Summary
Two-pore domain, outwardly rectifying potassium (TOK) channels are exclusively expressed in fungi. Human fungal pathogen TOK channels are potential antifungal targets, but TOK channel modulation in general is poorly understood. Here, we discovered that Candida albicans TOK (CaTOK) is regulated by extracellular pH, in contrast to TOK channels from other fungal species tested. Low pH increased CaTOK channel outward currents (pKa = 6.0), hyperpolarized the voltage-dependence of TOK activation, and increased pore selectivity for K+ over Na+, shifting the reversal potential (EREV) toward EK. Mutating H144 in the S1-S2 extracellular linker partially diminished pH sensitivity, suggesting H144 forms part of the CaTOK pH sensor. Functional analysis of chimeras made with pH-insensitive Saccharomyces cerevisiae TOK and point mutants revealed that CaTOK V462 and S466 in the final transmembrane segment complete the pH-responsive elements. A tripartite network of residues thus endows CaTOK with the ability to respond functionally to changes in pH.
Subject areas: Natural sciences, Biological sciences, Microbiology, Microbial physiology
Graphical abstract
Highlights
-
•
The Candida albicans TOK (CaTOK) K+ channel senses extracellular pH
-
•
Lowering extracellular pH augments CaTOK outward current and K+ selectivity
-
•
A tripartite network of residues comprises the complete CaTOK pH sensor
-
•
CaTOK may help facilitate pH-dependent hyphal formation, a key virulence trait
Natural sciences; Biological sciences; Microbiology; Microbial physiology
Introduction
First described in the baker’s yeast Saccharomyces cerevisiae,1 the two-pore domain, outwardly rectifying potassium (TOK) channel comprises eight membrane spanning domains and two pore loops (Figure 1A). Interestingly, despite being a tandem-pore potassium channel, TOK is evolutionarily distinct from two-pore domain potassium (K2P) channels in humans (Figure 1B), and shares no homologs in animals, plants, or insects. TOK channels have been cloned and characterized from several fungi, including the filamentous root fungus Neurospora crassa (NcTOK) and human fungal pathogens Candida albicans (CaTOK), Aspergillus fumigatus (AfTOK), and Cryptococcus neoformans var. neoformans (CnTOK).2,3 A recent systematic review estimated that ∼6.5 million people globally are affected by fungal infections, including 2.1 million with invasive Aspergillus, 1.8 million with chronic pulmonary aspergillosis, 1.5 million with candidemia, and 194,000 with cryptococcal meningitis.4 Together, fungal infections contribute to more than 3.75 million deaths per year, with 2.5 million being directly attributable to the infection.4 TOK channels have been posited to have potential as antimicrobial targets, with several studies proposing their activation by small molecules or peptides as a possible molecular switch for instigating programmed cell death and apoptosis.5,6,7,8,9,10 However, little is known about pharmacological and environmental factors that modulate TOK channels from human fungal pathogens. Previously, S. cerevisiae TOK (ScTOK) was shown to be insensitive to changes in extracellular pH, a trait postulated to have evolved to facilitate regulation of intracellular homeostasis of electrogenic ions despite inhabiting environments with different and changing pH levels.11,12 Whether insensitivity to extracellular pH is a trait shared by other TOK channels, notably those cloned from human fungal pathogens, has remained undetermined. Here, we report that CaTOK channel function is highly sensitive to pH and is CaTOK-specific among the species tested. Extracellular acidification potentiates CaTOK channel currents, induces a negative-shift in the voltage dependence of activation, and changes the ion selectivity of the conduction pore, hyperpolarizing the resting membrane potential. Using chimeras and site-directed mutagenesis, we reveal that the molecular mechanism underpinning CaTOK channel sensitivity to external pH is a sensor comprised of a residue in the extracellular S1-S2 linker and S8 residues contributing to the second pore domain.
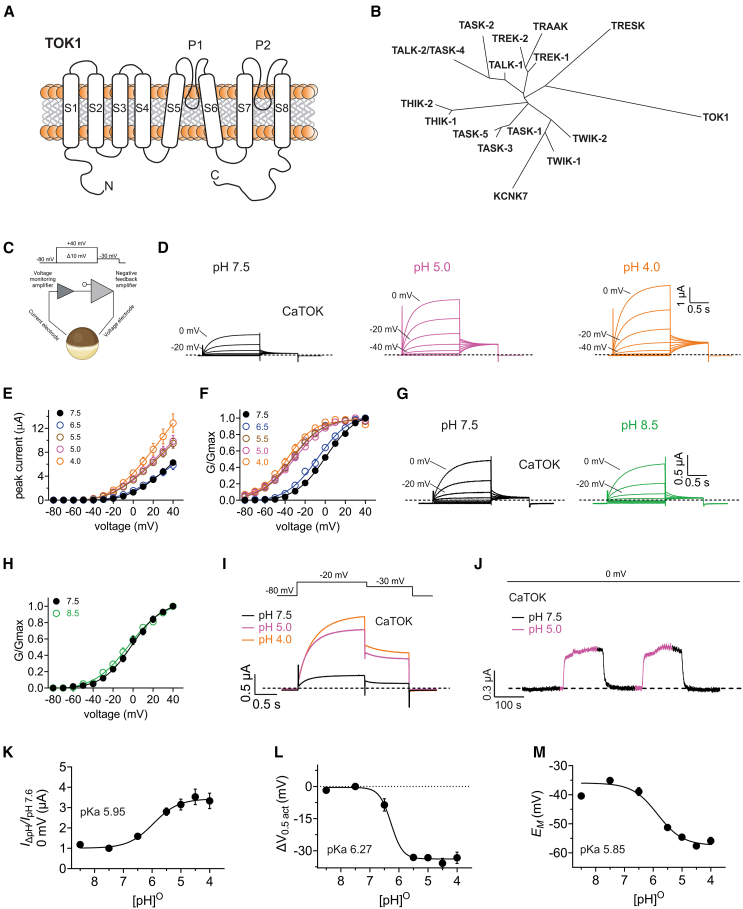
Figure 1.
Extracellular acidification significantly potentiates CaTOK channel currents and induces a hyperpolarizing shift in the voltage-dependence of activation
Data are represented as mean ± SEM.
(A) Cartoon depicting the topology of fungal TOK channel with eight pore spanning transmembrane domains (S1-S8) and two pore loops (P1 and P2).
(B) Dendrogram depicting the evolutionary relationship between human tandem-pore domain potassium (K2P) channels and TOK1.
(C) Schematic of two electrode voltage-clamp setup and the Xenopus oocyte expression system used to measure TOK channel biophysical properties. Upper inset: voltage protocol used to generate current-voltage relationships. Created with BioRender.com.
(D) Averaged traces for CaTOK channels as indicated, expressed in oocytes in the presence of pH 7.5, pH 5.0, and pH 4.0. Scale bar upper right; n = 8–26 per group.
(E) Mean peak current (measured during prepulse) from traces as in D; n = 8–26 per group.
(F) Mean normalized tail current (G/Gmax) for traces as in D; n = 8–26 per group.
(G) Averaged traces for CaTOK channels as indicated, expressed in oocytes in the presence of pH 7.5 or pH 8.5. Scale bar upper right; n = 9 per group.
(H) Mean normalized tail current (G/Gmax) for traces as in G; n = 9 per group.
(I) Averaged traces for CaTOK channel current increase at −20 mV as indicated, in the presence of pH 7.5, pH 5.0, and pH 4.0. Voltage protocol inset; n = 8–26 per group.
(J) Exemplar trace showing wash-in pH 5.0 (magenta) and washout pH 7.5 (black) at 0 mV on CaTOK channels expressed in oocytes (n = 6).
(K) Mean current increase versus (pH)O at 0 mV for oocytes expressing CaTOK; n = 8–26 per group.
(L) Mean V0.5act versus (pH)O for oocytes expressing CaTOK; n = 8–26 per group.
(M) Mean EM versus (pH)O for oocytes expressing CaTOK; n = 8–26 per group.
