Abstract
Background
Accumulating evidence have demonstrated that tobacco smoke exposure (TSE) causes damage to human mental issues. However, previous studies almost focus on the individual smoking exposure patterns and some inconsistent results are reported. Serum cotinine is a reliable and quantitative biomarker of TSE. This study aims to explore the association of serum cotinine with depression and sleep disorders and the potential gender differences.
Methods
Data from the National Health and Nutrition Examination Survey (NHANES) 2007-2014 was used. Weighted multiple logistic regression methods, generalized additive models (GAM), and restricted cubic spline (RCS) models were used for association analyses. Moreover, gender-stratified analyses were conducted.
Results
Of 12,599 individuals included in the final analysis, 1,295 had depression, 3245 had trouble sleeping and 1152 had diagnostic sleep disorders. After adjusting for potential covariates, linear relationship suggested higher serum cotinine levels were positively associated with risk of depression and sleep disorders, including self-reported trouble sleeping and diagnostic sleep disorders in the total sample and female participants, and serum cotinine levels were positively correlated with depression and trouble sleeping in male participants. Additionally, inverted L-shaped associations between serum cotinine and depression and sleep disorders were detected, and at the same cotinine level, females have a higher risk of experiencing depression and sleep disorders.
Conclusions
In this study, higher serum cotinine increased the risk of depression and sleep disorders and there was stronger association in females than males. These findings provided novel evidence about how TSE affected the mental condition of the general US population.
Keywords: tobacco smoke exposure, serum cotinine, human mental health, depression, sleep disorders, gender differences
1. Introduction
Accumulating epidemiological evidence has demonstrated a close relationship between tobacco smoke exposure (TSE) and various health damages and diseases, leading to an increasing global burden on society and economy (1, 2). It has been widely known that TSE increases the risk of lung cancer (3), respiratory diseases (3), cardiovascular diseases (4), reproductive and developmental diseases (5), and mental health issues (6). It is estimated that more than 200 million deaths have been caused by smoking tobacco use over the past 30 years and over eight million premature deaths and over 1 trillion dollars economic costs are attributable to TSE annually (7, 8).
Depression and sleep disorders are significant public health issues that seriously impair mental health and increase risks of various chronic diseases and mortality (6, 9, 10). Moreover, depression and sleep disorders exhibit a high degree of comorbidity, with sleep disorders potentially being both a consequence and a contributing factor to depression (11–13). Previous studies demonstrate that TSE is a main behavior risk of depression and sleep disorders (14). Smoking is even reported to have a causal effect on depression (15). Not only is the active smoking widely considered a risk factor for these mental health disorders, but secondhand smoke (SHS) has also been linked to mental health issues including depression, anxiety, and sleep disorders (6). Although majority of studies report that both active cigarette smoking and passive smoke exposure are found to be associated with sleep disturbance (16), inconsistent research results are presented. For example, Wang et al. reported an association between SHS and poor sleep quality in self-reported never-smokers in China (17), while a nationwide study using data from the National Health and Nutrition Examination Survey (NHANES) reported no significant associations between SHS and sleep disorders (18).
However, previous studies mainly evaluate TSE based on self-reported smoking status, and the definitions are inconsistent, introducing bias into the associations between TSE and research outcomes. Moreover, self-reported estimates of both active smoking and passive smoke exposure have been shown to be much lower than biomarker-determined exposure (19). Recent studies have sought to replace self-reported smoking status with serum cotinine levels as a more accurate and objective measure for assessing TSE (20, 21). Previous studies have explored the association between serum cotinine exposure and various health issues such as liver disease (22), Alzheimer’s disease (23), and diabetes (24, 25). However, research examining the association of cotinine levels with depression and sleep disorders remains scarce.
In this study, a cross-sectional study is conducted based on data from 4 cycles of NHANES 2007-2014. We aim to investigate the association of serum cotinine concentrations with depression and sleep disorders and to determine whether the gender differences in the associations exist.
2. Methods
2.1. Data source and study population
NHANES is a cross-sectional and nationwide survey conducted by the National Center for Health Statistics of the Centers for Disease Control and Prevention (CDC), aiming to assess the health and nutrition status of children and adults in the US. The survey is conducted every two years, and demographic data, dietary data, examination data, and laboratory data were collected. Complex, multistage, and probability sampling methods are used to obtain representative data of the general population in the United States. The relevant information of NHANES can be accessed at https://www.cdc.gov/nchs/nhanes/about_nhanes.htm.
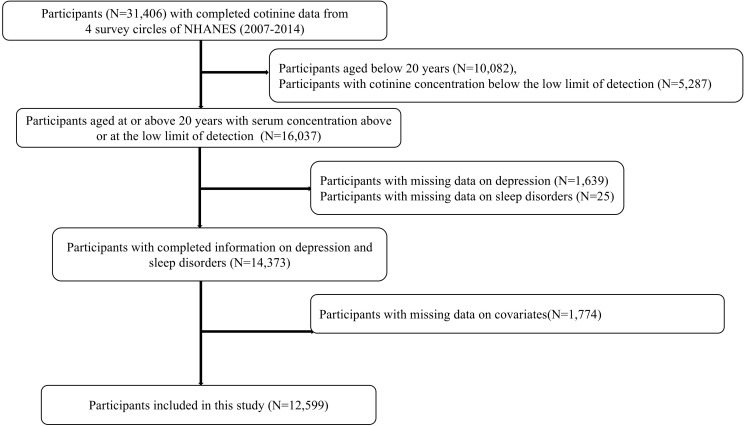
Data used in this study was from four cycles of datasets from NHANES (2007-2014). Among 31,406 participants with completed serum cotinine data from NHANES 2007-2014, we excluded 10,082 participants aged below 20 years, 5,287 participants with cotinine concentration below the low limit of detection, 1,639 participants with missing data on depression, 25 participants with missing data on sleep disorders, and 1,774 with missing data on covariates. Finally, a total of 12,599 participants were recruited in the study. Additional details of the study design, sampling, and exclusion procedures were illustrated in Figure 1 .
Figure 1.
Flow chart population included in the analysis.
2.2. Serum cotinine measurement
Serum cotinine is measured by an isotope-dilution high-performance liquid chromatography/atmospheric pressure chemical ionization tandem mass spectrometric (ID HPLC-APCI MS/MS) method. Briefly, the serum sample is spiked with methyl-D3-cotinine and methyl-D3-hydroxycotinine as internal standards. The cotinine was measured by m/z 80 product ion from the m/z 177 quasi-molecular ion. Analyte concentrations are derived from the area ratios of native-to-labeled compounds in the sample by comparisons to a standard curve. Details of the serum cotinine testing methods is available https://wwwn.cdc.gov/Nchs/Nhanes/2013-2014/COT_H.htm.
