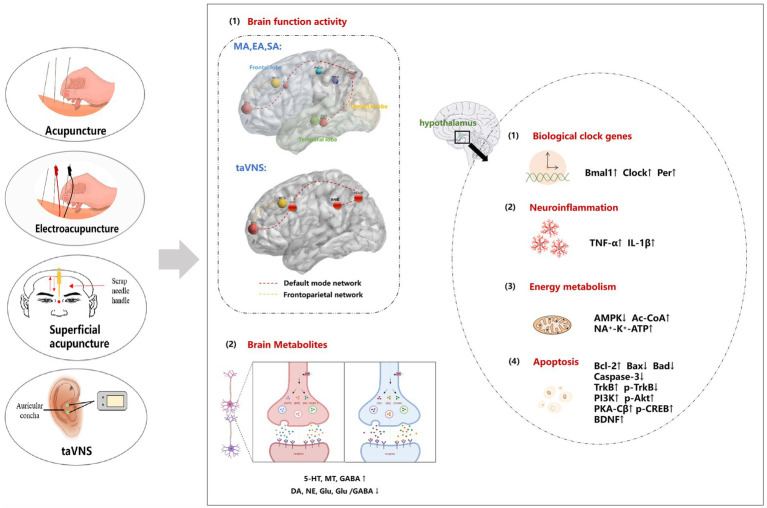
Previous studies have confirmed the efficacy of acupuncture in resolving PI status, improving the sleep quality of PI patients, prolonging sleep time and minimizing adverse consequences. The combination of acupuncture with drug therapy has been proven to improve the therapeutic efficacy of drugs while reducing the required dose and related adverse reactions. The results of this study showed that acupuncture can regulate the neural function network in the brain, the expression of neurotransmitters, the inflammatory reaction and the energy metabolism of the peripheral immune system; inhibit apoptosis; improve sleep rhythm and sleep quality; and alleviate emotional memory dysfunction caused by insomnia (Figure 3 for details). In addition, the regulatory effects of single acupuncture and cumulative acupuncture on brain functional areas are similar. Acupuncture, as a feasible nondrug intervention measure, has considerable potential for the clinical management of PI. This treatment method demonstrates the ability not only to improve the quality of sleep in patients but also to regulate the emotional memory dysfunction caused by insomnia. The results of this study can provide a reference for basic research on acupuncture and will also provide theoretical guidance for clinical research.
Figure 3.
The potential central regulatory mechanism of acupuncture for PI. (1) Acupuncture improves brain function by regulating brain regions such as the default network and frontal lobe network. (2) Acupuncture improves the sleep–wake cycle by increasing the expression of neurotransmitters such as 5-HT, MT and GABA and reducing the expression of DA NE, and Glu and the Glu/GABA ratio. (3) Acupuncture affects sleep rhythm by regulating the expression of circadian clock genes, specifically by upregulating the clock, Bmal1, and Per genes. () The therapeutic effects of acupuncture in relieving brain tissue damage involve the promotion of inflammatory factor synthesis and modulation of neurotransmitter release. (5) Acupuncture influences energy metabolism by regulating AMPK-related pathways. (6) The neuroprotective effects of acupuncture are attributed to its ability to inhibit neuronal apoptosis through the regulation of the PI3K/AKT, PKA/CREB or BDNF/TrkB signaling pathway, thereby restoring nerve function. MA, manual acupuncture; EA, electroacupuncture; taVNS, transcutaneous auricular vagus nerve stimulation; SA, superficial acupuncture; 5-HT, 5-hydroxytryptamine; NE, noradrenaline; DA, dopamine; MT, melatonin; Glu, glutamic acid; GABA, γ-aminobutyric acid; Per1, period1; Bmal1, bain and muscle arnt-like protein-1; Clock, circadian locomotor output cycles kaput; TNF-α, tumour necrosis factor alpha; IL-1β, interleukin-1beta; AMPK, adenosine 5′-monophosphate (AMP)-activated protein kinase; Ac-CoA, acetyl-coenzyme A; ATP, adenosine triphosphate; TrkB, tropomysin related kinase B; PI3K, phosphoinositide 3-kinase; Akt, protein kinase B; PKA-Cβ, catalytic subunit of protein kinase A; p-CREB, phosphorylated cAMP-responsive element-binding protein; BDNF, brain-derived neurotrophic factor.
Funding Statement
The author(s) declare that financial support was received for the research, authorship, and/or publication of this article. This work was supported by National Natural Science Foundation of China (Grant No. 82074548), Natural Science Foundation of Jilin Province (Joint Funds) (Permit Number: YDZJ202101ZYTS103), Science and Technology Program of Jilin Provincial Department of Education (grant number. JJKH20241068KJ), Natural Science Foundation of Jilin Province (Free exploration of general projects) (Permit Number: YDZJ202401680ZYTS), Natural Science Foundation of Jilin Province (Free exploration of general projects) (Permit Number: YDZJ202401697ZYTS), and Changchun University of Chinese Medicine Young Excellent Discipline Backbone Training Programme (Permit Number: 202312).