Results
CaTOK channel activity is stimulated by extracellular acidification
From a starting point of pH 7.5 (standard physiological buffer), CaTOK outward currents were significantly increased by lowering the bath pH < 6.5, with a 2-fold increase observed at +40 mV at pH 4.0 (Figures 1D and 1E). Concurrently, CaTOK currents were also significantly increased at negative membrane potentials in response to increasingly acidic pH (Figures 1D, 1E, S1A and S1B), shifting the midpoint voltage dependence of CaTOK channel activation (V0.5act) by −30.25 mV at pH 5.0 (from −1.81 ± 1.55 mV at pH 7.5 to −32.33 ± 1.33 mV at pH 5.0) (Figure 1F).
In contrast, shifting from pH 7.5 to a more alkaline pH (pH 8.5) did not alter CaTOK activity or V0.5act (Figures 1G and 1H). Figure 1I shows an example of the pH dependence of CaTOK currents at a single voltage (−20 mV) for direct comparison. Wash-in and washout experiments at 0 mV revealed immediate, robust potentiation of CaTOK channel outward currents upon wash-in, immediately reversible upon commencing washout (Figure 1J).
We next sought to establish the pKa of the effect, by measuring CaTOK channel current at 0 mV in a range of extracellular pH values. There was a 3-fold difference in peak current between pH 4.0 and pH 8.5. The resulting pH-response curve revealed a pKa of 6.0 ± 0.2 (Figure 1K). Likewise, fitting the V0.5act as a function of pH gave a pKa of 6.2 ± 0.1 (Figure 1I), while the pKa for EM of unclamped oocytes expressing CaTOK was 5.8 ± 0.1 (Figure 1M). The pKa value of ∼6 and the rapid onset and reversal of the effects of extracellular acidification suggested an extracellularly exposed imidazole side chain of histidine (pKa ∼6) as the prime candidate for pH sensing in CaTOK channels.
Histidine protonation in the S1-S2 linker forms part of the CaTOK pH sensor
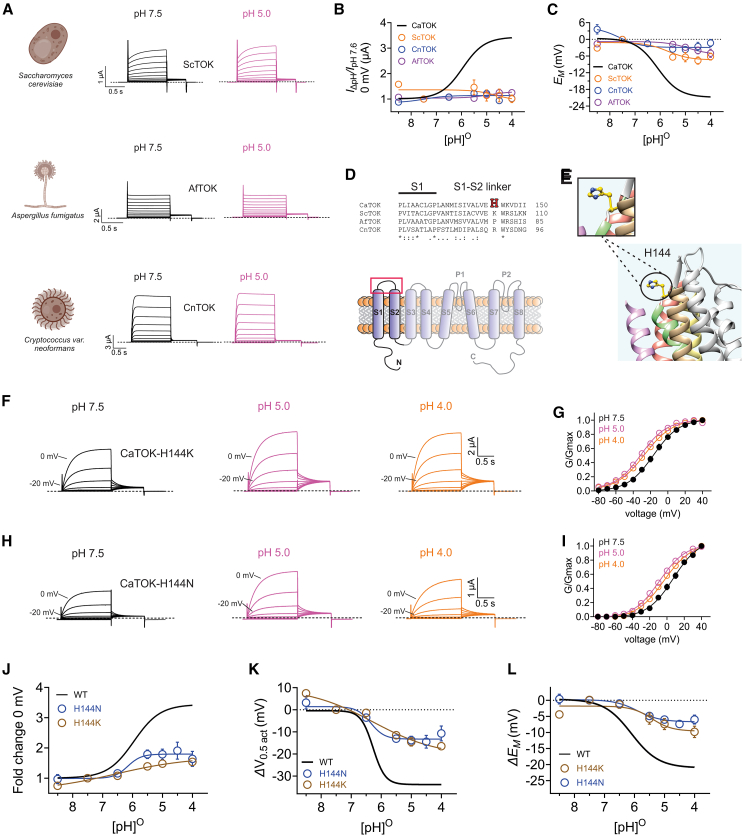
Next, we sought to determine the pH sensitivity of TOK channels cloned from S. cerevisiae (ScTOK), A. fumigatus (AfTOK), and C. neoformans var neoformans (CnTOK) (Figure 2A). Interestingly, ScTOK, CnTOK, and AfTOK exhibited limited pH sensitivity, with no pH-dependent change in current density observed across all pH ranges (Figure 2B: upper; Figures S2A–S2F) and almost no pH-dependent shift in the EM of unclamped oocytes, except for AfTOK, which exhibited a modest −5 mV shift at pH 4.0 (Figure 2C). Sequence alignments revealed a histidine (H144) located in the predicted S1-S2 extracellular linker of CaTOK that was absent from the other TOK channels (Figure 2D). To provide a better insight into the environment of the histidine residue, we used AlphaFold and UCSF Chimera13,14,15 to predict and visualize the structure of CaTOK because no high-resolution structure is currently available for any of the TOK channels (Figure S3). The H144 sidechain is predicted to be exposed to the external medium on the extracellular face of the S1-S2 linker of CaTOK (Figure 2E). Its extracellular exposure, absence in the pH-insensitive TOK channels, and a pKa matching that of the pH response-curve for CaTOK (∼6) strongly suggested H144 as the pH sensor. To confirm this, we performed site-directed mutagenesis mutating H144 to either a lysine (H144K), which is constitutively protonated at physiological pH, or to an asparagine (H144N), which is neutral at physiological pH. Baseline analysis of CaTOK-H144K compared to wild type revealed an almost 2-fold increase in current density at +40 mV from 5.6 ± 0. 6 μA to 9.9 ± 1.1 μA. Furthermore, H144K exhibited a −10.2 ± 1.7 mV left shift in the V0.5act at pH 7.5 compared to wild-type CaTOK. The H144N mutation, in contrast, had no effect on either current density or the V0.5act (Figures S4A and S4B). CaTOK-H144K exhibited only modest pH sensitivity compared to wild type, with attenuated increases in outward currents at pH 5.0 and pH 4.0. Additionally, the shift in V0.5act for H144K was reduced by almost half, with a hyperpolarizing shift of −15.7 ± 1.3 mV at pH 5.0 and −16.9 ± 1.4 mV at pH 4.0 (Figures 2F and 2G; Figures S4C–S4E). Similarly, H144N exhibited reduced sensitivity to low extracellular pH compared to wild-type CaTOK, with only minimal increases in current and a −11.1 mV ± 1.3 mV shift in the V0.5act at pH 4.0 (Figures 2H and 2I; Figures S4F–S4H). H144K and H144N mutations each blunted the pH-dependent increase in current at 0 mV, reducing the nearly 3.5-fold increase at pH 5.0 compared to pH 7.5 observed for wild type CaTOK to a <2-fold increase (Figure 2J). Each mutation also attenuated the CaTOK ΔV0.5act (Figure 2K) and the ΔEM of unclamped oocytes in response to lowered extracellular pH (Figure 2L). Interestingly, despite significantly blunting CaTOK response to extracellular acidification, mutating H144 failed to completely abolish the effect on current density and V0.5act, suggesting that this residue may form only part of a more intricate pH sensing mechanism. To test this hypothesis, we introduced a histidine at the homologous positions in ScTOK, AfTOK, and CnTOK. As expected, introduction of a histidine failed to endow pH sensitivity, with no pH-dependent augmentation in current magnitude or shift in the ΔEM of unclamped oocytes observed in across pH 7.5–5.0 (Figures S5A–S5D).
Figure 2.
H144 in the CaTOK S1-S2 linker contributes to pH-dependent current augmentation and voltage-dependent activation
Data are represented as mean ± SEM.
(A) Cartoons depicting Saccharomyces cerevisiae cell (Top), Aspergillus fumigatus (middle), and Cryptococcus neoformans (bottom). Averaged traces for ScTOK (Top), AfTOK (Middle), and CnTOK (Bottom) channels as indicated, expressed in oocytes in the presence of pH 7.5 or pH 5.0. Scale bars lower left for each trace; n = 5–11 per group. Created using Biorender.com.
(B) Mean current fold increase versus (pH)O at 0 mV for ScTOK (magenta), AfTOK (green), and CnTOK (orange), with CaTOK data from Figure 1 for comparison; n = 5–11 per group.
(C) Mean ΔEM versus (pH)O for oocytes expressing ScTOK (magenta), AfTOK (green), and CnTOK (orange), with CaTOK data from Figure 1 for comparison; n = 5–11 per group.