The lower limit of detection (LLOD) of serum cotinine was 0.015 ng/mL, and participants with serum cotinine concentrations below LLOD were excluded from this study referring to the previous study (21).
2.3. Assessment of depression and sleep disorders
The depressive symptoms were measured by the Patient Health Questionnaire-9 (PHQ-9), a good self-report instrument that assessed depression symptoms over the past 2 weeks. The PHQ-9 questionnaire contains nine items, in which response categories “not at all” “several days” “more than half the days” and “nearly every day” were given a point ranging from 0 to 3 and summing up a total scale range of 0 to 27. A total score ≥ 10 was considered as depression (13, 26, 27).
Sleep disorders were assessed by Sleep Disorder Questionnaire in NHANES. Participants were asked whether they have ever told a doctor or other health professional that they have trouble sleeping and whether they have been told by a doctor or other health professional that they have a sleep disorder. The participants who answered “Yes” were considered with trouble sleeping or diagnostic sleep disorder. The answers of “Do not know” and “Refused” were considered missing.
2.4. Covariates
Demographic characteristics and data on lifestyle and diabetes were collected through home interviews. The race was classified into four categories: non-Hispanic white, non-Hispanic black, Mexican American, and other or multiracial (28). Family income level was evaluated by family income-to-poverty ratio (FIPR) (≤1.3, 1.3–3.5, >3.5) (29). Educational levels were classified into four categories: less than high school graduated, high school graduated, some college or AA degree and college graduate or above. Body mass index (BMI) was calculated through dividing weight by square of height (kg/m2) and divided into three stratifications: ≤24.9, 25-29.9, and >30 kg/m2 (30). Drinking status was classified into two groups: no (those who had less than 12 alcohol drinks <12 times per year) and yes (those who had at least 12 alcohol drinks per year).
The physical activity was assessed by the Global Physical Activity Questionnaire (GPAQ), which included questions related to daily activities, leisure time activities, and sedentary activities. Physical activity levels were further categorized by metabolic equivalent (MET) scores using the recommended MET score for each activity type in NHANES. Finally, participants were divided into three groups: no physical activity group (0 MET-h/week), low intensity physical activity group (≤48 MET-h/wk) and high intensity physical activity group (> 48 MET-h/wk) (31, 32).
In addition, Healthy Eating Index-2015 (HEI-2015) score was calculated for each participant to assess diet quality based upon the Dietary Guidelines for Americans 2015–2020 (33).
Age and HEI-2015 scores were treated as continuous variables in subsequent analyses.
2.5. Statistical analysis
Analyses were conducted according to the guidelines for the analysis of NHANES data. Considering the complex survey design, an appropriate weight was used to provide nationally representative estimates and the weighted data was employed for subsequent analyses.
Serum cotinine concentrations were divided into four categories by quartiles in the weighted samples. The cut-off values were 0.031, 0.139, and 128.000 ng/mL, respectively. Descriptive information about demographic characteristics stratified by cotinine groups was represented as numbers (n) and percentages (%) for categorical variables and the mean and standard deviation (SD) or median and inter-quartile range (IQR) for continuous variables. Differences among groups were tested by using the Scott-Rao chi-square tests for categorical variables and ANOVA or Kruskal-Wallis tests for continuous variables.
The weighted logistic regression models were built to access the association between cotinine exposure and sleep disorders, in which the serum cotinine concentrations were employed as continuous variable and quartile categories (the first quartile was assigned as the reference group) exposure variables. Two models were used to explore the linear relationships. Model 1 was adjusted for age, gender and race; Model 2 was further adjusted for educational levels, FIPR, drinking status, physical activity, diabetes status, and HEI-2015 and added depression for sleep disorders (trouble sleeping and diagnostic sleep disorders) and trouble sleeping for depression, respectively. In addition, the ln-transformed serum cotinine concentrations were also used as continuous variables in the models.
Restricted cubic spline (RCS) and generalized additive model (GAM) were statistical approaches for fitting nonlinear relationships and were applied to analyze the nonlinear and dose-response relationships between serum cotinine concentrations and depression and sleep disorders. RCS is characterized by its linear restriction at the data range extremities, which helps in curbing overfitting, particularly during extrapolation. GAM extends beyond generalized linear models (GLM) by relaxing strict linearity assumptions, allowing for a variety of functions, including splines, local regression, or kernel functions, to model the relationships between variables. All the weighted models were adjusted for age, gender, race, educational levels, FIPR, drinking status, physical activity, diabetes status, and HEI-2015 and depression for sleep disorders (trouble sleeping and diagnostic sleep disorders) and trouble sleeping for depression, respectively. Generally, the effective degree of freedom (EDF) was obtained to estimate non-linearity, where EDF = 1 was considered as linearity and EDF > 1 was considered as non-linearity (34).
In addition, subgroups analyses were preformed stratified by gender as the gender differences were reported in previous studies. We constructed gender-stratified the smooth curve fitting methods and RCS analyses in subpopulations to estimate the nonlinear and dose-response relationships.
All statistical analyses were performed by using R software (version 4.2.2; R Core Team) and EmpowerStats (version 3.0). A two-sided P <0.05 considered statistically significant.
3. Results
3.1. Characteristics of the study population
The baseline characteristics of the study population stratified by serum cotinine concentrations (Q1: 0.015–0.031 ng/mL, Q2: 0.031–0.139 ng/mL, Q3: 0.139–128.000 ng/mL, Q4: 128.000-1820.000 ng/mL) were summarized in Tables 1 and 2 . Of 12,599 participants included in the analysis, 6,611 were males, 1,295 had depression, 3245 had trouble sleeping and 1152 had diagnostic sleep disorders. As shown in Tables 1 and 2 , age, gender, race, educational levels, physical activity, alcohol drinking, BMI levels, HEI-2015 and diabetes prevalence of participants in the four subgroups were summarized and significant differences were detected among the four subgroups. In addition, the prevalence of depression, trouble sleeping and diagnostic sleep disorder were found to increase in correlation with serum cotinine levels across quartiles.
Table 1.