Author contributions
LY: Writing – original draft, Writing – review & editing. YL: Writing – original draft, Writing – review & editing. ML: Writing – review & editing. HZ: Writing – review & editing. MS: Writing – review & editing. MH: Writing – review & editing. ZZ: Writing – review & editing. SM: Writing – review & editing. HH: Writing – review & editing. HW: Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fneur.2024.1406485/full#supplementary-material
References
- 1.Sutton EL. Insomnia. Ann Intern Med. (2021) 174:ITC33–48. doi: 10.7326/AITC202103160, PMID: [DOI] [PubMed] [Google Scholar]
- 2.Shekleton JA, Flynn-Evans EE, Miller B, Epstein LJ, Kirsch D, Brogna LA, et al. Neurobehavioral performance impairment in insomnia: relationships with self-reported sleep and daytime functioning. Sleep. (2014) 37:107–16. doi: 10.5665/sleep.3318, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carey TJ, Moul DE, Pilkonis P, Germain A, Buysse DJ. Focusing on the experience of insomnia. Behav Sleep Med. (2005) 3:73–86. doi: 10.1207/s15402010bsm0302_2, PMID: [DOI] [PubMed] [Google Scholar]
- 4.Duan D, Kim LJ, Jun JC, Polotsky VY. Connecting insufficient sleep and insomnia with metabolic dysfunction. Ann N Y Acad Sci. (2023) 1519:94–117. doi: 10.1111/nyas.14926, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Z, Xu Z, Wang H, Zhao ZQ, Rao Y. Influence of ethanol on the metabolism of alprazolam. Expert Opin Drug Metab Toxicol. (2018) 14:551–9. doi: 10.1080/17425255.2018.1483338, PMID: [DOI] [PubMed] [Google Scholar]
- 6.Ree M, Junge M, Cunnington D. Australasian Sleep Association position statement regarding the use of psychological/behavioral treatments in the management of insomnia in adults. Sleep Med. (2017) 36:S43–7. doi: 10.1016/j.sleep.2017.03.017, PMID: [DOI] [PubMed] [Google Scholar]
- 7.Riemann D, Baglioni C, Bassetti C, Bjorvatn B, Dolenc Groselj L, Ellis JG, et al. European guideline for the diagnosis and treatment of insomnia. J Sleep Res. (2017) 26:675–700. doi: 10.1111/jsr.12594, PMID: [DOI] [PubMed] [Google Scholar]
- 8.Wilson S, Anderson K, Baldwin D, Dijk D-J, Espie A, Espie C, et al. British Association for Psychopharmacology consensus statement on evidence-based treatment of insomnia, parasomnias and circadian rhythm disorders: An update. J Psychopharmacol. (2019) 33:923–47. doi: 10.1177/0269881119855343, PMID: [DOI] [PubMed] [Google Scholar]
- 9.Zhao T, Xu H, Zhao T, Jiao J-Y, Li G, Lei Z-Q, et al. Effect of Jiannao Anshen acupuncture on insomnia: a functional magnetic resonance imaging study. Zhongguo Zhen Jiu. (2021) 41:767–73. doi: 10.13703/j.0255-2930.20200325-0006, PMID: [DOI] [PubMed] [Google Scholar]
- 10.Zhang X-W, Hou W-B, Pu F-L, Wang X-F, Wang Y-R, Yang M, et al. Acupuncture for cancer-related conditions: An overview of systematic reviews. Phytomedicine. (2022) 106:154430. doi: 10.1016/j.phymed.2022.154430, PMID: [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y-Q, Lu L, Xu N, Tang X, Shi X, Carrasco-Labra A, et al. Increasing the usefulness of acupuncture guideline recommendations. BMJ. (2022) 376:e070533. doi: 10.1136/bmj-2022-070533, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Zhang Z, Huang S, Qiu X, Lao L, Huang Y, et al. Acupuncture for cancer-related insomnia: A systematic review and meta-analysis. Phytomedicine. (2022) 102:154160. doi: 10.1016/j.phymed.2022.154160, PMID: [DOI] [PubMed] [Google Scholar]
- 13.Lu Y, Zhu H, Wang Q, Tian C, Lai H, Hou L, et al. Comparative effectiveness of multiple acupuncture therapies for primary insomnia: a systematic review and network meta-analysis of randomized trial. Sleep Med. (2022) 93:39–48. doi: 10.1016/j.sleep.2022.03.012, PMID: [DOI] [PubMed] [Google Scholar]
- 14.Gould A, MacPherson H. Patient perspectives on outcomes after treatment with acupuncture. J Altern Complement Med. (2001) 7:261–8. doi: 10.1089/107555301300328133, PMID: [DOI] [PubMed] [Google Scholar]
- 15.Cao H, Pan X, Li H, Liu J. Acupuncture for treatment of insomnia: a systematic review of randomized controlled trials. J Altern Complement Med. (2009) 15:1171–86. doi: 10.1089/acm.2009.0041, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fu C, Zhao N, Liu Z, Yuan L-H, Xie C, Yang W-J, et al. Acupuncture improves Peri-menopausal insomnia: A randomized controlled trial. Sleep. (2017) 40:153. doi: 10.1093/sleep/zsx153, PMID: [DOI] [PubMed] [Google Scholar]
- 17.Yin X, Gou M, Xu J, Dong B, Yin P, Masquelin F, et al. Efficacy and safety of acupuncture treatment on primary insomnia: a randomized controlled trial. Sleep Med. (2017) 37:193–200. doi: 10.1016/j.sleep.2017.02.012, PMID: [DOI] [PubMed] [Google Scholar]
- 18.Cheng C-H, Yi P-L, Lin J-G, Chang F-C. Endogenous opiates in the nucleus tractus solitarius mediate electroacupuncture-induced sleep activities in rats. Evid Based Complement Alternat Med. (2011) 2011:159209. doi: 10.1093/ecam/nep132, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spence DW, Kayumov L, Chen A, Lowe A, Jain U, Katzman MA, et al. Acupuncture increases nocturnal melatonin secretion and reduces insomnia and anxiety: a preliminary report. J Neuropsychiatr Clin Neurosci. (2004) 16:19–28. doi: 10.1176/jnp.16.1.19, PMID: [DOI] [PubMed] [Google Scholar]