(D) Top: Sequence alignment of CaTOK, ScTOK, AfTOK, and CnTOK partial S1 and S1-S2 linker with histidine colored (red). ∗Residues are identical at this position in all sequences in the alignment; “:”, conserved substitutions at this position in all sequences in the alignment; “.”, semi-conserved substitutions at this position in all sequences in the alignment. Bottom: Red box highlighting the location of the histidine on the S1-S2 linker of CaTOK channel.
(E) Alpha-Fold predicted structure of CaTOK showing the predicted location of H144 (yellow).
(F) Averaged traces for CaTOK-H144K channels as indicated, expressed in oocytes in the presence of pH 7.5, pH 5.0, and pH 4.0. Scale bar upper right; n = 6–10 per group.
(G) Mean normalized tail current (G/Gmax) for traces as in F; n = 6–10 per group.
(H) Averaged traces for CaTOK-H144N channels as indicated, expressed in oocytes in the presence of pH 7.5, pH 5.0, and pH 4.0. Scale bar upper right; n = 8–16 per group.
(I) Mean normalized tail current (G/Gmax) for traces as in H; n = 8–16 per group.
(J) Mean current fold increase versus (pH)O at 0 mV for CaTOK-H144K (orange) and CaTOK-H144N (blue), with CaTOK data from Figure 1 for comparison; n = 6–16 per group.
(K) Mean ΔV0.5act versus (pH)O for oocytes expressing for CaTOK-H144K (orange) and CaTOK-H144N (blue), with CaTOK data from Figure 1 for comparison; n = 6–16 per group.
(L) Mean ΔEM versus (pH)O for oocytes expressing for CaTOK-H144K (orange) and CaTOK-H144N (blue), with CaTOK data from Figure 1 for comparison; n = 6–16 per group.
Extracellular acidification makes CaTOK more selective for potassium
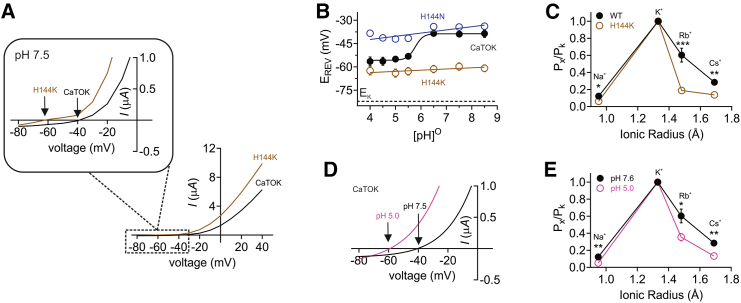
Previously, we showed that CaTOK is less selective for K+ over Na+ than ScTOK, correlating with a more depolarized EREV.3 In this context, it was notable that the reversal potential of CaTOK-H144K was more hyperpolarized than for wild-type CaTOK (∼−65 mV, compared to ∼ −40 mV for CaTOK) (Figure 3A). By plotting the reversal potential (EREV) as a function of pH, we found that CaTOK selectivity for K+ increases as extracellular pH is lowered toward pH 5.0, pushing the EREV closer to EK, as predicted by the Nernst equation (Figure 3B). Similarly, CaTOK-H144K EREV stays constant at ∼ −65 mV across the pH range, closely mimicking the effect seen for CaTOK at pH 5.0. In contrast, CaTOK-H144N current EREV remains constant at ∼ −40 mV across the pH range, similar to CaTOK at pH 7.5 (Figure 3B). One possible explanation for this observed shift in EREV is that acidic pH is causing conformational changes in the pore, near or at the selectivity filter, altering the ion selectivity of CaTOK. Thus, we conducted pseudo-bi-ionic substitution with monovalent cations to estimate the permeability ratios for CaTOK and CaTOK-H144K at pH 7.5 using a modification of the GHK voltage equation (Equation 2). For wild-type CaTOK, our data replicate our previous findings for all monovalent ions tested (Figure 3C).3 CaTOK-H144K altered ion selectivity, making the channel more selective for K+, substantially decreasing relative Na+, Rb+, and Cs+ permeation, explaining the hyperpolarizing shift observed for the EREV (Figure 3C). As CaTOK-H144K mimics the protonated state of a histidine at acidic pH, we next sought to confirm whether a similar effect on ion permeability and EREV was recapitulated by wild-type CaTOK at pH 5.0. Accordingly, switching to pH 5.0 resulted in a hyperpolarizing shift in the EREV of wild-type CaTOK to ∼ −60 mV (Figure 3D) and decreased the permeability of Na+, Rb+, and Cs+, as observed for CaTOK-H144K (Figure 3E).
Figure 3.
Extracellular protonation increases potassium selectivity via modulation of the pore
Data are represented as mean ± SEM.
(A) Mean peak current (measured during prepulse) for wild type CaTOK (black) and H144K (brown) in the presence of pH 7.5, upper right quadrant shows the reversal potential for each channel as indicated by the arrow; n = 10–26 per group.
(B) Extracellular PH (pHO) versus reversal potential (EREV) for wild-type and H144 mutant CaTOK channels as indicated, expressed in oocytes. Calculated from traces as in Figure 2; n = 6–26 per group.
(C) Relative ion permeabilities of CaTOK and H144K channels in the presence of pH 7.5 for rubidium (p = 0.0005), sodium (p = 0.0190), and cesium (p = 0.0046); n = 10. Statistical analysis by One-way ANOVA.
(D) Mean peak current (measured during prepulse) for CaTOK in the presence of pH 7.5 (black) and pH 5.0 (pink), the reversal potential of CaTOK in pH 7.5 and pH 5.0 is indicated by the arrow; n = 22–26 per group.
(E) Relative ion permeabilities of CaTOK in the presence of pH 7.5 (black) and pH 5.0 (pink) for rubidium (p = 0.0172), sodium (p = 0.0066), and cesium (p = 0.0014); n = 10. Statistical analysis by One-way ANOVA.
The second pore domain of CaTOK coordinates a gating mechanism sensitive to extracellular acidification
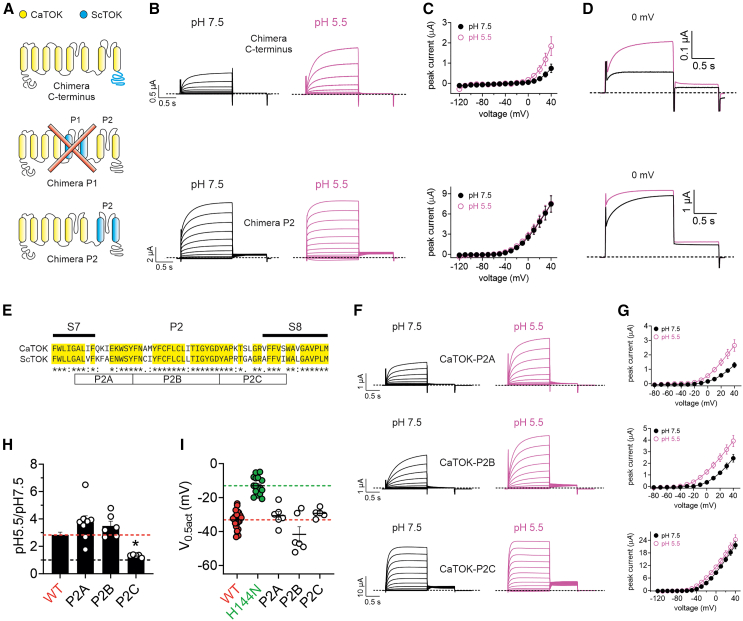
To investigate the role of the pore domain in CaTOK modulation by extracellular pH, we first generated three chimeras in which we substituted the first pore (P1), second pore (P2), or the C-terminal domains of CaTOK with the equivalent domains of pH insensitive ScTOK (Figures 4A and 4B). Chimera P1 failed to produce discernable currents and therefore could not be studied (data not shown). The C-terminal chimera exhibited reduced current density compared to wild type and failed to produce measurable tail currents (Figures 4C and S6A) but it could be studied to demonstrate that the substitution did not alter sensitivity to pH, with a shift to pH 5.5 increasing currents 5.5-fold at 0 mV (Figures 4C and 4D, upper).
Figure 4.
Residues in CaTOK S8 are required for current augmentation, but not hyperpolarization of V0.5act, by acidic pH
Data are represented as mean ± SEM.
(A) Cartoons depicting the topology of CaTOK (yellow) and ScTOK (blue) chimeras. Top: C-terminal chimera; Bottom: P2 chimera. Averaged traces for CaTOK-ScTOK chimeric channels as indicated, expressed in oocytes in the presence of pH 7.5 and pH 5.5. Scale bar lower left; n = 8–10 per group.