Baseline data of participants in groups stratified by serum cotinine concentrations.
| Total (N=12599) | Q1 (0.015-0.031) (N=3102) | Q2 (0.031-0.139) (N=3188) | Q3 (0.139-128.000) (N=3283) | Q4(128.000)-(N=3026) | p | |
|---|---|---|---|---|---|---|
| Age, year | 45.49 ± 16.45 | 52.01 ± 16.42 | 49.38 ± 17.29 | 44.02 ± 16.46 | 45.95 ± 14.39 | <0.001 |
| Gender, n (weighted %) | <0.001 | |||||
| Male | 6611 (52.4) | 1676 (55.0) | 1583 (48.7) | 1503 (45.7) | 1226 (41.0) | |
| Female | 5988 (47.6) | 1426 (45.0) | 1605 (51.3) | 1780 (54.3) | 1800 (59.0) | |
| Race, n (weighted %) | <0.001 | |||||
| Mexican American | 2777 (12.6) | 899 (15.0) | 790 (14.5) | 777 (15.2) | 311 (5.7) | |
| Non-Hispanic White | 5804 (68.2) | 1333 (68.2) | 1349 (65.7) | 1347 (63.1) | 1775 (76.0) | |
| Non-Hispanic Black | 2920 (12.4) | 510 (8.4) | 934 (11.7) | 934 (16.5) | 768 (13.1) | |
| Other or multiracial | 1908 (6.7) | 360 (8.4) | 225 (8.1) | 225 (5.2) | 172 (5.2) | |
| FIPR, n (weighted %) | <0.001 | |||||
| ≤1.30 | 4521 (25.2) | 757 (15.4) | 946 (20.3) | 1329 (29.5) | 1489 (35.9) | |
| 1.30-3.50 | 4608 (36.2) | 1146 (33.8) | 1234 (37.4) | 1227 (37.6) | 1001 (35.8) | |
| >3.50 | 3470 (38.6) | 1199 (50.8) | 1008 (42.4) | 727 (32.9) | 536 (28.3) | |
| Educational levels, n (weighted %) | <0.001 | |||||
| Less than high school graduated | 3276 (18.5) | 617 (11.9) | 755 (15.8) | 900 (19.8) | 1004 (26.8) | |
| High school graduated | 3145 (24.9) | 598 (17.4) | 726 (23.8) | 882 (26.2) | 939 (32.1) | |
| Some college or AA degree | 3775 (32.8) | 923 (32.1) | 948 (32.1) | 1038 (35.2) | 866 (31.9) | |
| College graduate or above | 2403 (23.7) | 964 (38.6) | 759 (28.2) | 463 (18.8) | 217 (9.2) | |
| Alcohol drinking, n (weighted %) | <0.001 | |||||
| No | 3076 (19.6) | 979 (24.9) | 1000 (25.0) | 669 (16.7) | 428 (11.6) | |
| Yes | 9523 (80.4) | 2123 (75.1) | 2188 (75.0) | 2614 (83.3) | 2598 (88.4) | |
| Diabetes, n (weighted %) | 2247 (13.5) | 629 (15.1) | 612 (14.4) | 554 (12.5) | 452 (12.2) | 0.01 |
| BMI levels, n (weighted %) | <0.001 | |||||
| <25 | 3640 (29.9) | 851 (29.4) | 837 (26.6) | 830 (26.7) | 1122 (37.1) | |
| 25 to <30 | 4118 (33.0) | 1076 (34.8) | 1047 (33.3) | 1038 (32.7) | 957 (31.1) | |
| ≥30 | 4841 (37.1) | 1175 (35.9) | 1304 (40.1) | 1415 (40.6) | 947 (31.8) | |
| Physical activity, n (weighted %) | <0.001 | |||||
| No | 3240 (22.0) | 767 (21.2) | 823 (20.9) | 822 (21.4) | 828 (24.3) | |
| Low | 5231 (43.2) | 1501 (50.4) | 1377 (44.8) | 1298 (42.3) | 1055 (35.2) | |
| High | 4128 (34.8) | 834 (28.4) | 988 (34.3) | 1163 (36.3) | 1143 (40.5) | |
| HEI-2015 | 52.09 ± 13.08 | 56.48 ± 13.15 | 54.24 ± 13.21 | 50.57 ± 12.10 | 47.04 ± 11.76 | <0.001 |
Sampling weights were applied for calculation of demographic descriptive statistics. The p value was calculated after weighting.
FIPR, family income-to-poverty index; BMI, body mass index; HEI-2015, healthy eating index 2015.
Table 2.
Information about depression and sleep disorders in different groups stratified by serum cotinine concentrations.
| Total(N=12599) | Q1(N=3102) | Q2(N=3188) | Q3(N=3283) | Q4(N=3026) | p | |
|---|---|---|---|---|---|---|
| Depression, n (weighted %) | <0.001 | |||||
| No | 12304 (91.0) | 2198 (95.2) | 2928 (93.4) | 2926 (90.4) | 2532 (85.1) | |
| Yes | 295 (9.0) | 184 (4.8) | 260 (6.6) | 357 (9.6) | 494 (14.9) | |
| Trouble sleeping, n (weighted %) | <0.001 | |||||
| No | 9354 (73.0) | 2394 (75.8) | 2478 (76.7) | 2415 (72.1) | 2058 (67.3) | |
| Yes | 3245 (27.0) | 708 (24.2) | 710 (23.3) | 868 (27.9) | 968 (32.7) | |
| Diagnostic sleep disorder, n (weighted %) | 0.007 | |||||
| No | 11447 (90.7) | 2840 (91.4) | 2917 (91.5) | 2993 (91.1) | 2697 (88.7) | |
| Yes | 1152 (9.3) | 262 (8.6) | 271 (8.5) | 290 (8.9) | 329 (11.3) |
Sampling weights were applied for calculation of demographic descriptive statistics. The p value was calculated after weighting.
As shown in Supplementary Table S1 in gender-stratified groups, the median (IQR) of serum cotinine concentrations were 0.139 (0.031, 128.000) ng/mL in total participants, 0.269 (0.036, 166.000) ng/mL in male participants, and 0.093 (0.028, 73.300) ng/mL in female participants, respectively. Female participants had lower serum cotinine concentrations and higher prevalence of depression and trouble sleeping.
3.2. The linear relationship analysis between serum cotinine and depression and sleep disorders
The linear relationships between serum cotinine and depression and sleep disorders were performed by weighted binary logistic regression models and the results were presented in Table 3 . After adjusting for potential covariables, serum cotinine concentrations showed significant association with depression, trouble sleeping and diagnostic sleep disorder. Compared with Q1 of serum cotinine concentrations, the ORs (quartile, 95%CIs) of depression were 1.59 (Q3, 1.28-1.98) and 2.39 (Q4, 1.81-3.17); the ORs (quartile, 95%CIs) of trouble sleeping were 1.31 (Q3, 1.09-1.58) and 1.46 (Q4, 1.21-1.75); and the OR (quartile, 95%CIs) of diagnostic sleep disorder was 1.40 (Q4, 1.13-1.75).
Table 3.
The relationship between serum cotinine and depression and sleep disorders.