- 20.Soligo M, Nori SL, Protto V, Florenzano F, Manni L. Acupuncture and neurotrophin modulation. Int Rev Neurobiol. (2013) 111:91–124. doi: 10.1016/B978-0-12-411545-3.00005-5 [DOI] [PubMed] [Google Scholar]
- 21.Han J-S. Acupuncture: neuropeptide release produced by electrical stimulation of different frequencies. Trends Neurosci. (2003) 26:17–22. doi: 10.1016/S0166-2236(02)00006-1, PMID: [DOI] [PubMed] [Google Scholar]
- 22.Manni L, Albanesi M, Guaragna M, Barbaro Paparo S, Aloe L. Neurotrophins and acupuncture. Auton Neurosci. (2010) 157:9–17. doi: 10.1016/j.autneu.2010.03.020, PMID: [DOI] [PubMed] [Google Scholar]
- 23.Li S, Chen K, Wu Y. Effects of warm needling at Zusanli(ST 36)on NO and IL-2 levels in the middle-aged and old people. J Tradit Chin Med. (2003) 23:127–8. doi: 10.3969/j.issn.0255-2922.2003.02.019 PMID: [DOI] [PubMed] [Google Scholar]
- 24.Huang W, Kutner N, Bliwise DL. Autonomic activation in insomnia: the case for acupuncture. J Clin Sleep Med. (2011) 7:95–102. doi: 10.5664/jcsm.28048, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu S, Wang Z-F, Su Y-S, Ray RS, Jing X-H, Wang Y-Q, et al. Somatotopic organization and intensity dependence in driving distinct NPY-expressing sympathetic pathways by electroacupuncture. Neuron. (2020) 108:436–450.e7. doi: 10.1016/j.neuron.2020.07.015, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y-K, Li T, Ha L-J, Lv Z-W, Wang F-C, Wang Z-H, et al. Effectiveness and cerebral responses of multi-points acupuncture for primary insomnia: a preliminary randomized clinical trial and fMRI study. BMC Complement Med Ther. (2020) 20:254. doi: 10.1186/s12906-020-02969-6, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yan C-Q, Liu C-Z, Wang X, Huo J-W, Zhou P, Zhang S, et al. Abnormal functional connectivity of anterior cingulate cortex in patients with primary insomnia: A resting-state functional magnetic resonance imaging study. Front Aging Neurosci. (2018) 10:167. doi: 10.3389/fnagi.2018.00167, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yousaf T, Dervenoulas G, Politis M. Advances in MRI methodology. Int Rev Neurobiol. (2018) 141:31–76. doi: 10.1016/bs.irn.2018.08.008 [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Wang Y. To evaluate the effect of acupuncture on brain function in patients with primary insomnia based on whole brain resting-state fMRI. Chin J Integ Med Cardio-Cerebrovas Dis. (2019) 17:3040–3. doi: 10.12102/j.issn.1672-1349.2019.19.047 [DOI] [Google Scholar]
- 30.Xu X, Wang S, Lu R. Alterations of whole brain networks degree centrality in patients with primary insomnia after acupuncture treatment: A voxel-based resting-state fMRI research. J Sun Yat-sen Univ Med Sci. (2017) 38:296–300. doi: 10.13471/j.cnki.j.sun.yat-sen.univ(med.sci).2017.0048 [DOI] [Google Scholar]
- 31.Zhao F-Y, Fu Q-Q, Kennedy GA, Conduit R, Zhang W-J, Wu W-Z, et al. Can acupuncture improve objective sleep indices in patients with primary insomnia? A systematic review and meta-analysis. Sleep Med. (2021) 80:244–59. doi: 10.1016/j.sleep.2021.01.053, PMID: [DOI] [PubMed] [Google Scholar]
- 32.Kim S-A, Lee S-H, Kim J-H, van den Noort M, Bosch P, Won T, et al. Efficacy of acupuncture for insomnia: a systematic review and meta-analysis. Am J Chin Med. (2021) 49:1135–50. doi: 10.1142/S0192415X21500543, PMID: [DOI] [PubMed] [Google Scholar]
- 33.Wang Z-J, Zhang Y, Guo W, Zhuang L-X, Gao X, Willcox ML, et al. Is single acupoint Sanyinjiao (SP 6) effective in managing insomnia? A systematic review of randomized controlled trials. Glob Health Med. (2020) 2:212–20. doi: 10.35772/ghm.2020.01010, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim S-H, Jeong J-H, Lim J-H, Kim B-K. Acupuncture using pattern-identification for the treatment of insomnia disorder: a systematic review and meta-analysis of randomized controlled trials. Integr Med Res. (2019) 8:216–26. doi: 10.1016/j.imr.2019.08.002, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon C-Y, Lee B, Cheong MJ, Kim T-H, Jang B-H, Chung SY, et al. Non-pharmacological treatment for elderly individuals with insomnia: A systematic review and network Meta-analysis. Front Psychol. (2020) 11:608896. doi: 10.3389/fpsyt.2020.608896, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gunja N. The clinical and forensic toxicology of Z-drugs. J Med Toxicol. (2013) 9:155–62. doi: 10.1007/s13181-013-0292-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sarris J, Byrne GJ. A systematic review of insomnia and complementary medicine. Sleep Med Rev. (2011) 15:99–106. doi: 10.1016/j.smrv.2010.04.001, PMID: [DOI] [PubMed] [Google Scholar]
- 38.Cabýoglu MT, Ergene N, Tan U. The mechanism of acupuncture and clinical applications. Int J Neurosci. (2006) 116:115–25. doi: 10.1080/00207450500341472 [DOI] [PubMed] [Google Scholar]
- 39.Zhu C, Wu S, Zhou M, Bao B. Analysis of the therapeutic effect of Shenmen acupoint. J Anhui Univ Chin Med. (2020) 39:47–50. doi: 10.3969/j.issn.2095-7246.2020.06.014 [DOI] [Google Scholar]
- 40.Waits A, Tang Y-R, Cheng H-M, Tai C-J, Chien L-Y. Acupressure effect on sleep quality: A systematic review and meta-analysis. Sleep Med Rev. (2018) 37:24–34. doi: 10.1016/j.smrv.2016.12.004, PMID: [DOI] [PubMed] [Google Scholar]