(B) Mean peak current (measured during prepulse) from traces as in A; n = 8–10 per group.
(C) Mean ΔEM of unclamped oocytes as in A; n = 8–10 per group.
(D) Averaged traces at 0 mV as indicated, expressed in oocytes in the presence of pH 7.5 and pH 5.5; n = 8–10 per group.
(E) Sequence alignment of CaTOK versus ScTOK S7-P2-S8 region. Boxes P2A, P2B, and P2C indicate clusters of divergent sequences. ∗Residues are identical at this position in all sequences in the alignment; “:”, conserved substitutions at this position in all sequences in the alignment; “.”, semi-conserved substitutions at this position in all sequences in the alignment.
(F) Averaged traces for P2A, P2B, and P2C mutant channels as indicated, expressed in oocytes in the presence of pH 7.5 and pH 5.5. Scale bar lower left; n = 5–8 per group.
(G) Mean peak current (measured during prepulse) from traces as in F; n = 5–8 per group.
(H) Fold increase at 0 mV as in G. Black dashed line indicates no change; Red dashed line indicates current-fold increase for wild type CaTOK; n = 5–26 per group. Statistical significance for relative current for P2C was ∗p = 0.0156 by One-way ANOVA.
(I) Mean ΔV0.5act for wild-type and mutant CaTOK channels. Green dashed line indicates the mean ΔV0.5act for CaTOK-H144N; Red dashed line indicates mean ΔV0.5act for wild-type CaTOK; n = 5–26 per group.
Chimera P2 produced robust outward currents comparable in magnitude to those observed for wild type CaTOK (Figures 4B and S6A). Strikingly, the V0.5act for chimera P2 was −75 mV, representing a −72 mV hyperpolarizing shift compared to wild-type CaTOK (Figure S6B). This substitution significantly weakened the response to extracellular acidification (Fig. 4CD, lower). Subsequent alignments of the P2 domain of CaTOK and ScTOK revealed three clusters containing divergent residues. Using site-directed mutagenesis, residues in CaTOK were substituted with the reciprocal residues in ScTOK, to generate three further chimeric constructs, which we designated P2A-C (Figure 4E). Mutants P2A and P2B exhibited reduced basal current density compared to wild-type CaTOK but had similar V0.5act (Figures 4F, 4G, S6A, S6C, and S6D). Mutant P2C increased basal currents 4-fold and significantly hyperpolarized the V0.5act (−47.9 ± 1.6 mV) (Figures 4F, 4G, S6A, and S6E). Both P2A and P2B exhibited similar pH sensitivity to wild type, with pH 5.5 increasing current density 3.8-fold and 3.5-fold at 0 mV, respectively. Strikingly, mutant P2C almost completely blunted the effect of pH 5.5 on current augmentation, to 1.3-fold at 0 mV, recapitulating the blunted pH effect observed with chimera P2, and effects on V0.5act (Figures 4H and 4I). Interestingly, despite chimera P2 and mutant P2C significantly impairing the effect of acidic pH on current potentiation, neither mutant suppressed the ability of pH 5.5 to hyperpolarize the V0.5act, with both mutants displaying similar ∼ −33 mV shifts as seen with wild-type CaTOK (Figures 4I and S6F–S6I).
A crosstalk mechanism between residues H144, V462, and S466 underpins the molecular basis for CaTOK pH sensitivity
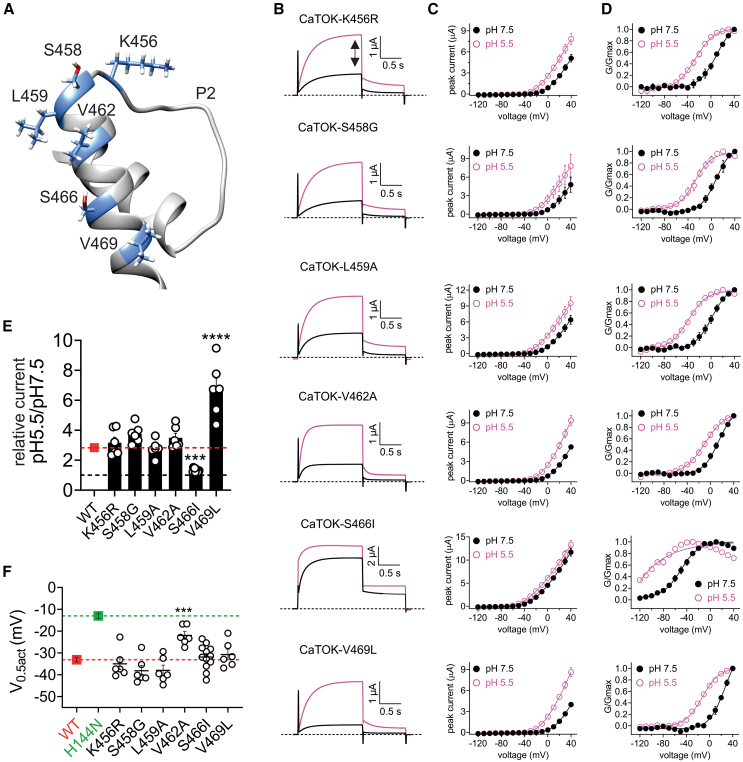
Mutant P2C spans a region comprising six amino acids located within the final transmembrane segment (S8) of CaTOK (Figure 5A). To study these residues, all six were mutated individually to the corresponding residues in the pH insensitive ScTOK. All mutants exhibited robust outward current similar in magnitude to that of wild type CaTOK, with the exception of S466I, which was ∼2-fold larger (Figure S7A). Mutants K456R, S458G, and L459A all had a V0.5act akin to that of wild-type CaTOK. Interestingly, V462A and V495L each right-shifted the V0.5act, while S466I hyperpolarized the V0.5act by −51mV (Figure S7B). Mutants K456R, S458G, L459A, V462A, and V469L all failed to prevent an increase in CaTOK currents at pH 5.5, with all mutants exhibiting a 3-fold or greater current increase at 0 mV (Figures 5B, 5C, 5E, and S7C). Similarly, the V0.5act of these mutants was shifted ∼ −33 mV at pH 5.5, with the exception of V462, which reduced the hyperpolarizing shift by −11 mV compared to wild type (Figures 5D, 5F, and S7C). Critically, mutant S466I essentially abolished current potentiation in response to pH 5.5, exhibiting only a 25%-fold increase in current at 0 mV (Figures 5B, 5C, 5E, and S7C). Conversely, S466I did not prevent the shift in the V0.5act with pH 5.5 inducing a shift of −31.9 ± 1.6 mV (Figures 5D and 5F). These data reveal that S466 in S8 near the external portion of the pore is integral for facilitating current augmentation in response to extracellular acidification but does not alone perturb the effect of pH on the V0.5act.
Figure 5.
S466 is the molecular determinant for current augmentation, but not hyperpolarization of V0.5act, by acidic pH
Data are represented as mean ± SEM.
(A) Alpha-Fold predicted structure of P2 and the extracellular end of S8 of CaTOK showing the predicted locations of the six residues within the P2C region (cornflower blue).
(B) Averaged traces for S8 mutant currents at 0 mV as indicated, in the presence of pH 7.5 and pH 5.5. Black dashed line indicates no change; Red dashed line indicates current-fold increase for wild type CaTOK; n = 6–11 per group.
(C) Mean peak current (measured during prepulse) from traces as in B; n = 6–11 per group.
(D) Mean normalized tail current (G/Gmax) for traces as in B; n = 6–12 per group.
(E) Fold increase at 0 mV as in G. Black dashed line indicates no change; Red dashed line indicates current-fold increase for wild type CaTOK; n = 6–12 per group. Statistical significance for comparison of relative current for S466I was ∗∗∗p = 0.0002 and V496L was ∗∗∗∗<0.0001 by One-way ANOVA.
(F) Mean ΔV0.5act for wild-type and mutant CaTOK channels. Green dashed line indicates the mean ΔV0.5act for CaTOK-H144N; Red dashed line indicates mean ΔV0.5act for wild-type CaTOK; n = 6–12 per group. Statistical significance for comparison of ΔV0.5act for V462A was ∗∗∗p = 0.0001 by One-way ANOVA.
