Abstract
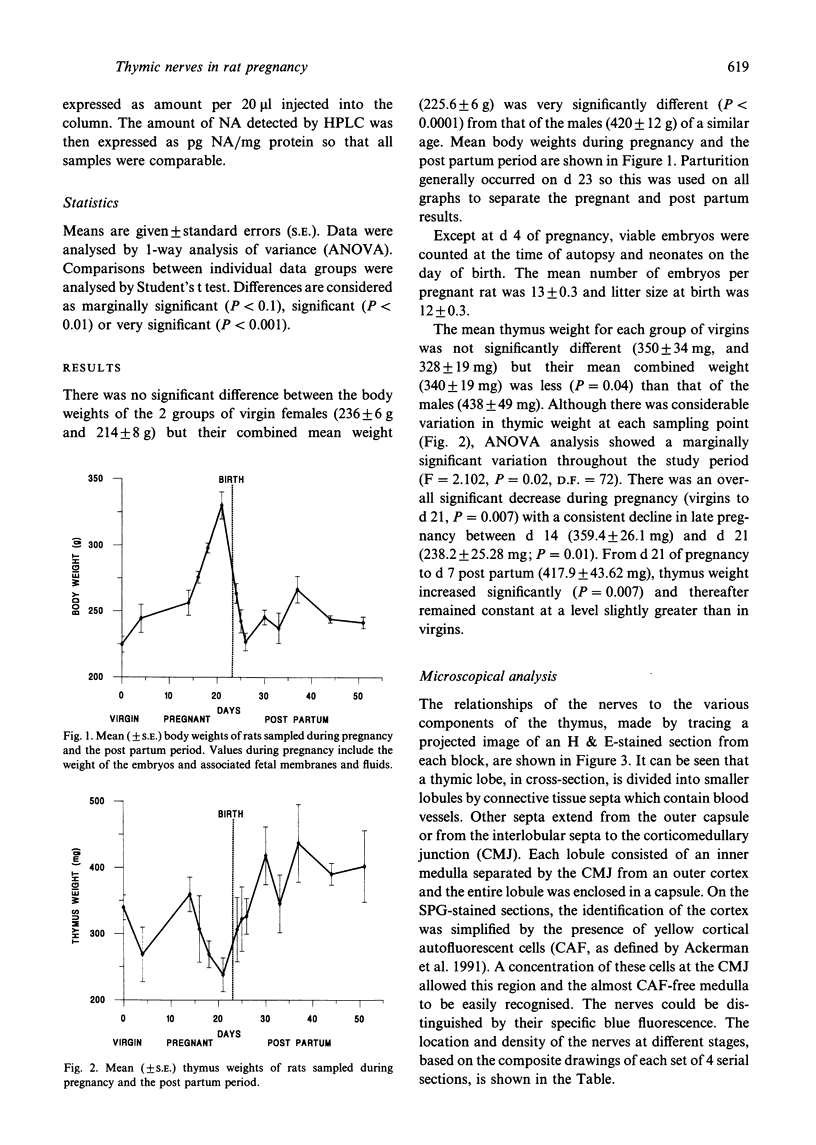
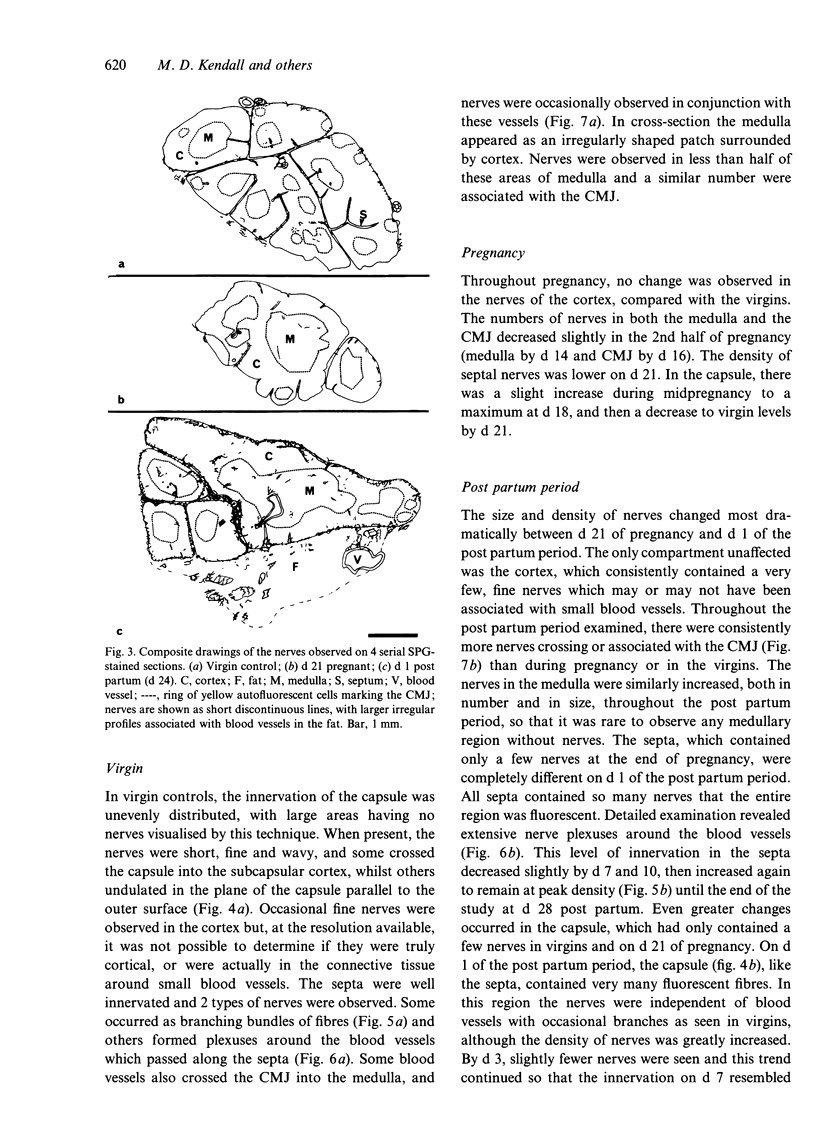
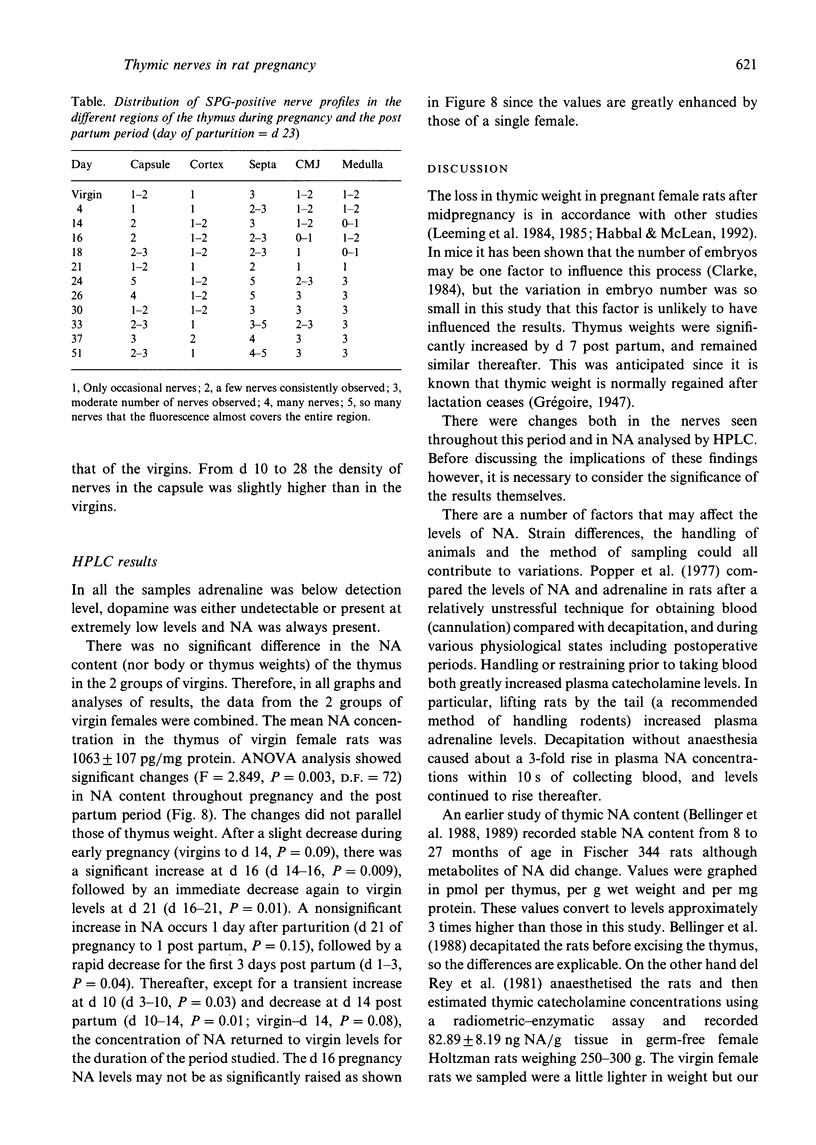
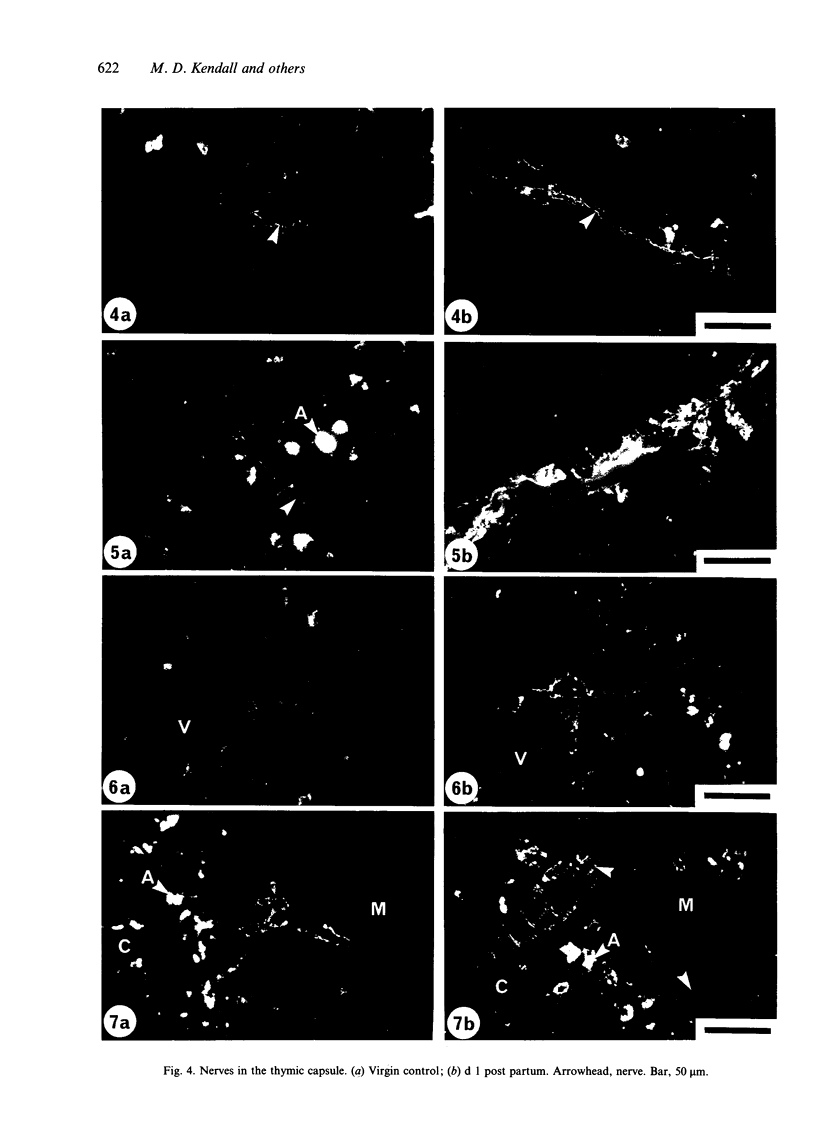
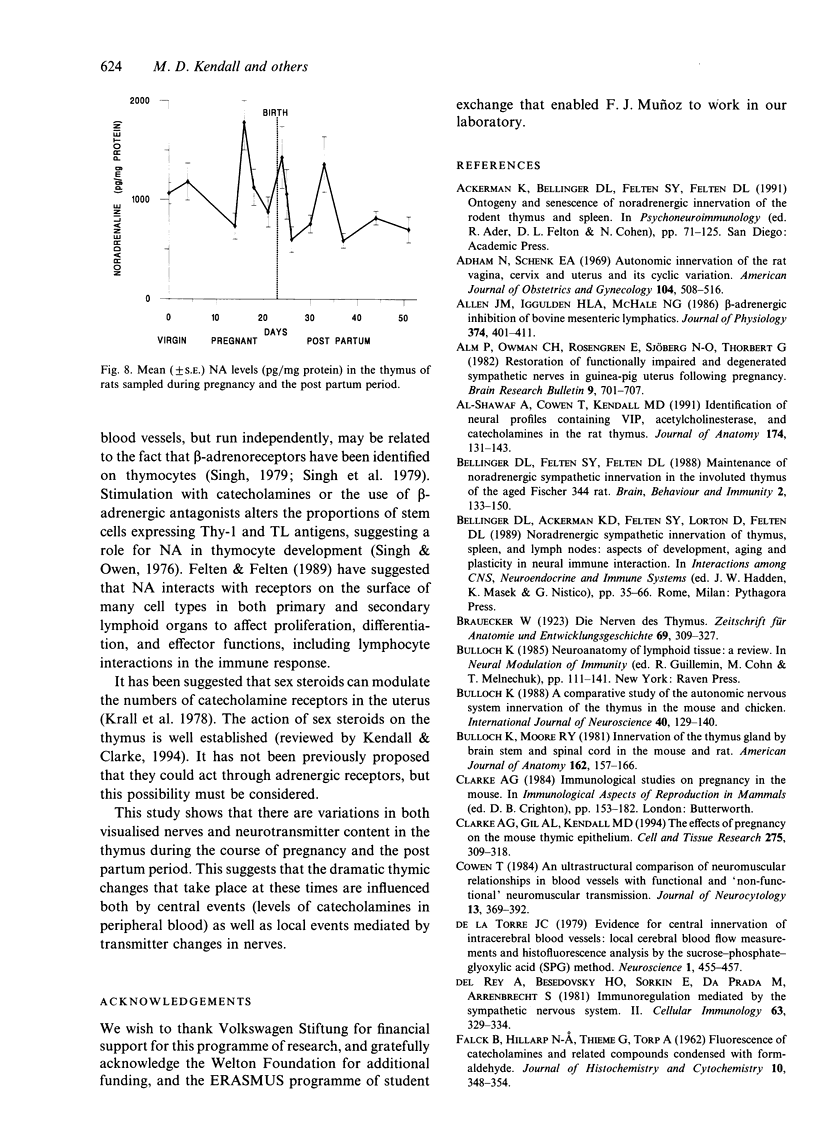
The noradrenergic innervation of the rat thymus during pregnancy and the post partum period was examined by a sucrose glyoxylic acid method for catecholamines, and by high pressure liquid chromatography. Fluorescent nerves decreased in number throughout pregnancy when there was an overall loss in thymic weight due to cortical involution. These changes are maximal by parturition. There was a dramatic increase in nerves between d 21 of pregnancy and d 1 after parturition, especially in the capsule and around blood vessels in the connective tissue septa. The neonates were removed at parturition and thymic weight was rapidly regained. The increased numbers of nerves remained throughout this post partum period. Noradrenaline levels in the thymus altered in a similar pattern throughout pregnancy and the post partum period, but did not parallel thymic weight changes. The mean noradrenaline concentration in the virgin thymus was 1063 +/- 107 pg/mg protein. Levels remained similar during early pregnancy and increased significantly at d 16. Virgin levels were regained by d 21. Values peaked after parturition but rapidly decreased over the next 3 days, and remained at or below virgin levels to d 28 except for a transient rise at d 10 post partum. Adrenaline