- 41.Shergis JL, Ni X, Jackson ML, Zhang AL, Guo X, Li Y, et al. A systematic review of acupuncture for sleep quality in people with insomnia. Complement Ther Med. (2016) 26:11–20. doi: 10.1016/j.ctim.2016.02.007, PMID: [DOI] [PubMed] [Google Scholar]
- 42.Cheng W, Zhang L, Chen B, Tian X, Yao Y, Zhang Z, et al. A study on the regularity of acupoint match based on association rules with SP6 as the main acupoint and its clinical application. Int J Gen Med. (2023) 16:5675–93. doi: 10.2147/IJGM.S441978, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cao Y, Yan Y-J, Xu J-Y, Liwayiding A, Liu Y-P, Yin X, et al. Acupuncture for insomnia after ischemic stroke: an assessor-participant blinded, randomized controlled trial. Acupunct Med. (2022) 40:443–52. doi: 10.1177/09645284221077106, PMID: [DOI] [PubMed] [Google Scholar]
- 44.Tian Q-Q, Cheng C, Yin Z-X, Yuan Y-Y, Wang C, Zeng X, et al. Combined transcutaneous auricular vagus stimulation (taVNS) with 0.1Hz slow breathing enhances insomnia treatment efficacy: a pilot study. Brain Stimul. (2024) 17:4–6. doi: 10.1016/j.brs.2023.11.015, PMID: [DOI] [PubMed] [Google Scholar]
- 45.Wu X, Zhang Y, Luo W-T, Mai R-R, Hou X-Y, Xia Z-Q, et al. Brain functional mechanisms determining the efficacy of transcutaneous auricular vagus nerve stimulation in primary insomnia. Front Neurosci. (2021) 15:609640. doi: 10.3389/fnins.2021.609640, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shi X, Hu Y, Zhang B, Li W, Chen JD, Liu F. Ameliorating effects and mechanisms of transcutaneous auricular vagal nerve stimulation on abdominal pain and constipation. JCI Insight. (2021) 6:150052. doi: 10.1172/jci.insight.150052, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schwartz RM, Shaam P, Williams MS, McCann-Pineo M, Ryniker L, Debnath S, et al. Understanding mental health needs and gathering feedback on transcutaneous auricular Vagus nerve stimulation as a potential PTSD treatment among 9/11 responders living with PTSD symptoms 20 years later: a qualitative approach. Int J Environ Res Public Health. (2022) 19:4847. doi: 10.3390/ijerph19084847, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen HY, Shi Y, Ng CS, Chan SM, Yung KKL, Zhang QL. Auricular acupuncture treatment for insomnia: a systematic review. J Altern Complement Med. (2007) 13:669–76. doi: 10.1089/acm.2006.6400, PMID: [DOI] [PubMed] [Google Scholar]
- 49.Qi S-Y, Lin D, Lin L-L, Huang X-Z, Lin S, Yu Y-Y, et al. Using nonlinear dynamics and multivariate statistics to analyze EEG signals of insomniacs with the intervention of superficial acupuncture. Evid Based Complement Alternat Med. (2020) 2020:8817843. doi: 10.1155/2020/8817843, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M, Wang R, Zhao M, Zhai J, Liu B, Yu D, et al. Abnormalities of thalamus volume and resting state functional connectivity in primary insomnia patients. Brain Imaging Behav. (2019) 13:1193–201. doi: 10.1007/s11682-018-9932-y, PMID: [DOI] [PubMed] [Google Scholar]
- 51.Schiel JE, Holub F, Petri R, Leerssen J, Tamm S, Tahmasian M, et al. Affect and arousal in insomnia: through a lens of neuroimaging studies. Curr Psychiatry Rep. (2020) 22:44. doi: 10.1007/s11920-020-01173-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seeley WW. The salience network: a neural system for perceiving and responding to homeostatic demands. J Neurosci. (2019) 39:9878–82. doi: 10.1523/JNEUROSCI.1138-17.2019, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Leerssen J, Wassing R, Ramautar JR, Stoffers D, Lakbila-Kamal O, Perrier J, et al. Increased hippocampal-prefrontal functional connectivity in insomnia. Neurobiol Learn Mem. (2019) 160:144–50. doi: 10.1016/j.nlm.2018.02.006, PMID: [DOI] [PubMed] [Google Scholar]
- 54.Ma X, Jiang G, Tian J, Liu M, Fang J, Xu Y, et al. Convergent and divergent functional connectivityalterations of hippocampal subregions between short-term and chronic insomnia disorder. Brain Imaging Behav. (2021) 15:986–95. doi: 10.1007/s11682-020-00306-6, PMID: [DOI] [PubMed] [Google Scholar]
- 55.Zhou Q, Yang D, Cui X. Cerebral fMRI in observation on the therapeutic mechanism of electro-acupuncture on Shenmen and Sanyinjiao for insomnia. Chin J Interv Imaging Treat. (2011) 8:204–7. doi: 10.13929/j.1672-8475.2011.03.007 [DOI] [Google Scholar]
- 56.Jiang Y, Shi X, Wang J. A task-ased fMRI study of acupuncture affect the brain regions of primary insomnia. Chin J Lab Diag. (2020) 24:903–6. [Google Scholar]
- 57.Gao L, Zhang M, Gong H, Bai L, Dai X-J, Min Y, et al. Differential activation patterns of FMRI in sleep-deprived brain: restoring effects of acupuncture. Evid Based Complement Alternat Med. (2014) 2014:465760. doi: 10.1155/2014/465760, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Allison T, Puce A, McCarthy G. Social perception from visual cues: role of the STS region. Trends Cogn Sci. (2000) 4:267–78. doi: 10.1016/S1364-6613(00)01501-1, PMID: [DOI] [PubMed] [Google Scholar]
- 59.Cao J, Chen X, Chen J, Ai M, Gan Y, Wang W, et al. Resting-state functional MRI of abnormal baseline brain activity in young depressed patients with and without suicidal behavior. J Affect Disord. (2016) 205:252–63. doi: 10.1016/j.jad.2016.07.002, PMID: [DOI] [PubMed] [Google Scholar]