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | ||
| Depression | |||||
| continuous | 1.14 (1.13,1.16) | <0.001 | 1.08 (1.06,1.10) | <0.001 | |
| Q1 | ref. | ref. | |||
| Q2 | 1.51 (1.15,1.97) | 0.003 | 1.34 (1.00,1.79) | 0.052 | |
| Q3 | 2.36 (1.91,2.92) | <0.001 | 1.59 (1.28,1.98) | <0.001 | |
| Q4 | 4.27 (3.40,5.36) | <0.001 | 2.39 (1.81,3.17) | <0.001 | |
| P for trend | <0.001 | <0.001 | |||
| Trouble sleeping | |||||
| Continuous | 1.06 (1.05,1.08) | <0.001 | 1.05 (1.03,1.07) | <0.001 | |
| Q1 | ref. | ref. | |||
| Q2 | 1.03 (0.89,1.18) | 0.682 | 0.98 (0.84,1.13) | 0.77 | |
| Q3 | 1.46 (1.23,1.74) | <0.001 | 1.31 (1.09,1.58) | 0.005 | |
| Q4 | 1.72 (1.46,2.03) | <0.001 | 1.46 (1.21,1.75) | <0.001 | |
| P for trend | <0.001 | <0.001 | |||
| Diagnostic sleep disorder | |||||
| continuous | 1.05 (1.02,1.07) | <0.001 | 1.05 (1.02,1.07) | <0.001 | |
| Q1 | ref. | ref. | |||
| Q2 | 1.03 (0.83,1.29) | 0.761 | 0.97 (0.78,1.22) | 0.808 | |
| Q3 | 1.19 (0.95,1.50) | 0.126 | 1.09 (0.85,1.39) | 0.503 | |
| Q4 | 1.46 (1.21,1.77) | <0.001 | 1.40 (1.13,1.75) | 0.003 | |
| P for trend | <0.001 | <0.001 | |||
Model 1 was adjusted by age, gender, race.
Model 2, for depression, was further adjusted by FIPR, educational level, physical activity, alcohol drinking, diabetes, BMI levels, HEI-2015 and trouble sleeping; for trouble sleeping and diagnostic sleep disorder was further adjusted by age, gender, race, FIPR, educational level, physical activity, alcohol drinking, diabetes, BMI levels, HEI-2015 and depression.
When performed ln-transformed serum cotinine concentrations as continuous variables in the weighted logistic regression models, the similar positive associations of serum cotinine concentrations and depression, trouble sleeping and diagnostic sleep disorder were remained. The multivariate-adjusted OR (95%CIs) reached 1.08 (1.06-1.10) for depression, 1.05 (1.03-1.07) for trouble sleeping, and 1.05 (1.02-1.07) for diagnostic sleep disorder.
3.3. The nonlinear relationships between serum cotinine and depression and sleep disorders
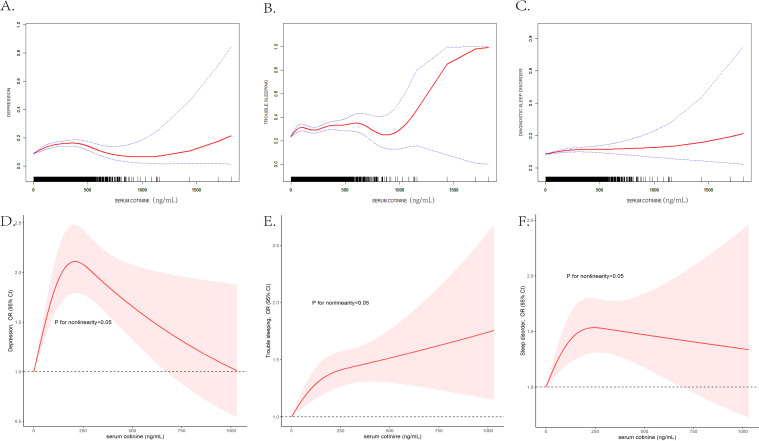
The GAM and RCS models were applied to estimate the nonlinear relationship between serum cotinine concentrations and depression and sleep disorders. As Figure 2 displayed, the nonlinear relationships between serum cotinine and depression (EDF = 4.507, p<0.001), trouble sleeping (EDF =6.704, p<0.001) and diagnostic sleep disorder (EDF = 2.667, p<0.001) were detected. In addition, RCS models presented inverted L-shape patterns in the associations.
Figure 2.
The non-linear association analyzed by the smooth curve fitting method between serum cotinine and depression (A), trouble sleeping (B), diagnostic sleep disorder (C) and analyzed by RCS analysis between serum cotinine and depression (D), trouble sleeping (E), and diagnostic sleep disorder (F). The X-axis showed the serum cotinine concentration, and the Y-axis showed the estimated risk for depression (A), trouble sleeping (B), diagnostic sleep disorder (C) and the estimated odds ratio for depression (D), trouble sleeping (E), and diagnostic sleep disorder (F).
3.4. The subgroup associations between serum cotinine and depression and sleep disorders stratified by gender
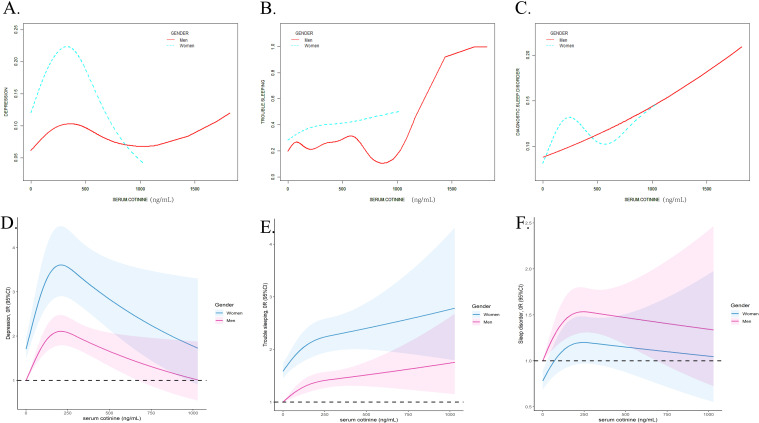
The linear relationships between serum cotinine and depression and sleep disorders stratified by gender were listed in Table 4 (for males) and Table 5 (for females). The smooth curve fitting methods and RCS curves were presented in Figure 3 . In male participants, serum cotinine showed positive associations with depression and trouble sleeping ( Table 4 ). In female participants, serum cotinine showed positive associations with depression, trouble sleeping, and diagnostic sleep disorder ( Table 5 ).
Table 4.
The relationship between serum cotinine and depression and sleep disorders in male participants.