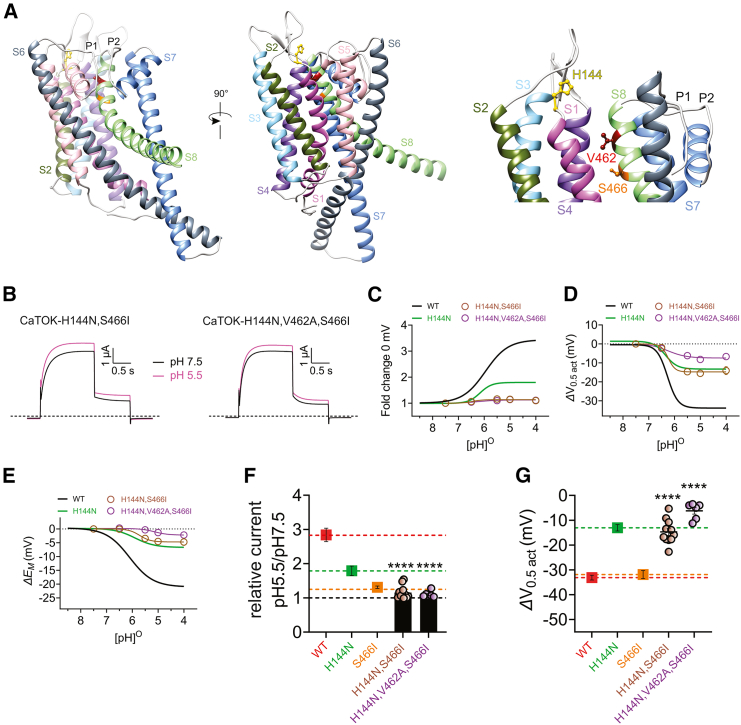
CaTOK pH modulation is coordinated by H144 in the S1-S2 linker and V462 and S466 in S8
Our data thus far revealed that mutating H144 in the S1-S2 linker reduces current augmentation and V0.5act by extracellular acidification, while mutating S466 in S8 abolishes current augmentation but not the effect of pH on V0.5act. Thus, it appears H144 and S466 (Figure 6A) are working in tandem to contribute to the different effects elicited by acidic pH. To validate this prediction, we generated a double mutant, CaTOK-H144N,S466I (Figures S8A and S8B), and found that this modification substantially reduced the effect of pH 5.5 on current augmentation, such that maximal peak current increase at 0 mV was reduced from 3-fold to being barely distinguishable from control (Figures 6B and 6C, Figures S8C and S8D). The effect of pH 5.5 on the V0.5act of CaTOK-H144N,S466I was almost identical to its effect on CaTOK-H144N, reducing the hyperpolarizing shift by −18.4 mV, to −14.7 ± 1.5 mV compared to −33 mV for wild-type CaTOK (Figure 6D; Figure S8E); this pattern was recapitulated in effects on EM of unclamped oocytes (Figure 6E). However, this meant that as for CaTOK-H144N, CaTOK-H144N,S466I still exhibited a hyperpolarizing shift in V0.5act in response to acidic pH, suggesting contribution from another residue. Earlier, we observed that V462A reduced the effect of pH 5.5 on the V0.5act by 11 mV, raising the possibility that this residue could also form part of the pH-responsive elements. Thus, we generated and characterized a triple mutant channel, Ca-TOK-H144N,S466I,V462A (Ca-TOK-x3, Figures S8A and S8B). Strongly supporting that all three residues constitute the pH-responsive element, the triple-mutant channel exhibited negligible low pH-induced current augmentation at 0 mV similar to CaTOK-H144N,S466I (Figures 6C and 6F; Figures S8F and S8G) and less than half the low pH-induced hyperpolarization of V0.5act observed for CaTOK-H144N,S466I (Figures 6D and 6G; Figure S8H) with a negligible low pH-induced hyperpolarization of the EM (Figure 6E).
Figure 6.
Residues H144, V462, and S446 participate cooperatively to sense and mechanically transduce CaTOK responses to pH
Data are represented as mean ± SEM.
(A) Alpha-Fold predicted structure showing the proximity of H144 (yellow) in the S1-S2 linker to V462 (red) and S466 (orange) in the extracellular end of CaTOK S8.
(B) Averaged traces for H144N-S466I (left) and H144N,V462A,S446I (right) mutant CaTOK currents at 0 mV as indicated, in the presence of pH 7.5 and pH 5.5; n = 5–11 per group.
(C) Mean current fold increase versus (pH)O at 0 mV for H144N-S466I (brown) and H144N,V462A,S466I (purple), with CaTOK data from Figure 1 and H144N data from Figure 2 for comparison; n = 5–11 per group.
(D) Mean ΔV0.5act versus (pH)O for H144N,S466I (brown) and H144N,V462A,S466I (purple), with CaTOK data from Figure 1 and H144N data from Figure 2 for comparison; n = 5–11 per group.
(E) Mean ΔEM versus (pH)O for H144N,S466I (brown) and H144N,V462A,S466I (purple), with CaTOK data from Figure 1 and H144N data from Figure 2 for comparison; n = 5–11 per group.
(F) Fold increase at 0 mV as in C for H144N,S466I (brown) and H144N,V462A,S466I (purple), with CaTOK (red dashed line), H144N (green dashed line), and S466I (orange dashed line) for comparison. Black dashed line reflects no change; n = 5–11 per group. Statistical significance for comparison of relative current for H144N, S466I was ∗∗∗∗<0.0001 and H144N, V462A, S466I was ∗∗∗∗<0.0001 by One-way ANOVA.
(G) Mean ΔV0.5act as in D for H144N,S466I (brown) and H144N,V462A,S466I (purple), with CaTOK (red dashed line), H144N (green dashed line), and S466I (orange dashed line) for comparison. Black dashed line reflects no change; n = 5–11 per group. Statistical significance for comparison of ΔV0.5act for H144N, S466I was ∗∗∗∗<0.0001 and H144N, V462A, S466I was ∗∗∗∗<0.0001 by One-way ANOVA.
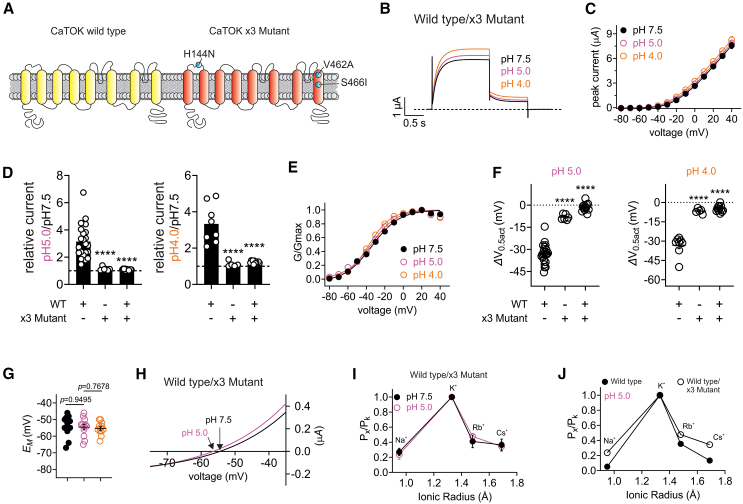
Coexpression of CaTOK wild-type and CaTOK-H144N,S466I,V462A reveals that both subunits are required for pH sensing
We next investigated whether both subunits of the CaTOK homodimer are required for pH sensitivity by coexpressing CaTOK wild type and CaTOK-H144N,S466I,V462A (Figure 7A). Interestingly, the effect of pH 5.0 and pH 4.0 was negligible, with no significant increases in current density observed across all voltage ranges (Figures 7B–7E). Acidic pH also had no effect on the V0.5act of channels formed after equal co-expression of wild type and CaTOK-H144N,S466I,V462A, reducing the hyperpolarizing shift by −31.8 mV, to −1.4 ± 0.8 at pH 5.0 and −28.4 mV, to −4.8 ± 0.7 at pH 4.0, almost identical to the reduction in voltage-dependent activation observed for CaTOK-H144N,S466I,V462A (Figures 7E and 7F). Similarly, no effect of acidic pH was observed on EM of unclamped oocytes (Figure 7G). In Figure 3 we revealed that acidic pH shifted the reversal potential of wild-type CaTOK closer to EK by altering ion selectivity, making CaTOK more selective for K+, an effect we hypothesize is due to a pH-dependent conformational change at the selectivity filter (Figures 3D and 3E). Thus, we wanted to investigate whether pH-dependent changes in ion selectivity still occur in channels formed by equal co-expression of wild type and CaTOK-H144N,S466I,V462A. Strikingly, the effect of acidic pH on the reversal potential was negligible (−54 mV at pH 7.5, compared to −55 mV at pH 5.0) compared to the ∼-20 mV shift observed for wild-type CaTOK at the same pH range, suggesting pH no longer effects ion selectivity (Figure 7H).