- 60.Thomas M, Sing H, Belenky G, Holcomb H, Mayberg H, Dannals R, et al. Neural basis of alertness and cognitive performance impairments during sleepiness. I. Effects of 24 h of sleep deprivation on waking human regional brain activity. J Sleep Res. (2000) 9:335–52. doi: 10.1046/j.1365-2869.2000.00225.x, PMID: [DOI] [PubMed] [Google Scholar]
- 61.Kim Y-B, Kim N, Lee JJ, Cho S-E, Na K-S, Kang S-G. Brain reactivity using fMRI to insomnia stimuli in insomnia patients with discrepancy between subjective and objective sleep. Sci Rep. (2021) 11:1592. doi: 10.1038/s41598-021-81219-2, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yang L, Lei Y, Wang L, Chen P, Cheng S, Chen S, et al. Abnormal functional connectivity density in sleep-deprived subjects. Brain Imaging Behav. (2018) 12:1650–7. doi: 10.1007/s11682-018-9829-9, PMID: [DOI] [PubMed] [Google Scholar]
- 63.Shi X-H, Wang Y-K, Li T, Liu H-Y, Wang X-T, Wang Z-H, et al. Gender-related difference in altered fractional amplitude of low-frequency fluctuations after electroacupuncture on primary insomnia patients: A resting-state fMRI study. Brain Behav. (2021) 11:e01927. doi: 10.1002/brb3.1927, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Smallwood J, Bernhardt BC, Leech R, Bzdok D, Jefferies E, Margulies DS. The default mode network in cognition: a topographical perspective. Nat Rev Neurosci. (2021) 22:503–13. doi: 10.1038/s41583-021-00474-4, PMID: [DOI] [PubMed] [Google Scholar]
- 65.Yu S, Guo B, Shen Z, Wang Z, Kui Y, Hu Y, et al. The imbalanced anterior and posterior default mode network in the primary insomnia. J Psychiatr Res. (2018) 103:97–103. doi: 10.1016/j.jpsychires.2018.05.013, PMID: [DOI] [PubMed] [Google Scholar]
- 66.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. (2010) 6:15–28. doi: 10.1038/nrneurol.2009.198, PMID: [DOI] [PubMed] [Google Scholar]
- 67.Chayer C, Freedman M. Frontal lobe functions. Curr Neurol Neurosci Rep. (2001) 1:547–52. doi: 10.1007/s11910-001-0060-4, PMID: [DOI] [PubMed] [Google Scholar]
- 68.Li S, Tian J, Li M, Wang T, Lin C, Yin Y, et al. Altered resting state connectivity in right side frontoparietal network in primary insomnia patients. Eur Radiol. (2018) 28:664–72. doi: 10.1007/s00330-017-5012-8, PMID: [DOI] [PubMed] [Google Scholar]
- 69.Kropf E, Syan SK, Minuzzi L, Frey BN. From anatomy to function: the role of the somatosensory cortex in emotional regulation. Braz J Psychiatry. (2019) 41:261–9. doi: 10.1590/1516-4446-2018-0183, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bai Y, Tan J, Liu X, Cui X, Li D, Yin H. Resting-state functional connectivity of the sensory/somatomotor network associated with sleep quality: evidence from 202 young male samples. Brain Imaging Behav. (2022) 16:1832–41. doi: 10.1007/s11682-022-00654-5, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Davidson RJ, Abercrombie H, Nitschke JB, Putnam K. Regional brain function, emotion and disorders of emotion. Curr Opin Neurobiol. (1999) 9:228–34. doi: 10.1016/S0959-4388(99)80032-4, PMID: [DOI] [PubMed] [Google Scholar]
- 72.Burruss JW, Hurley RA, Taber KH, Rauch RA, Norton RE, Hayman LA. Functional neuroanatomy of the frontal lobe circuits. Radiology. (2000) 214:227–30. doi: 10.1148/radiology.214.1.r00ja43227, PMID: [DOI] [PubMed] [Google Scholar]
- 73.Dang-Vu TT, Desseilles M, Petit D, Mazza S, Montplaisir J, Maquet P. Neuroimaging in sleep medicine. Sleep Med. (2007) 8:349–72. doi: 10.1016/j.sleep.2007.03.006 [DOI] [PubMed] [Google Scholar]
- 74.Lin D, Huang X-Z, Zhuang W-Y, Yu Y-Y, Wu Q. Analysis on insomniac electroencephalogram data after treatment with superficial needling based on approximate entropy and correlation dimensionality. Zhen Ci Yan Jiu. (2018) 43:180–4. doi: 10.13702/j.1000-0607.170373, PMID: [DOI] [PubMed] [Google Scholar]
- 75.Henri-Bhargava A, Stuss DT, Freedman M. Clinical assessment of prefrontal lobe functions. Continuum (Minneap Minn). (2018) 24:704–26. doi: 10.1212/CON.0000000000000609, PMID: [DOI] [PubMed] [Google Scholar]
- 76.Baxter MG. Involvement of medial temporal lobe structures in memory and perception. Neuron. (2009) 61:667–77. doi: 10.1016/j.neuron.2009.02.007, PMID: [DOI] [PubMed] [Google Scholar]
- 77.Flores LP. Occipital lobe morphological anatomy: anatomical and surgical aspects. Arq Neuropsiquiatr. (2002) 60:566–71. doi: 10.1590/S0004-282X2002000400010, PMID: [DOI] [PubMed] [Google Scholar]
- 78.Peng Z, Chen C, Wang Y. Acupuncture plus infrared radiation on Wuzang Tiaoshen points combined with drugs in treating 40 patients with insomnia. J Shandong Univ Tradit Chin Med. (2020) 44:51–4. doi: 10.16294/j.cnki.1007-659x.2020.01.011 [DOI] [Google Scholar]
- 79.Holst SC, Landolt H-P. Sleep-Wake Neurochemistry. Sleep Med Clin. (2022) 17:151–60. doi: 10.1016/j.jsmc.2022.03.002, PMID: [DOI] [PubMed] [Google Scholar]
- 80.Yang J-L, Zhang R, Du L, Yang Y-S, Liu X-C. Clinical observation on the neurotransmitters regulation in patients of insomnia differentiated as yang deficiency pattern treated with warm acupuncture and auricular point sticking therapy. Zhongguo Zhen Jiu. (2014) 34:1165–8. doi: 10.13703/j.0255-2930.2014.12.005 PMID: [DOI] [PubMed] [Google Scholar]
- 81.Monti JM. Serotonin control of sleep-wake behavior. Sleep Med Rev. (2011) 15:269–81. doi: 10.1016/j.smrv.2010.11.003, PMID: [DOI] [PubMed] [Google Scholar]