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | ||
| Depression | |||||
| continuous | 1.11 (1.07,1.15) | <0.001 | 1.05 (1.01,1.09) | 0.014 | |
| Q1 | ref. | ref. | |||
| Q2 | 1.20 (0.81,1.76) | 0.351 | 1.13 (0.68,1.90) | 0.623 | |
| Q3 | 1.84 (1.31,2.58) | <0.001 | 1.07 (0.65,1.75) | 0.799 | |
| Q4 | 2.85 (2.00,4.07) | <0.001 | 1.68 (1.07,2.66) | 0.027 | |
| P for trend | <0.001 | 0.020 | |||
| Trouble sleeping | |||||
| continuous | 1.05 (1.02,1.07) | <0.001 | 1.04 (1.02,1.07) | <0.001 | |
| Q1 | ref. | ref. | |||
| Q2 | 1.24 (1.00,1.53) | 0.049 | 1.24 (1.00,1.54) | 0.053 | |
| Q3 | 1.61 (1.30,1.99) | <0.001 | 1.58 (1.26,1.99) | <0.001 | |
| Q4 | 1.54 (1.24,1.92) | <0.001 | 1.58 (1.25,1.99) | <0.001 | |
| P for trend | <0.001 | <0.001 | |||
| Diagnostic sleep disorder | |||||
| continuous | 1.03 (0.99,1.06) | 0.122 | 1.04 (1.00,1.07) | 0.065 | |
| Q1 | ref. | ref. | |||
| Q2 | 0.94 (0.71,1.26) | 0.678 | 0.94 (0.70,1.26) | 0.666 | |
| Q3 | 1.15 (0.83,1.58) | 0.394 | 1.15 (0.83,1.61) | 0.388 | |
| Q4 | 1.15 (0.82,1.61) | 0.397 | 1.28 (0.92,1.79) | 0.138 | |
| P for trend | 0.292 | 0.426 | |||
Model 1 was adjusted by age, race.
Model 2, for depression, was adjusted by FIPR, educational level, physical activity, alcohol drinking, diabetes, BMI levels, HEI-2015 and trouble sleeping; for trouble sleeping and diagnostic sleep disorder was adjusted by age, gender, race, FIPR, educational level, physical activity, alcohol drinking, diabetes, BMI levels, HEI-2015 and depression.
Table 5.
The relationship between serum cotinine and depression and sleep disorders in female participants.
| Model 1 | Model 2 | ||||
|---|---|---|---|---|---|
| OR (95%CI) | p | OR (95%CI) | p | ||
| Depression | |||||
| continuous | 1.16 (1.14,1.19) | <0.001 | 1.10 (1.08,1.13) | <0.001 | |
| Q1 | ref. | ref. | |||
| Q2 | 1.47 (0.99,2.18) | 0.059 | 1.32 (0.87,2.00) | 0.185 | |
| Q3 | 2.17 (1.55,3.03) | <0.001 | 1.53 (1.08,2.17) | 0.018 | |
| Q4 | 4.70 (3.33,6.62) | <0.001 | 2.75 (1.88,4.04) | <0.001 | |
| P for trend | <0.001 | <0.001 | |||
| Trouble sleeping | |||||
| continuous | 1.08 (1.06,1.10) | <0.001 | 1.05 (1.03,1.08) | <0.001 | |
| Q1 | ref. | ref. | |||
| Q2 | 0.97 (0.8,1.18) | 0.779 | 0.90 (0.73,1.12) | 0.335 | |
| Q3 | 1.27 (0.98,1.63) | 0.066 | 1.09 (0.84,1.43) | 0.499 | |
| Q4 | 1.92 (1.54,2.4) | <0.001 | 1.47 (1.15,1.88) | 0.003 | |
| P for trend | <0.001 | <0.001 | |||
| Diagnostic sleep disorder | |||||
| continuous | 1.07 (1.04,1.10) | <0.001 | 1.06 (1.02,1.09) | 0.003 | |
| Q1 | ref. | ref. | |||
| Q2 | 1.10 (0.79,1.54) | 0.560 | 0.98 (0.68,1.40) | 0.896 | |
| Q3 | 1.14 (0.83,1.58) | 0.405 | 0.97 (0.69,1.37) | 0.873 | |
| Q4 | 1.88 (1.39,2.56) | <0.001 | 1.62 (1.16,2.26) | 0.006 | |
| P for trend | <0.001 | <0.001 | |||
Model 1 was adjusted by age, race.
Model 2, for depression, was adjusted by FIPR, educational level, physical activity, alcohol drinking, diabetes, BMI levels, HEI-2015 and trouble sleeping; for trouble sleeping and diagnostic sleep disorder was adjusted by age, gender, race, FIPR, educational level, physical activity, alcohol drinking, diabetes, BMI levels, HEI-2015 and depression.
Figure 3.
The gender-stratified non-linear association analyzed by the GAM between serum cotinine and depression (A), trouble sleeping (B), diagnostic sleep disorder (C), and analyzed by RCS analysis between serum cotinine and depression (D), trouble sleeping (E), and diagnostic sleep disorder (F). The X-axis showed the serum cotinine concentration, and the Y-axis showed the estimated risk for depression (A), trouble sleeping (B), diagnostic sleep disorder (C) and the estimated odds ratio for depression (D), trouble sleeping (E), and diagnostic sleep disorder (F).
The GAM, smooth curve fitting methods and RCS models applied to estimate the dose–response curve of associations between serum cotinine and mental health disorders. As Figure 3 displayed, in female participants, the nonlinear associations between serum cotinine and depression (EDF = 2.722, p<0.001), trouble sleeping (EDF = 2.512, p<0.001) and diagnostic sleep disorder (EDF = 2.844, p<0.001) were detected; in female participants, the nonlinear associations between serum cotinine and depression (EDF = 3.153, p<0.001) and trouble sleeping (EDF = 7.041, p<0.001) were detected and the association between serum cotinine and of diagnostic sleep disorder was quiet different (EDF = 1.004, p=0.034). In addition, the nonlinear relationships stratified by gender were detected in RCS models, and the trend was different from smooth curve fitting method. In addition, the associations of serum cotinine concentrations with depression, trouble sleeping and diagnostic sleep disorder were stronger in female participants than males.
4. Discussion
In the current study, we examined the correlation between serum cotinine levels and depression and sleep disorders in a representative sample of adults in the United States. Our findings revealed that higher serum cotinine levels were associated with an increased risk of depression and sleep disorders (self-reported trouble sleeping and diagnostic sleep disorders). This association was significant in both the total sample and female participants. Additionally, we identified non-linear relationships between serum cotinine levels and depression and sleep disorders and the associations were stronger in females than males.