Figure 7.
CaTOK requires both subunits to confer pH sensitivity
Data are represented as mean ± SEM.
(A) Schematic of CaTOK wild type (yellow) and H144N,V462A,S446I mutant (red).
(B) Averaged traces for CaTOK wild type/x3 mutant currents at 0 mV as indicated, in the presence of pH 7.5, pH 5.0, pH 4.0: n = 12 per group.
(C) Mean peak current (measured during prepulse) from traces as in B; n = 12 per group.
(D) Fold increase at 0 mV as in C for CaTOK wild type, ×3 mutant (H144N,V462A,S466I), and wild type/x3 mutant heterodimer in the presence of pH 5.0 (left) and pH 4.0 (right). Black dashed line reflects no change; n = 8–22 per group. Statistical significance for comparison of relative current for ×3 mutant (H144N,V462A,S466I) was ∗∗∗∗<0.0001 and wild type/x3 mutant heterodimer was ∗∗∗∗<0.0001 at pH 5.0 and ×3 mutant (H144N,V462A,S466I) was ∗∗∗∗<0.0001 and wild type/x3 mutant heterodimer was ∗∗∗∗<0.0001 at pH 4.0 by One-way ANOVA..
(E) Mean normalized tail current (G/Gmax) for traces as in B; n = 12 per group.
(F) Mean ΔV0.5act as in E for CaTOK wild type, ×3 mutant (H144N,V462A,S466I), and wild type/x3 mutant heterodimer in the presence of pH 5.0 (left) and pH 4.0 (right). Black dashed line reflects no change; n = 8–22 per group. Statistical significance for comparison of ΔV0.5act for ×3 mutant (H144N,V462A,S466I) was ∗∗∗∗<0.0001 and wild type/x3 mutant heterodimer was ∗∗∗∗<0.0001 at pH 5.0 and ×3 mutant (H144N,V462A,S466I) was ∗∗∗∗<0.0001 and wild type/x3 mutant heterodimer was ∗∗∗∗<0.0001 at pH 4.0 by One-way ANOVA..
(G) Mean EM versus (pH)O as in B; n = 12 per group. Statistical analysis was by One-way ANOVA.
(H) Mean peak current (measured during prepulse) for wild type/x3 mutant heterodimers in the presence of pH 7.5 (black) and pH 5.0 (pink) as in C showing the reversal potential for as indicated by the arrow; n = 12 per group.
(I) Relative ion permeabilities of wild type/x3 mutant heterodimers in the presence of pH 7.5 (black) and pH 5.0 (pink); n = 5–8.
(J) Relative ion permeabilities of wild-type CaTOK (black circles) and wild type/x3 mutant heterodimers (open circles) in the presence of pH 5.0; n = 8–10.
To confirm our suspicions, we conducted pseudo-bi-ionic substitution experiments. As expected, switching to pH 5.0 had no effect on the permeability of Na+, Rb+, and Cs+, explaining the reduced hyperpolarizing shift observed for the EREV (Figure 7I). This is in stark contrast to wild-type CaTOK, which exhibited markedly decreased permeability of Na+, Rb+, and Cs+ at pH 5.0 (Figure 7J). Assuming a binomial distribution of dimeric CaTOK channel types formed by equal co-expression of wild type and mutant CaTOK, one would expect ¼ channels to be wild type, ¼ homomeric mutant, and ½ heteromeric wild-type/mutant. The most likely explanation from the data are therefore that heteromeric wild-type/mutant CaTOK channels behave similarly to homomeric mutant channels, i.e., are pH-insensitive, and only a small fraction of the channels (the homomeric wild-type channels) are pH-sensitive. Thus, the pH sensor of CaTOK requires all of the pH-sensing amino acids on both of the subunits within the homodimer to confer pH sensitivity.
Discussion
C. albicans is unusual among fungal pathogens in its ability to colonize in a broad range of external pH, surviving in both extremely acidic (pH ≤ 2) and extremely alkaline (pH ≥ 10) conditions. This is due in part to C. albicans having a malleable cytosolic pH, which can range between pH 5.8 and pH 9.0 compared to non-pathogenic yeast species such as S. cerevisiae, which maintain a cytosolic pH between 6.0 and 7.0.16 In fact, significant alkalinization of the cytosol precedes hyphal formation in C. albicans, with an internal pH of 7.0 initiating the outgrowth of germ tubes.17 It is thought that Pma1p, a plasma membrane H+-ATPase, mediates this process and is also a major driver in setting yeast membrane potential via H+ movement across the membrane.18 This pH gradient drives nutrient uptake via secondary transporters, including K+ uptake, which can reach 200–300 mM inside the yeast cell. In the absence of energy supply, where Pma1p is no longer helping to dictate the membrane potential, or in the presence of some other stressor, yeast cells will depolarize.
Several studies have implicated TOK channels in critical processes such as K+ homeostasis, membrane potential regulation, and osmoregulation.19,20 Rather than setting membrane potential per se (as do analogous K+ channels in higher animals), TOK channels are thought to mitigate the negative impact of yeast cell depolarization, i.e., play a gatekeeper role in maintaining membrane potential under stress. When membrane potential shifts positive to EK, TOK channels become active and stabilize the membrane potential, preventing further excessive depolarization which would likely result in cell death. They do not switch on at a set voltage (as do voltage gated K+ channels) but rather at the membrane potential determined by EK under the specific conditions the cell is experiencing. Therefore, while some studies have shown Candida resting membrane potential to be as hyperpolarized as, e.g., −120 mV, with internal K+ at 200–300 mM, external K+ at around 4 mM (body fluid) and at a host mammalian body temp of 37°C, the Nernst equation predicts TOK activation at around −100 to −115 mV, which would be in the protective range for the microbe under those conditions. The shifting voltage dependence of TOK activation permits the yeast to adapt to different environmental K+ conditions or fluctuations in internal K+. The proposed model for TOK channels’ capacity to only pass current positive to EK (thus ensuring they are outward rectifiers despite lacking a traditional voltage sensing apparatus) is that the K+ reversal potential determines ion occupancy and therefore conductivity of a selectivity filter that can sample both intracellular and extracellular ion concentrations and thus acts as the channel gate (similar two mammalian K2P channels; see in the further text).3
Prasad and Hofer measured resting membrane potential (EM) for C. albicans at a range of external pH values and found it to be between −72 and −78 mV at pH 4.5–5.5, hyperpolarizing to −100 mV at pH 7.5 and >−120 mV at pH 8.5.21 In their study, EM was measured indirectly from the distribution of tetraphenylphosphonium ions, which may even overestimate EM compared to when quantified by direct microelectrode measurement (i.e., the actual values may be less negative).22 At pH 4.0–5.5 we observed a substantial negative shift in the voltage dependence of CaTOK activation and overall current augmentation such that it was open even as far negative at −80 mV (Figure 1F). Thus, the low pH-induced shift in voltage dependence of CaTOK activation coincides with a low pH-induced shift in C. albicans EM, which would be predicted to endow CaTOK with a greater capacity to protect against depolarization of C. albicans EM under acidic extracellular conditions.