- 82.Hasegawa E, Miyasaka A, Sakurai K, Cherasse Y, Li Y, Sakurai T. Rapid eye movement sleep is initiated by basolateral amygdala dopamine signaling in mice. Science. (2022) 375:994–1000. doi: 10.1126/science.abl6618, PMID: [DOI] [PubMed] [Google Scholar]
- 83.Benson KL, Bottary R, Schoerning L, Baer L, Gonenc A, Eric Jensen J, et al. (1)H MRS measurement of cortical GABA and glutamate in primary insomnia and major depressive disorder: relationship to sleep quality and depression severity. J Affect Disord. (2020) 274:624–31. doi: 10.1016/j.jad.2020.05.026, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kjaerby C, Andersen M, Hauglund N, Untiet V, Dall C, Sigurdsson B, et al. Memory-enhancing properties of sleep depend on the oscillatory amplitude of norepinephrine. Nat Neurosci. (2022) 25:1059–70. doi: 10.1038/s41593-022-01102-9, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhao F-Y, Spencer SJ, Kennedy GA, Zheng Z, Conduit R, Zhang W-J, et al. Acupuncture for primary insomnia: effectiveness, safety, mechanisms and recommendations for clinical practice. Sleep Med Rev. (2024) 74:101892. doi: 10.1016/j.smrv.2023.101892, PMID: [DOI] [PubMed] [Google Scholar]
- 86.Jiang J, Zhao B, Ha L. Effects of different waveform Electroacupuncture on the 5-HT, Glu and GABA contents of the hypothalamus in rats with PCPA-induced insomnia. Shanghai J Acupunct Moxibustion. (2015) 34:678–81. doi: 10.13460/j.issn.1005-0957.2015.07.0678 [DOI] [Google Scholar]
- 87.Jiang J, Zhao B, Zhao H. Effects of different waveform of electroacupuncture on the levels of monoamine neurotransmitters in hypothalamus of insomnia rats induced by PCPA. Guiding J Tradit Chin Med Pharm. (2015) 21:34–6. doi: 10.13862/j.cnki.cn43-1446/r.2015.21.012 [DOI] [Google Scholar]
- 88.Wu J, Wang T, Wang Y. Research of electro-nape-acupuncture on the contents of monoamine neurotransmitters in brainstem of insomnia rats. J Changchun Univ Chin Med. (2017) 33:358–61. doi: 10.13463/j.cnki.cczyy.2017.03.005 [DOI] [Google Scholar]
- 89.Young MW. Life’s 24-hour clock: molecular control of circadian rhythms in animal cells. Trends Biochem Sci. (2000) 25:601–6. doi: 10.1016/S0968-0004(00)01695-9, PMID: [DOI] [PubMed] [Google Scholar]
- 90.Zielinski MR, Gibbons AJ. Neuroinflammation, sleep, and circadian rhythms. Front Cell Infect Microbiol. (2022) 12:853096. doi: 10.3389/fcimb.2022.853096, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Timmons GA, Carroll RG, O’Siorain JR, Cervantes-Silva MP, Fagan LE, Cox SL, et al. The circadian clock protein BMAL1 acts as a metabolic sensor in macrophages to control the production of pro IL-1β. Front Immunol. (2021) 12:700431. doi: 10.3389/fimmu.2021.700431, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wang X, Wang Z, Cao J, Dong Y, Chen Y. Melatonin ameliorates anxiety-like behaviors induced by sleep deprivation in mice: role of oxidative stress, neuroinflammation, autophagy and apoptosis. Brain Res Bull. (2021) 174:161–72. doi: 10.1016/j.brainresbull.2021.06.010, PMID: [DOI] [PubMed] [Google Scholar]
- 93.Shukla C, Basheer R. Metabolic signals in sleep regulation: recent insights. Nat Sci Sleep. (2016) 8:9–20. doi: 10.2147/NSS.S62365, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu P, Berto S, Kulkarni A, Jeong B, Joseph C, Cox KH, et al. NPAS4 regulates the transcriptional response of the suprachiasmatic nucleus to light and circadian behavior. Neuron. (2021) 109:3268–3282.e6. doi: 10.1016/j.neuron.2021.07.026, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Chen C-R, Zhong Y-H, Jiang S, Xu W, Xiao L, Wang Z, et al. Dysfunctions of the paraventricular hypothalamic nucleus induce hypersomnia in mice. eLife. (2021) 10:9909. doi: 10.7554/eLife.69909, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Liu Z, Wang S. Effects of acupuncture on the expression of clock genes and amino acid neurotransmitters in the brain tissue of insomniac rats by acupuncture on Si Shen Cong and Bai Hui. Chin J Gerontol. (2015) 35:6067–9. doi: 10.3969/j.issn.1005-9202.2015.21.027 [DOI] [Google Scholar]
- 97.Guo B-J, Yu S-Y, Shen Z-F, Hu Y-P. Effect of acupuncture at points in heel vessel for circadian clock genes of period 1 and period 2 mRNAs in the suprachiasmatic nucleus in insomnia rats. Zhen Ci Yan Jiu. (2017) 42:507–9. doi: 10.13702/j.1000-0607.2017.06.007, PMID: [DOI] [PubMed] [Google Scholar]
- 98.Wei X-R, Wei G-W, Zheng X-N, Wu X-F, Chen X-L, Liu L, et al. Effect of acupuncture stimulation of different acupoint combinations on sleep and expression of circadian clock and Bmal 1 genes in hypothalamus of insomnia rats. Zhen Ci Yan Jiu. (2017) 42:429–33. doi: 10.13702/j.1000-0607.2017.05.010 PMID: [DOI] [PubMed] [Google Scholar]
- 99.Tang L, You F, Hu X, Li Y-F. Electroacupuncture improves insomnia by down-regulating peripheral benzodiazepine receptor expression in hippocampus, and up-regulating 5-HT, 5-HIAA, TNF-α and IL-1β contents in hypothalamus in insomnia rats. Zhen Ci Yan Jiu. (2019) 44:560–5. doi: 10.13702/j.1000-0607.180610, PMID: [DOI] [PubMed] [Google Scholar]
- 100.Zhu Y-H, Yang C-H, He L-X, Qiao L, Cheng C-S. Effect of electroacupuncture at “Shenmen”(HT7) and “Sanyinjiao”(SP6) on energy metabolism in paraventricular nucleus of hypothalamus of insomnia rats. Zhen Ci Yan Jiu. (2019) 44:170–5. doi: 10.13702/j.1000-0607.170996, PMID: [DOI] [PubMed] [Google Scholar]