Current evidence demonstrated that TSE increased risk of depression and sleep disorders. A mendelian randomization study have indicated a causal relationship between smoking including lifetime smoking and smoking initiation, and depression (15). It was remarkably, however, that the association between SHS and depression reported inconsistent results in previous studies, although majority of them reported significant association (6, 35). Self-reported smoking was found to be significantly associated with increased difficulty of initiating sleep (36). Furthermore, a cross-sectional study conducted in Korea found a significant association between urinary cotinine levels and depression symptoms (37). Elevated urinary cotinine levels have also been linked to a higher likelihood of experiencing sleep problems (38). Similarly, our findings indicated that higher TSE is associated with increased risks of depression and sleep disorders.
Furthermore, the non-linear relationships observed in our study suggested the presence of potential inflection points in the effect of serum cotinine on mental health disorders. This finding potentially supported the self-medication hypothesis that smokers may experience withdrawal symptoms and addiction when nicotine levels decreased, leading them to seek more nicotine to alleviate these symptoms (11, 39, 40). The inflection level exceeded 200 ng/mL, that accounts for majority of the cotinine-detectable population in our study. This indicated that individuals with nicotine addiction might reach higher nicotine levels to fulfill their physical and psychological needs, unexpectedly alleviating their symptoms depression and sleep disorders. Additionally, evidence suggested that the association between smoking and depression may be bidirectional, as chronic smokers may attempt to alleviate depressive symptoms by smoking, which can affect sleep architecture (11, 41). In general, from a public health perspective, controlling TSE is more beneficial to people’s health, especially for those who are passively exposed to tobacco (generally considered to have serum cotinine levels below 10 ng/mL), as it can reduce the risk of depression and sleep disorders.
Interestingly, our results showed that females had lower serum cotinine levels but a higher prevalence of depression and trouble sleeping, along with stronger associations between serum cotinine levels and both depression and sleep disorders. Previous studies on gender differences in TSE and depression and sleep disorders have yielded inconsistent findings. Park et al. reported a significant association between urinary cotinine levels and depression symptoms only in females (37). A meta-analysis including 24 studies revealed no gender difference in the association between SHS and depressive symptoms (42). Zandy et al. (38) reported stronger associations of TSE and sleep problems in females, while Mehari (36) and Ioannidou (43) et al. reported no gender differences. These differences might be caused by the different diagnosis of smoking status, criteria or types of depressive systems and sleep disorders, and study population. The gender difference may be explained by the fact that females are more likely to develop mental disorders and are more vulnerable to TSE. Previous studies have suggested that ovarian hormones could affect the hypothalamic-pituitary-adrenal (HPA) axis, dopaminergic, noradrenergic, and gamma-aminobutyric acid (GABA) systems, which are also the targets of nicotine (44, 45). Estrogen could accelerate nicotine metabolism (46). In addition, females are more susceptible to the effects of nicotine on neuro-steroid production due to the menstrual cycle phase and sex hormones (40, 47). These enhance the women’s sensitivity to nicotine’s rewarding and reinforcing effects, making women more vulnerable to TSE. Moreover, several social psychological theories propose explanations for the gender gap in depression (48, 49), demonstrating that females were more vulnerable to nicotine dependence due to their responses to stress and negative mood (40, 50, 51). Taken together, these factors contributed to the increased susceptibility of females to TSE.
Our study had several clear strengths. First, different from the evaluation of smoking patterns in previous studies, serum cotinine concentrations were used as a more objective and accurate biomarker to reflect TSE in this study. Second, to the best of our knowledge, this study was the first to systematically investigate the associations between cotinine levels and depression and sleep disorders in the general US population, providing new epidemiological evidence on the impact of tobacco smoke on mental health. Third, we accounted for complex sampling weights in our analysis, which was conducted using data from four cycles of the NHANES program and involved a large sample size, thereby increasing the robustness of our findings. Finally, the use of smooth curve fitting methods and restricted cubic spline models allowed us to identify non-linear relationships between serum cotinine levels and mental health disorders, providing a deeper understanding of the risks associated with TSE. However, it should be noted that the differences observed in the two non-linear relationship models may be due to variations in the equations used in the gender-stratified models.
Some limitations in our study should be noted. First, NHANES was a cross-sectional survey so that the temporal relationship between exposure and outcome was uncertain. Second, the definitions of depression in our study defined by PHQ-9, and sleep disorders (trouble sleeping and sleep disorders) were verified by questionnaire survey method, which may cause diagnosis bias. It seemed limited to classify sleep disorders as mental health issues, but there were studies that reported sleep disorder as mental health (6, 52). Third, the study population with serum cotinine concentrations above LLOD was from NHANES program and the generalization of our conclusion to other population should be cautious. Lastly, although we accounted for numerous covariates in our model, there remains the possibility that unmeasured variables, such as genetic factors, medication use, and occupational habits, could influence our findings.
5. Conclusion
In this study, we detected nonlinearity relationships between serum cotinine and depression and sleep disorders. The results suggested that TSE was an independent the risk of depression and sleep disorders and there was stronger association in females than males. Our findings provide novel epidemiological evidence about direct and/or secondhand tobacco smoke exposure affects the mental health condition of the general US population, suggesting that prohibiting TSE in public spaces is beneficial to public health. The causal relationship and potential mechanisms of the sex-difference relation between TSE and mental health need to be further investigated.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This work was supported by grants from the Shenzhen Science and Technology Innovation Committee [grant number JCYJ20230807115915033], the Major Technological Project of Shenzhen Nanshan District Health System [grant number NSZD2023024, 2024028], and the Key Project of Shenzhen Nanshan District of Science and Technology [grant number NS2023018], and Sanming Project of Medicine.
Data availability statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.
Ethics statement
The data come from NHANES. No Ethics approval and consent to participate is needed.