A particularly striking finding of our present study was that only CaTOK was pH sensitive among the TOK channels tested. Despite belonging to the same family, the homology between the channels is relatively low, with ScTOK at 36% showing the closest homology to CaTOK. One possible explanation could lie with the route of infection of the host organism. C. albicans is uniquely tuned to its environment, using changes in temperature, high CO2, nutrient deprivation, and pH to initiate its transition from a commensal round yeast into a virulent filamentous hyphal form.23,24,25,26 This is, particularly advantageous for C. albicans when it colonizes within the commensal flora of the oral, genital, and intestinal mucosa,27 where these parameters fluctuate regularly. The primary reservoir of C. albicans is in the gastrointestinal (GI) tract, which has perhaps the most dynamic range of pH (<2.0 to >10.0), from highly acidic in the stomach to neutral or alkaline in other parts of the gut.28 This is in stark contrast to A. fumigatus and C. neoformans, which do not infect via these routes but rather attach to airway epithelia or within the pulmonary alveoli which are subject to less dynamic changes in pH. Thus, it could be speculated that CaTOK pH sensitivity arises from an evolutionary advantage for C. albicans to be responsive to pH changes, while the insensitivity of AfTOK and CnTOK results from a lack of evolutionary pressure on A. fumigatus and C. neoformans to be responsive to pH.
Differential modulation by pH is not, however, exclusive to TOK channels, even among channels with two P domains. TASK1 and TASK3 were named for their acid-sensitivity, whereby protonation of a His in the first pore domain inhibits the channel across the physiologic pH range.29,30,31 TREK-1 and TREK-2 are regulated by a range of stimuli, including mechanical stretch, osmolarity, heat, polyunsaturated fatty acids, and pH.32,33,34,35,36 TREK channel responses to pH are contrasting, with TREK-1 robustly inhibited by acidic pH, while TREK-2 is activated by external acidification, differences attributed to the electrostatic interactions between the conserved histidine and their neighboring residues in the distal end of the P2 domain.37 The mechanism of TREK-1 inhibition by external pH is governed by two histidine residues (H87 and H141) in the external S1-S2 linker of the channel. Protonation of these histidine residues is thought to facilitate the collapse of the selectivity filter region, which acts as the TREK-1 channel gate, through hydrogen bond formation with a proximal glutamate (E84).38 However, the inhibitory effect of acidic pH on TREK-1 currents is diminished significantly by gain-of-function (GOF) mutations to residues lining the pore, suggesting that these GOF mutations act by uncoupling the sensory and gating apparatus of TREK-1.39
The AlphaFold-generated structure prediction for CaTOK shows H144 in close proximity to V462 and S466; however, how these residues interact in order to confer pH sensitivity remains to be elucidated. In TREK-1 and TREK-2, a histidine in the first pore loop is thought to interact with charged residues in the second pore to confer inhibition and activation, respectively.37 It was proposed that negatively charged residues in the P2 domain of TREK-1 attract the protonated imidazole side chain of the histidine and induce a conformational change more likely to favor the closed-state. Whereas in TREK-2, the same region is comprised of basic residues and likely repels the protonated histidine, resulting in the opposite effect.37 There is no charge difference in the region P2C domain of CaTOK and pH insensitive ScTOK, suggesting the electrostatic mechanism model of pH gating for TREK channels does not apply here.
The role of TOK channels in fungal physiology is currently incompletely understood. Deletion or overexpression of TOK1 in S. cerevisiae has been shown to depolarize and hyperpolarize the membrane potential, respectively,19 as well providing the molecular basis for both killer toxin activity and immunity.9,10 Conversely, TOK1 inhibition has been proposed as integral in protecting yeast during hyperosmotic stress, whereby phosphorylation of the mitogen-activated protein kinase Hog1P inhibits TOK1 channel activity leading to membrane depolarization and reduced secondary ion flow into the cell.40 Our study identifies CaTOK as a potential molecular sensor for C. albicans pH sensitivity that may contribute to pH-dependent hyphal formation, a key virulence trait, and therefore highlights CaTOK as a potential therapeutic target.
Limitations of the study
This study was performed in vitro using cellular electrophysiology of currents from TOK channels heterologously expressed in Xenopus oocytes; future studies could examine TOK function in the context of Candida itself. In addition, we rely on AlphaFold-predicted TOK channel structures in the absence of high-resolution experimental data of the TOK channel structure.
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Dr. Geoffrey W. Abbott (abbottg@hs.uci.edu).
Materials availability
This study did not generate new unique reagents; channel constructs are available upon request.
Data and code availability
-
•
Electrophysiology source data have been deposited at Dryad and are publicly available as of the date of publication: Accession numbers are listed in the key resources table.
-
•
There are no applicable codes.
-
•
There are no other applicable items.
Acknowledgments
This study was supported by the National Institutes of Health, National Institute of General Medical Sciences (GM130377) to G.W.A., The Royal Society (RGS\R1\201026) to RWM and Biotechnology and Biological Sciences Research Council (BBSRC), UK, grant number BB/J006114/1 to A.L.
Author contributions
R.W.M., G.W.A., S.A.N.G., and A.L. conceived the study; A.L. and Z.A.M. generated and performed initial characterization of some constructs; R.W.M. and C.L.I. conducted TEVC studies and analyzed data; R.W.M., G.W.A., S.A.N.G., and A.L. oversaw and/or obtained funding for the project; R.W.M. prepared the figures, R.W.M. and G.W.A. wrote the manuscript; all authors contributed to editing the manuscript.
Declaration of interests
The authors declare no competing interests.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Critical commercial assays | ||
| mMessage mMACHINE T7 Transcription Kit | Thermo Fisher | Cat#AM1344 |
| QuikChange Site-Directed Mutagenesis kit | Stratagene | N/A |
| Deposited data | ||
| Electrophysiology Data Excel Files | This paper | https://doi.org/10.5061/dryad.t76hdr893 |
| Experimental models: Organisms/strains | ||
| Stage V & VI Xenopus laevis oocytes | Xenopus1 | xenopus1.com |
| Recombinant DNA | ||
| CaTOK pMAX plasmid | Lewiset al.3 | N/A |
| ScTOK pMAX plasmid | Lewiset al.3 | N/A |
| AfTOK pMAX plasmid | Lewiset al.3 | N/A |
| CnTOK pMAX plasmid | Lewiset al.3 | N/A |
| CaTOK-H144K pMAX plasmid | This paper | N/A |
| CaTOK-H144N pMAX plasmid | This paper | N/A |
| CaTOK-H144N pMAX plasmid | This paper | N/A |
| CaTOK-ScTOK C-terminus chimera pMAX plasmid | This paper | N/A |
| CaTOK-ScTOK chimera 1 pMAX plasmid | This paper | N/A |
| CaTOK-ScTOK chimera 2 pMAX plasmid | This paper | N/A |
| CaTOK-P2A pMAX plasmid | This paper | N/A |
| CaTOK-P2B pMAX plasmid | This paper | N/A |
| CaTOK-P2C pMAX plasmid | This paper | N/A |
| CaTOK-K456R pMAX plasmid | This paper | N/A |
| CaTOK-S458G pMAX plasmid | This paper | N/A |
| CaTOK-L459A pMAX plasmid | This paper | N/A |
| CaTOK-V462A pMAX plasmid | This paper | N/A |
| CaTOK-S466I pMAX plasmid | This paper | N/A |
| CaTOK-V469L pMAX plasmid | This paper | N/A |
| CaTOK-H144N, S466I pMAX plasmid | This paper | N/A |
| CaTOK-H144N, V462A, S466I pMAX plasmid | This paper | N/A |
| Software and algorithms | ||
| pClamp 9.2 | Molecular Devices | moleculardevices.com |
| Clampfit 11.2 | Molecular Devices | moleculardevices.com |
| GraphPad Prism 10 | Prism | www.graphpad.com |
| Biorender | Biorender | www.biorender.com |
Experimental model details
We used defolliculated stage V and VI Xenopus laevis oocytes (Xenoocyte, Dexter, MI, US) for electrophysiological recordings. We incubated the oocytes at 16°C in ND96 oocyte storage solution containing penicillin and streptomycin, with daily washing, for 1 day prior to two-electrode voltage-clamp (TEVC) recording.
Method details
Channel subunit cRNA preparation and Xenopus laevis oocyte injection
We generated cRNA transcripts encoding TOK channels from Candida albicans, Aspergillus fumigatus, Cryptococcus neoformans var. neoformans, and Saccharomyces cerevisiae, by in vitro transcription using the T7 mMessage mMachine kit (Thermo Fisher Scientific), after vector linearization, from cDNA sub-cloned into plasmids (pMAX) incorporating Xenopus laevis β-globin 5′ and 3′ UTRs flanking the coding region to enhance translation and cRNA stability. Mutant TOK channel cDNAs were generated by Genscript (Piscataway, NJ, USA) or using QuickChange Site-Directed Mutagenesis kit (Stratagene, La Jolla, CA), and cRNAs made as above. We injected defolliculated stage V and VI Xenopus laevis oocytes (Xenoocyte, Dexter, MI, US) with TOK cRNAs (1–5 ng). We incubated the oocytes at 16°C in ND96 oocyte storage solution containing penicillin and streptomycin, with daily washing, for 1 day prior to two-electrode voltage-clamp (TEVC) recording.