- 101.Cao F, Xu Y, Zhang M, Li X, Chen Y, Zhi M, et al. Baihui (DU20), Shenmen (HT7) and Sanyinjiao (SP6) target the cAMP/CREB/BDNF and PI3K/Akt pathways to reduce central nervous system apoptosis in rats with insomnia. Heliyon. (2022) 8:e12574. doi: 10.1016/j.heliyon.2022.e12574, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lina Q, Yinan S, Lianhong T, Yanshu J, Yongsheng Y. Efficacy of electroacupuncture stimulating Shenmen (HT7), Baihui (GV20), Sanyinjiao (SP6) on spatial learning and memory deficits in rats with insomnia induced by Para-chlorophenylalanine: a single acupoint combined acupoints. J Tradit Chin Med. (2023) 43:704–14. doi: 10.19852/j.cnki.jtcm.20230308.001, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Xu Y, Li X, Man D, Su X. iTRAQ-based proteomics analysis on insomnia rats treated with Mongolian medical warm acupuncture. Biosci Rep. (2020) 40:1517. doi: 10.1042/BSR20191517, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yujuan YE, Yuting W, Jing J, Xingke Y. Efficacy of needling Baihui (GV20), Neiguan (PC6), Shenmen (HT7) and Taichong (LR3) on cerebral cortical blood oxygen level in rats with insomnia. J Tradit Chin Med. (2023) 43:523–32. doi: 10.19852/j.cnki.jtcm.20230404.005, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang J, Ge X, Zhang K, Qi Y, Ren S, Zhai X. Acupuncture for Parkinson’s disease-related constipation: current evidence and perspectives. Front Neurol. (2023) 14:1253874. doi: 10.3389/fneur.2023.1253874, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang J, Ji C, Zhai X, Ren S, Tong H. Global trends and hotspots in research on acupuncture for stroke: a bibliometric and visualization analysis. Eur J Med Res. (2023) 28:359. doi: 10.1186/s40001-023-01253-w, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Jiang T-F, Chen Z-Y, Liu J, Yin X-J, Tan Z-J, Wang G-L, et al. Acupuncture modulates emotional network resting-state functional connectivity in patients with insomnia disorder: a randomized controlled trial and fMRI study. BMC Complement Med Ther. (2024) 24:311. doi: 10.1186/s12906-024-04612-0, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Zheng M, Zheng G, He F. A resting-state functional magnetic resonance imaging study of superficial needling at Shangen point for treatment of primary insomnia. J Fujian Univ Tradit Chin Med. (2014) 24:5–9. doi: 10.13261/j.cnki.jfutcm.002983 [DOI] [Google Scholar]
- 109.Zhao B, Bi Y, Zhang L. Central mechanism study of transcutaneous auricular vagus nerve stimulation based on ReHo in the treatment of primary insomnia. China J Trad Chin Med Pharm. (2020) 35:2585–8. doi: 10.16068/j.1000-1719.2020.05.08 [DOI] [Google Scholar]
- 110.Zhang S, He J-K, Meng H, Zhao B, Zhao Y-N, Wang Y, et al. Effects of transcutaneous auricular vagus nerve stimulation on brain functional connectivity of medial prefrontal cortex in patients with primary insomnia. Anat Rec (Hoboken). (2021) 304:2426–35. doi: 10.1002/ar.24785, PMID: [DOI] [PubMed] [Google Scholar]
- 111.Zhao B, Li L, Zhang J-L, Zhang L, Wang J-Y, Wang Y, et al. Instant adjustive effect of auricular electroacupuncture on brain default model network of patients with primary insomnia. Zhen Ci Yan Jiu. (2019) 44:884–7. doi: 10.13702/j.1000-0607.190562, PMID: [DOI] [PubMed] [Google Scholar]
- 112.He J, Wang C, Fang J. Brain mechanism of transcutaneous auricular electroacupuncture in alleviating daytime sleepiness symptoms of primary insomnia: A rest-state fMRI study. China J Trad Chin Med Pharm. (2021) 36:4637–41. [Google Scholar]
- 113.He J, Jia B, Li S. Instant modulational effect of transcutaneous auricular vagus nerve electrical stimulation on multiple networks in primary insomnia patients. China J Trad Chin Med Pharm. (2021) 36:6422–7. [Google Scholar]
- 114.Zhao B, Bi Y, Li L, Zhang J, Hong Y, Zhang L, et al. The instant spontaneous neuronal activity modulation of transcutaneous auricular vagus nerve stimulation on patients with primary insomnia. Front Neurosci. (2020) 14:205. doi: 10.3389/fnins.2020.00205, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhao B, Bi Y, Chen Y, Zhang J, Zhang S, Zhang D, et al. Altered functional connectivity of the thalamus in patients with insomnia disorder after transcutaneous auricular vagus nerve stimulation therapy. Front Neurol. (2023) 14:1164869. doi: 10.3389/fneur.2023.1164869, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xu Y, Wang Z, Chen D. Controlled observation of instant effect of concentration of brain oxyhemoglobin in patients with primary insomnia by acupuncture. J Liaoning Univ Tradit Chin Med. (2022) 24:82–7. doi: 10.13194/j.issn.1673-842x.2022.12.016 [DOI] [Google Scholar]
- 117.Zhang L, Deng Y, Hui R, Tang Y, Yu S, Li Y, et al. The effects of acupuncture on clinical efficacy and steady-state visual evoked potentials in insomnia patients with emotional disorders: A randomized single-blind sham-controlled trial. Front Neurol. (2022) 13:1053642. doi: 10.3389/fneur.2022.1053642, PMID: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhao Y, Wang Y, Zhang J. Effects of auricular point stimulation via the vagus nerve on polysomnographic features in patients with insomnia and depression. China J Trad Chin Med Pharm. (2022) 37:6168–72. [Google Scholar]