Author contributions
HY: Formal analysis, Methodology, Software, Writing – original draft, Writing – review & editing. YL: Formal analysis, Software, Writing – original draft. ZH: Validation, Writing – original draft. GD: Formal analysis, Funding acquisition, Writing – original draft.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fpsyt.2024.1434116/full#supplementary-material
References
- 1. Burki TK. Smoking and mental health. Lancet Respir Med. (2016) 4:437. doi: 10.1016/s2213-2600(16)30109-6 [DOI] [PubMed] [Google Scholar]
- 2. Hodson R. Smoking: an avoidable health disaster explained. Nature. (2023) 618:S2–s3. doi: 10.1038/d41586-023-01837-w [DOI] [PubMed] [Google Scholar]
- 3. Darawshy F, Abu Rmeileh A, Kuint R, Berkman N. Waterpipe smoking: a review of pulmonary and health effects. Eur Respir Rev. (2021) 30. doi: 10.1183/16000617.0374-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bonaldi C, Pasquereau A, Hill C, Thomas D, Moutengou E, Thanh VN, et al. Hospitalizations for cardiovascular diseases attributable to tobacco smoking in France in 2015. Eur J Prev Cardiol. (2021) 28:1327–33. doi: 10.1177/2047487319885462 [DOI] [PubMed] [Google Scholar]
- 5. Gustavson K, Ystrom E, Stoltenberg C, Susser E, Surén P, Magnus P, et al. Smoking in pregnancy and child ADHD. Pediatrics. (2017) 139. doi: 10.1542/peds.2016-2509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. van der Eijk Y, Woh J. Is secondhand smoke associated with mental health issues? A narrative review of the evidence and policy implications. Health Policy. (2023) 136:104900. doi: 10.1016/j.healthpol.2023.104900 [DOI] [PubMed] [Google Scholar]
- 7. World Heath Organization . Newsroom/Fact sheets/Detail/Tobacco (2020). Available online at: https://www.who.int/news-room/fact-sheets/detail/tobacco. (accessed October 15, 2023)
- 8. GBD 2019 Tobacco Collaborators . Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: a systematic analysis from the Global Burden of Disease Study 2019. Lancet. (2021) 397:2337–60. doi: 10.1016/s0140-6736(21)01169-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Meng R, Yu C, Liu N, He M, Lv J, Guo Y, et al. Association of depression with all-cause and cardiovascular disease mortality among adults in China. JAMA Netw Open. (2020) 3:e1921043. doi: 10.1001/jamanetworkopen.2019.21043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Li W, Chen D, Ruan W, Peng Y, Lu Z, Wang D. Associations of depression, sleep disorder with total and cause-specific mortality: A prospective cohort study. J Affect Disord. (2022) 298:134–41. doi: 10.1016/j.jad.2021.10.131 [DOI] [PubMed] [Google Scholar]
- 11. Jaehne A, Unbehaun T, Feige B, Lutz UC, Batra A, Riemann D. How smoking affects sleep: a polysomnographical analysis. Sleep Med. (2012) 13:1286–92. doi: 10.1016/j.sleep.2012.06.026 [DOI] [PubMed] [Google Scholar]
- 12. Huang J, Shi P, Zhao Y, Zhang H, Gao T, Wang X. Associations between smoking, sex steroid hormones, trouble sleeping, and depression among U.S. adults: a cross-sectional study from NHANES (2013-2016). BMC Public Health. (2024) 24:1541. doi: 10.1186/s12889-024-19045-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Huang AA, Huang SY. Use of machine learning to identify risk factors for insomnia. PloS One. (2023) 18:e0282622. doi: 10.1371/journal.pone.0282622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Taylor GM, Lindson N, Farley A, Leinberger-Jabari A, Sawyer K, Te Water Naudé R, et al. Smoking cessation for improving mental health. Cochrane Database Syst Rev. doi: 10.1002/14651858.CD013522.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wootton RE, Richmond RC, Stuijfzand BG, Lawn RB, Sallis HM, Taylor GMJ, et al. Evidence for causal effects of lifetime smoking on risk for depression and schizophrenia: a Mendelian randomisation study. Psychol Med. (2020) 50:2435–43. doi: 10.1017/s0033291719002678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McNamara JP, Wang J, Holiday DB, Warren JY, Paradoa M, Balkhi AM, et al. Sleep disturbances associated with cigarette smoking. Psychol Health Med. (2014) 19:410–9. doi: 10.1080/13548506.2013.832782 [DOI] [PubMed] [Google Scholar]
- 17. Wang L, Heizhati M, Li M, Wang Z, Yang Z, Abudereyimu R, et al. Secondhand smoke is associated with poor sleep quality in self-reported never-smokers of Northwest China: a cross-sectional study. Sleep Breath. (2022) 26:1417–26. doi: 10.1007/s11325-021-02505-x [DOI] [PubMed] [Google Scholar]
- 18. Davila EP, Lee DJ, Fleming LE, LeBlanc WG, Arheart K, Dietz N, et al. Sleep disorders and secondhand smoke exposure in the U.S. population. Nicotine Tob Res. (2010) 12:294–9. doi: 10.1093/ntr/ntp193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jung-Choi KH, Khang YH, Cho HJ. Hidden female smokers in Asia: a comparison of self-reported with cotinine-verified smoking prevalence rates in representative national data from an Asian population. Tob Control. (2012) 21:536–42. doi: 10.1136/tobaccocontrol-2011-050012 [DOI] [PubMed] [Google Scholar]
- 20. Raja M, Garg A, Yadav P, Jha K, Handa S. Diagnostic methods for detection of cotinine level in tobacco users: A review. J Clin Diagn Res. (2016) 10:Ze04–6. doi: 10.7860/jcdr/2016/17360.7423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lei T, Li M, Zhu Z, Yang J, Hu Y, Hua L. Comprehensive evaluation of serum cotinine on human health: Novel evidence for the systemic toxicity of tobacco smoke in the US general population. Sci Total Environ. (2023) 892:164443. doi: 10.1016/j.scitotenv.2023.164443 [DOI] [PubMed] [Google Scholar]
- 22. Kim NH, Jung YS, Hong HP, Park JH, Kim HJ, Park DI, et al. Association between cotinine-verified smoking status and risk of nonalcoholic fatty liver disease. Liver Int. (2018) 38:1487–94. doi: 10.1111/liv.13701 [DOI] [PubMed] [Google Scholar]
- 23. Echeverria V, Zeitlin R, Burgess S, Patel S, Barman A, Thakur G, et al. Cotinine reduces amyloid-β aggregation and improves memory in Alzheimer's disease mice. J Alzheimers Dis. (2011) 24:817–35. doi: 10.3233/jad-2011-102136 [DOI] [PubMed] [Google Scholar]