values were consistently below detection levels. This study shows that there are variations in both nerves visualised, and in neurotransmitter (noradrenaline) content in the thymus during the course of pregnancy and the post partum period. Thus thymic function could be influenced by central events (levels of catecholamines in peripheral blood) as well as local events mediated by transmitter changes in nerves.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adham N., Schenk E. A. Autonomic innervation of the rat vagina, cervix, and uterus and its cyclic cariation. Am J Obstet Gynecol. 1969 Jun 15;104(4):508–516. doi: 10.1016/s0002-9378(16)34239-9. [DOI] [PubMed] [Google Scholar]
- Allen J. M., Iggulden H. L., McHale N. G. Beta-adrenergic inhibition of bovine mesenteric lymphatics. J Physiol. 1986 May;374:401–411. doi: 10.1113/jphysiol.1986.sp016087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alm P., Owman C., Rosengren E., Sjöberg N. O., Thorbert G. Restoration of functionally impaired and degenerated sympathetic nerves in guinea-pig uterus following pregnancy. Brain Res Bull. 1982 Jul-Dec;9(1-6):701–707. doi: 10.1016/0361-9230(82)90175-7. [DOI] [PubMed] [Google Scholar]
- Bellinger D. L., Felten S. Y., Felten D. L. Maintenance of noradrenergic sympathetic innervation in the involuted thymus of the aged Fischer 344 rat. Brain Behav Immun. 1988 Jun;2(2):133–150. doi: 10.1016/0889-1591(88)90014-1. [DOI] [PubMed] [Google Scholar]