- 119.Ruan J. Effects of electroacupuncture on the content of glutamic acid and aminobutyric acid and expression of GABAA receptor of insomnia rats. China J Trad Chin Med Pharm. (2013) 28:3657–60. [Google Scholar]
- 120.Liu Z, Tang C, Yu M. Effect of different intensities of electroacupuncture on the expression of GABA and GABRA1 in hypothalamus of insomnia rats by PCPA. Life Sci Res. (2011) 15:236–40. doi: 10.16605/j.cnki.1007-7847.2011.03.003 [DOI] [Google Scholar]
- 121.Hu J, Wang Z, Qi Y. The effect of acupuncture at the five visceral points on the monoamine neurotransmitters in insomniac rats. J Changchun Univ Chin Med. (2008) 121:369–74. doi: 10.1097/00029330-200802020-00016, PMID: 18304472 [DOI] [Google Scholar]
- 122.Liu L, Dong B, Liu X. Mechanism of acupuncture at Sanyinjiao(SP6), Shenmen(HT7) and Neiguan(PC6) on hypothalamic 5-HT content and imbalance of Glu/GABA Gln metabolic pathway in insomnia rats. Chin Arch Tradit Chin Med. (2022) 40:65–8. doi: 10.13193/j.issn.1673-7717.2022.03.016 [DOI] [Google Scholar]
- 123.Xing C, Chen Y, Sun Z. Improvement of symptoms and spontaneous activity of insomniac rats by regulating GABA-Gln metabolism. Chin J Comp Med. (2021) 31:47–52. doi: 10.3969/j.issn.1671-7856.2021.05.008 [DOI] [Google Scholar]
- 124.Xie L, Xie Z, Guo X. Effects of acupuncture treatment based on meridians and Acupoint selection on MT content and MT receptor expressions in VLPO in insomnia rats. Chin J Inform Tradit Chin Med. (2018) 25:40–4. doi: 10.3969/j.issn.1005-5304.2018.12.011 [DOI] [Google Scholar]
- 125.Zhen X-N, Wu X-F, Guo X, Xie L-N. Manual acupuncture stimulation of paired Acupoints can relieve sleep disorder possibly by upregulating pineal melatonin protein and its receptor mRNA levels in the suprachiasmatic nucleus in insomnia rats. Zhen Ci Yan Jiu. (2018) 43:360–4. doi: 10.13702/j.1000-0607.170409, PMID: [DOI] [PubMed] [Google Scholar]
- 126.Wu X, Yuan J, Zheng X. Effect of acupuncture treatment on 5-HT1a and 5-HT2a mRNA expression in hypothalamus of insomnia rats. Chin J Rehabilit. (2018) 33:360–4. doi: 10.3870/zgkf.2018.05.002 [DOI] [Google Scholar]
- 127.Wei A, Wei G, Zheng X. Effect of acupuncture with acupoint selection along the meridians on the level of melatonin in rats with insomnia. J Hunan Univ Chin Med. (2018) 38:787–90. doi: 10.3969/j.issn.1674-070X.2018.07.016 [DOI] [Google Scholar]
- 128.Zhang D, Sun Z, Xu X. The effect on the content of 5-HT and 5-HIAA in the insomnia rat hypothalamus caused by Sishencong (EX-HN1) acupuncture. Chin Arch Tradit Chin Med. (2009) 27:1975–7. doi: 10.13193/j.archtcm.2009.09.185.zhangdq.007 [DOI] [Google Scholar]
- 129.Zhou Y-L, Gao X-Y, Wang P-Y, Ren S. Effect of acupuncture at different acupoints on expression of hypothalamic GABA and GABA(A) receptor proteins in insomnia rats. Zhen Ci Yan Jiu. (2012) 37:302–7. doi: 10.13702/j.1000-0607.2012.04.010 PMID: [DOI] [PubMed] [Google Scholar]
- 130.Mu R, Wang Y. Effect of monk’s warmth on dopamine content in brain tissue of insomniac rats. J Med Pharm Chin Minorit. (2014) 20:18–9. doi: 10.16041/j.cnki.cn15-1175.2014.08.050 [DOI] [Google Scholar]
- 131.Lan N, Zhao C, Xu Y. Timing study of 5-HT, 5-HIAA and NE in insomnia rats. Heilongjiang J Tradit Chin Med. (2013) 42:48–9. [Google Scholar]
- 132.Zhang F, Zhang X, Peng Q, Tang L. Electroacupuncture of the cymba concha alleviates p-chlorophenylalanine-induced insomnia in mice. Acupunct Med. (2023) 41:345–53. doi: 10.1177/09645284231160193, PMID: [DOI] [PubMed] [Google Scholar]
- 133.Luo B, Wang Y, Zhang Y. Study of three acupunture methods affecting Total RNA expressions of 5-HT1A and 5-HT2A in Hippocampus of PCPA insomnia rats. Chin Arch Tradit Chin Med. (2017) 35:2630–3. doi: 10.13193/j.issn.1673-7717.2017.10.041 [DOI] [Google Scholar]
- 134.Mu R, Wang Y. Effect of monk’s therapeutic treatment on norepinephrine content in brain tissue of insomniac rats. J Med Pharm Chin Minorit. (2014) 20:50–1. doi: 10.16041/j.cnki.cn15-1175.2014.07.040 [DOI] [Google Scholar]
- 135.Luo B, Wang Y, Zhang Y. Study of three aupuncture Methock on affecting the content of 5-HT, 5-HIAA in hippocampal of PCPA insomnia rats. J Basic Chin Med. (2016) 22:1517–9. doi: 10.19945/j.cnki.issn.1006-3250.2016.11.034 [DOI] [Google Scholar]
- 136.Wang Y, Peng F, Chen T. Effects of warm acupuncture on monoamine neurotransmitters in the brainstem of insomniac rats. Lishizhen Med Materia Med Res. (2015) 26:1784–5. [Google Scholar]
- 137.Zhou Y. Experimental study on the effect of acupuncture on neurotransmitters 5-HT and DA in the brain of rats with insomnia model. J Basic Chin Med. (2012) 18:887–8. doi: 10.19945/j.cnki.issn.1006-3250.2012.08.036 [DOI] [Google Scholar]
- 138.Li W, Liu C-Y, Gao X-Y, Li Y-J. Effect of Yuanshu points on p38MAPK in brain tissue of insomnia model rats. Shaanxi J Tradit Chin Med. (2024) 45:883–7. doi: 10.3969/j.issn.1000-7369.2024.07.004 [DOI] [Google Scholar]
- 139.Zhao M, Gao L, Gao X-Y, Chen X-W. Effect of acupuncture at Back-Shu points on expression of PI3K and Akt in brain tissue of insomnia model rats. J Liaoning Univ Tradit Chin Med. (2023) 25:52–5. doi: 10.13194/j.issn.1673-842x.2023.06.010 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.