- 24. Merianos AL, Hossain MM, Khoury JC, Matt GE, Mahabee-Gittens EM. Serum cotinine and hemoglobin A1c among a national sample of adolescents without known diabetes. Nicotine Tob Res. (2018) 20:474–81. doi: 10.1093/ntr/ntx115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Alshaarawy O, Elbaz HA. Serum cotinine levels and diabetes mellitus in never smokers. J Diabetes Complications. (2015) 29:1032–6. doi: 10.1016/j.jdiacomp.2015.08.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. (2001) 16:606–13. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang AA, Huang SY. Exploring depression and nutritional covariates amongst US adults using shapely additive explanations. Health Sci Rep. (2023) 6:e1635. doi: 10.1002/hsr2.1635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tsai J, Homa DM, Gentzke AS, Mahoney M, Sharapova SR, Sosnoff CS, et al. Exposure to secondhand smoke among nonsmokers - United States, 1988-2014. MMWR Morb Mortal Wkly Rep. (2018) 67:1342–6. doi: 10.15585/mmwr.mm6748a3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma Y, Hu Q, Yang D, Zhao Y, Bai J, Mubarik S, et al. Combined exposure to multiple metals on serum uric acid in NHANES under three statistical models. Chemosphere. (2022) 301:134416. doi: 10.1016/j.chemosphere.2022.134416 [DOI] [PubMed] [Google Scholar]
- 30. Dreimüller N, Lieb K, Tadić A, Engelmann J, Wollschläger D, Wagner S. Body mass index (BMI) in major depressive disorder and its effects on depressive symptomatology and antidepressant response. J Affect Disord. (2019) 256:524–31. doi: 10.1016/j.jad.2019.06.067 [DOI] [PubMed] [Google Scholar]
- 31. Tian X, Xue B, Wang B, Lei R, Shan X, Niu J, et al. Physical activity reduces the role of blood cadmium on depression: A cross-sectional analysis with NHANES data. Environ pollut. (2022) 304:119211. doi: 10.1016/j.envpol.2022.119211 [DOI] [PubMed] [Google Scholar]
- 32. Yang H, Liu Y, Huang Z, Deng G. The Healthy Eating Index 2020 components contributed unequally to systemic inflammatory biomarkers: A large national cross-sectional study. Food Sci Nutr. (2024) 12:7212–22. doi: 10.1002/fsn3.4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang K, Zhao Y, Nie J, Xu H, Yu C, Wang S. Higher HEI-2015 score is associated with reduced risk of depression: result from NHANES 2005-2016. Nutrients. (2021) 13. doi: 10.3390/nu13020348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ravindra K, Rattan P, Mor S, Aggarwal AN. Generalized additive models: Building evidence of air pollution, climate change and human health. Environ Int. (2019) 132:104987. doi: 10.1016/j.envint.2019.104987 [DOI] [PubMed] [Google Scholar]
- 35. Dickerson AS, Wu AC, Liew Z, Weisskopf M. A scoping review of non-occupational exposures to environmental pollutants and adult depression, anxiety, and suicide. Curr Environ Health Rep. (2020) 7:256–71. doi: 10.1007/s40572-020-00280-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Mehari A, Weir NA, Gillum RF. Gender and the association of smoking with sleep quantity and quality in American adults. Women Health. (2014) 54:1–14. doi: 10.1080/03630242.2013.858097 [DOI] [PubMed] [Google Scholar]
- 37. Park MB, Kwan Y, Sim B, Lee J. Association between urine cotinine and depressive symptoms in non-smokers: National representative sample in Korea. J Affect Disord. (2021) 294:527–32. doi: 10.1016/j.jad.2021.07.039 [DOI] [PubMed] [Google Scholar]
- 38. Zandy M, Chang V, Rao DP, Do MT. Tobacco smoke exposure and sleep: estimating the association of urinary cotinine with sleep quality. Health Promot Chronic Dis Prev Can. (2020) 40:70–80. doi: 10.24095/hpcdp.40.3.02 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ziedonis D, Hitsman B, Beckham JC, Zvolensky M, Adler LE, Audrain-McGovern J, et al. Tobacco use and cessation in psychiatric disorders: National Institute of Mental Health report. Nicotine Tob Res. (2008) 10:1691–715. doi: 10.1080/14622200802443569 [DOI] [PubMed] [Google Scholar]
- 40. O'Dell LE, Torres OV. A mechanistic hypothesis of the factors that enhance vulnerability to nicotine use in females. Neuropharmacology. (2014) 76 Pt B:566–80. doi: 10.1016/j.neuropharm.2013.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moreno-Coutiño A, Calderón-Ezquerro C, Drucker-Colín R. Long-term changes in sleep and depressive symptoms of smokers in abstinence. Nicotine Tob Res. (2007) 9:389–96. doi: 10.1080/14622200701188901 [DOI] [PubMed] [Google Scholar]
- 42. Han C, Liu Y, Gong X, Ye X, Zhou J. Relationship between secondhand smoke exposure and depressive symptoms: A systematic review and dose⁻Response meta-analysis. Int J Environ Res Public Health. (2019) 16. doi: 10.3390/ijerph16081356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ioannidou D, Kalamaras G, Kotoulas SC, Pataka A. Smoking and obstructive sleep apnea: is there an association between these cardiometabolic risk factors?-gender analysis. Medicina (Kaunas). (2021) 57. doi: 10.3390/medicina57111137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Epperson CN, O'Malley S, Czarkowski KA, Gueorguieva R, Jatlow P, Sanacora G, et al. Sex, GABA, and nicotine: the impact of smoking on cortical GABA levels across the menstrual cycle as measured with proton magnetic resonance spectroscopy. Biol Psychiatry. (2005) 57:44–8. doi: 10.1016/j.biopsych.2004.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Verplaetse TL, Weinberger AH, Smith PH, Cosgrove KP, Mineur YS, Picciotto MR, et al. Targeting the noradrenergic system for gender-sensitive medication development for tobacco dependence. Nicotine Tob Res. (2015) 17:486–95. doi: 10.1093/ntr/ntu280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P, 3rd. Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. (2006) 79:480–8. doi: 10.1016/j.clpt.2006.01.008 [DOI] [PubMed] [Google Scholar]
- 47. Lynch WJ. Sex and ovarian hormones influence vulnerability and motivation for nicotine during adolescence in rats. Pharmacol Biochem Behav. (2009) 94:43–50. doi: 10.1016/j.pbb.2009.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hyde JS, Mezulis AH, Abramson LY. The ABCs of depression: integrating affective, biological, and cognitive models to explain the emergence of the gender difference in depression. Psychol Rev. (2008) 115:291–313. doi: 10.1037/0033-295x.115.2.291 [DOI] [PubMed] [Google Scholar]
- 49. Salk RH, Hyde JS, Abramson LY. Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull. (2017) 143:783–822. doi: 10.1037/bul0000102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Torres OV, O'Dell LE. Stress is a principal factor that promotes tobacco use in females. Prog Neuropsychopharmacol Biol Psychiatry. (2016) 65:260–8. doi: 10.1016/j.pnpbp.2015.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Weinberger AH, McKee SA. Gender differences in smoking following an implicit mood induction. Nicotine Tob Res. (2012) 14:621–5. doi: 10.1093/ntr/ntr198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Lai MC, Kassee C, Besney R, Bonato S, Hull L, Mandy W, et al. Prevalence of co-occurring mental health diagnoses in the autism population: a systematic review and meta-analysis. Lancet Psychiatry. (2019) 6:819–29. doi: 10.1016/s2215-0366(19)30289-5 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes/index.htm.