- Bulloch K. A comparative study of the autonomic nervous system innervation of the thymus in the mouse and chicken. Int J Neurosci. 1988 May;40(1-2):129–140. doi: 10.3109/00207458808985734. [DOI] [PubMed] [Google Scholar]
- Bulloch K., Moore R. Y. Innervation of the thymus gland by brain stem and spinal cord in mouse and rat. Am J Anat. 1981 Oct;162(2):157–166. doi: 10.1002/aja.1001620207. [DOI] [PubMed] [Google Scholar]
- Clarke A. G., Gil A. L., Kendall M. D. The effects of pregnancy on the mouse thymic epithelium. Cell Tissue Res. 1994 Feb;275(2):309–318. doi: 10.1007/BF00319429. [DOI] [PubMed] [Google Scholar]
- Cowen T. An ultrastructural comparison of neuromuscular relationships in blood vessels with functional and 'non-functional' neuromuscular transmission. J Neurocytol. 1984 Jun;13(3):369–392. doi: 10.1007/BF01148329. [DOI] [PubMed] [Google Scholar]
- Habbal O., McLean J. Lymphoid tissue changes during early syngeneic pregnancy in the RT1u rat. Acta Anat (Basel) 1992;144(4):363–370. doi: 10.1159/000147331. [DOI] [PubMed] [Google Scholar]
- Kendall M. D. Functional anatomy of the thymic microenvironment. J Anat. 1991 Aug;177:1–29. [PMC free article] [PubMed] [Google Scholar]
- Kendall M. D., al-Shawaf A. A. Innervation of the rat thymus gland. Brain Behav Immun. 1991 Mar;5(1):9–28. doi: 10.1016/0889-1591(91)90004-t. [DOI] [PubMed] [Google Scholar]
- Krall J. F., Mori H., Tuck M. L., LeShon S. L., Korenman S. G. Demonstration of adrenergic catecholamine receptors in rat myometrium and their regulation by sex steroid hormones. Life Sci. 1978 Sep 11;23(10):1073–1081. doi: 10.1016/0024-3205(78)90669-0. [DOI] [PubMed] [Google Scholar]
- Lederman R. P., McCann D. S., Work B., Jr, Huber M. J. Endogenous plasma epinephrine and norepinephrine in last-trimester pregnancy and labor. Am J Obstet Gynecol. 1977 Sep 1;129(1):5–8. doi: 10.1016/0002-9378(77)90809-2. [DOI] [PubMed] [Google Scholar]
- Leeming G., McLean J. M., Gibbs A. C. The cell content and proliferative response of the rat thymus during first syngeneic and allogeneic pregnancy, and the effects of strain difference. Thymus. 1985;7(4):247–255. [PubMed] [Google Scholar]
- Leeming G., McLean J., Gibbs A. C. Thymic and body weight during first syngeneic and allogeneic pregnancy in the rat, and the effects of strain difference. Thymus. 1984;6(3):153–165. [PubMed] [Google Scholar]
- Lindvall O., Björklund A. The glyoxylic acid fluorescence histochemical method: a detailed account of the methodology for the visualization of central catecholamine neurons. Histochemistry. 1974 Apr 22;39(2):97–127. doi: 10.1007/BF00492041. [DOI] [PubMed] [Google Scholar]
- Livnat S., Felten S. Y., Carlson S. L., Bellinger D. L., Felten D. L. Involvement of peripheral and central catecholamine systems in neural-immune interactions. J Neuroimmunol. 1985 Nov;10(1):5–30. doi: 10.1016/0165-5728(85)90031-1. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Melo R. C., Machado C. R. Noradrenergic and acetylcholinesterase-positive nerve fibres of the uterus in sexually immature and cycling rats. Histochem J. 1993 Mar;25(3):213–218. doi: 10.1007/BF00163817. [DOI] [PubMed] [Google Scholar]
- Owman C., Alm P., Rosengren E., Sjöberg N., Thorbert G. Variations in the level of uterine norepinephrine during pregnancy in the guinea pig. Am J Obstet Gynecol. 1975 Aug 15;122(8):961–964. doi: 10.1016/0002-9378(75)90356-7. [DOI] [PubMed] [Google Scholar]
- Popper C. W., Chiueh C. C., Kopin I. J. Plasma catecholamine concentrations in unanesthetized rats during sleep, wakefulness, immobilization and after decapitation. J Pharmacol Exp Ther. 1977 Jul;202(1):144–148. [PubMed] [Google Scholar]
- Rosengren E., Sjöberg N. O. Changes in the amount of adrenergic transmitter in the female genital tract of rabbit during pregnancy. Acta Physiol Scand. 1968 Apr;72(4):412–424. doi: 10.1111/j.1748-1716.1968.tb03866.x. [DOI] [PubMed] [Google Scholar]
- Singh U. Effect of catecholamines on lymphopoiesis in fetal mouse thymic explants. J Anat. 1979 Sep;129(Pt 2):279–292. [PMC free article] [PubMed] [Google Scholar]
- Singh U., Millson D. S., Smith P. A., Owen J. J. Identification of beta adrenoreceptors during thymocyte ontogeny in mice. Eur J Immunol. 1979 Jan;9(1):31–35. doi: 10.1002/eji.1830090108. [DOI] [PubMed] [Google Scholar]
- Singh U., Owen J. J. Studies on the maturation of thymus stem cells. The effects of catecholamines, histamine and peptide hormones on the expression of T cell alloantigens. Eur J Immunol. 1976 Jan;6(1):59–62. doi: 10.1002/eji.1830060113. [DOI] [PubMed] [Google Scholar]
- Sporrong B., Alm P., Owman C., Sjöberg N. O., Thorbert G. Pregnancy is associated with extensive adrenergic nerve degeneration in the uterus. An electronmicroscopic study in the guinea-pig. Neuroscience. 1981;6(6):1119–1126. doi: 10.1016/0306-4522(81)90076-2. [DOI] [PubMed] [Google Scholar]
- Webber R. H., DeFelice R., Ferguson R. J., Powell J. P. Bone marrow response to stimulation of the sympathetic trunks in rats. Acta Anat (Basel) 1970;77(1):92–97. doi: 10.1159/000143531. [DOI] [PubMed] [Google Scholar]
- al-Shawaf A. A., Kendall M. D., Cowen T. Identification of neural profiles containing vasoactive intestinal polypeptide, acetylcholinesterase and catecholamines in the rat thymus. J Anat. 1991 Feb;174:131–143. [PMC free article] [PubMed] [Google Scholar]
- de la Torre J. C. Evidence for central innervation of intracerebral blood vessels: local cerebral blood flow measurements and histofluorescence analysis by the sucrose-phosphate-glyoxylic acid (SPG) method. Neuroscience. 1976 Dec;1(6):455–457. doi: 10.1016/0306-4522(76)90096-8. [DOI] [PubMed] [Google Scholar]
- del Rey A., Besedovsky H. O., Sorkin E., da Prada M., Arrenbrecht S. Immunoregulation mediated by the sympathetic nervous system, II. Cell Immunol. 1981 Sep 15;63(2):329–334. doi: 10.1016/0008-8749(81)90012-5. [DOI] [PubMed] [Google Scholar]