Two-electrode voltage clamp (TEVC)
We performed TEVC (Figure 1C) at room temperature using an OC-725C amplifier (Warner Instruments, Hamden, CT) and pClamp10 software (Molecular Devices, Sunnyvale, CA) 1 day after cRNA injection. Oocytes were placed in a small-volume oocyte bath (Warner) and viewed with a dissection microscope. Oocytes expressing TOK channels were recorded in 4 mM extracellular KCl bath solution containing (in mM): 96 NaCl, 4 KCl, 1 MgCl2, 1 CaCl2 unless otherwise indicated. Extracellular 4 mM KCl solutions were buffered (in mM) with: 10 MES (pH 4.0 to 5.5), 10 HEPES (pH 6.0 to 7.5), 5 CHES (pH 8.0 to 9.5). Pipettes were of 1–2 MΩ resistance when filled with 3 M KCl. We recorded currents in response to voltage pulses between −120/-80 mV and +40 mV at 10 mV intervals from a holding potential of −80 mV, to yield current-voltage relationships and examine activation kinetics. We plotted raw or normalized tail currents for CaTOK versus prepulse voltage and fitted with a single Boltzmann function:
| (Equation 1) |
where g is the normalized tail conductance, A1 is the initial value at -∞ (very negative membrane potential), A2 is the final value at +∞ (very positive membrane potential), V1/2 is the half-maximal voltage of activation and Vs the slope factor. We analyzed data using Clampfit (Molecular Devices) and Graphpad Prism software (GraphPad, San Diego, CA, USA), stating values as mean ± SEM.
Relative permeability calculations
The Goldman-Hodgkin-Katz (GHK) equation states:
| (Equation 2) |
Where Vm is the absolute reversal potential and P is the permeability. By knowing the intracellular and extracellular concentrations of each ion, one can modify this equation to calculate the relative permeability of each ion. Here, we modified the equation to determine the relative permeability of two ions in a system in which only the extracellular ion concentration was known. By plotting the I/V relationships for CaTOK in the presence of 100 mM Rb+, Na+, and Cs+ we can compare their relative permeability to 100 mM K+. Permeability ratios for each ion (x) compared to K+ were calculated as,
| (Equation 3) |
CaTOK structure prediction
We used AlphaFold13,14 to generate a predicted structure for CaTOK. We visualized the CaTOK channel structure in UCSF Chimera,15 which we also used to generate figures.
Quantification and statistical analysis
All values are expressed as mean ± SEM. Comparison of two groups was conducted using a t-test; all p values were two-sided. One-way ANOVA was applied to all tests comparing more than two groups; all p values were two-sided. Individual statistical details for each experiment are shown in figure legends.
Published: November 22, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.111451.
Supplemental information
References
- 1.Ketchum K.A., Joiner W.J., Sellers A.J., Kaczmarek L.K., Goldstein S.A. A new family of outwardly rectifying potassium channel proteins with two pore domains in tandem. Nature. 1995;376:690–695. doi: 10.1038/376690a0. [DOI] [PubMed] [Google Scholar]
- 2.Roberts S.K. TOK homologue in Neurospora crassa: first cloning and functional characterization of an ion channel in a filamentous fungus. Eukaryot. Cell. 2003;2:181–190. doi: 10.1128/EC.2.1.181-190.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lewis A., McCrossan Z.A., Manville R.W., Popa M.O., Cuello L.G., Goldstein S.A.N. TOK channels use the two gates in classical K(+) channels to achieve outward rectification. Faseb. J. 2020;34:8902–8919. doi: 10.1096/fj.202000545R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Denning D.W. Global incidence and mortality of severe fungal disease. Lancet Infect. Dis. 2024;24:e428–e438. doi: 10.1016/s1473-3099(23)00692-8. [DOI] [PubMed] [Google Scholar]
- 5.AndréS M.T., Viejo-DíAz M., Fierro J.F. Human Lactoferrin Induces Apoptosis-Like Cell Death inCandida albicans: Critical Role of K+-Channel-Mediated K+Efflux. Antimicrob. Agents Chemother. 2008;52:4081–4088. doi: 10.1128/aac.01597-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baev D., Rivetta A., Li X.S., Vylkova S., Bashi E., Slayman C.L., Edgerton M. Killing of Candida albicans by Human Salivary Histatin 5 Is Modulated, but Not Determined, by the Potassium Channel TOK1. Infect. Immun. 2003;71:3251–3260. doi: 10.1128/iai.71.6.3251-3260.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee W., Lee D.G. Potential role of potassium and chloride channels in regulation of silymarin-induced apoptosis in Candida albicans. IUBMB Life. 2018;70:197–206. doi: 10.1002/iub.1716. [DOI] [PubMed] [Google Scholar]
- 8.Yun J., Lee D.G. Role of potassium channels in chlorogenic acid-induced apoptotic volume decrease and cell cycle arrest in Candida albicans. Biochim. Biophys. Acta Gen. Subj. 2017;1861:585–592. doi: 10.1016/j.bbagen.2016.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Ahmed A., Sesti F., Ilan N., Shih T.M., Sturley S.L., Goldstein S.A. A molecular target for viral killer toxin. Cell. 1999;99:283–291. doi: 10.1016/s0092-8674(00)81659-1. [DOI] [PubMed] [Google Scholar]
- 10.Sesti F., Shih T.M., Nikolaeva N., Goldstein S.A. Immunity to K1 killer toxin: internal TOK1 blockade. Cell. 2001;105:637–644. doi: 10.1016/s0092-8674(01)00376-2. [DOI] [PubMed] [Google Scholar]
- 11.Lesage F., Guillemare E., Fink M., Duprat F., Lazdunski M., Romey G., Barhanin J. A pH-sensitive Yeast Outward Rectifier K+ Channel with Two Pore Domains and Novel Gating Properties. J. Biol. Chem. 1996;271:4183–4187. doi: 10.1074/jbc.271.8.4183. [DOI] [PubMed] [Google Scholar]
- 12.Bertl A., Bihler H., Reid J.D., Kettner C., Slayman C.L. Physiological characterization of the yeast plasma membrane outward rectifying K + channel, DUK1 (TOK1), in situ. J. Membr. Biol. 1998;162:67–80. doi: 10.1007/s002329900343. [DOI] [PubMed] [Google Scholar]
- 13.Jumper J., Evans R., Pritzel A., Green T., Figurnov M., Ronneberger O., Tunyasuvunakool K., Bates R., Žídek A., Potapenko A., et al. Highly accurate protein structure prediction with AlphaFold. Nature. 2021;596:583–589. doi: 10.1038/s41586-021-03819-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Varadi M., Anyango S., Deshpande M., Nair S., Natassia C., Yordanova G., Yuan D., Stroe O., Wood G., Laydon A., et al. AlphaFold Protein Structure Database: massively expanding the structural coverage of protein-sequence space with high-accuracy models. Nucleic Acids Res. 2022;50:D439–D444. doi: 10.1093/nar/gkab1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pettersen E.F., Goddard T.D., Huang C.C., Couch G.S., Greenblatt D.M., Meng E.C., Ferrin T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 16.Rane H.S., Hayek S.R., Frye J.E., Abeyta E.L., Bernardo S.M., Parra K.J., Lee S.A. Candida albicans Pma1p Contributes to Growth, pH Homeostasis, and Hyphal Formation. Front. Microbiol. 2019;10:1012. doi: 10.3389/fmicb.2019.01012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stewart E., Gow N.A., Bowen D.V. Cytoplasmic alkalinization during germ tube formation in Candida albicans. J. Gen. Microbiol. 1988;134:1079–1087. doi: 10.1099/00221287-134-5-1079. [DOI] [PubMed] [Google Scholar]
- 18.Goossens A., De La Fuente N., Forment J., Serrano R., Portillo F. Regulation of yeast H+-ATPASe by protein kinases belonging to a family dedicated to activation of plasma membrane transporters. Mol. Cell Biol. 2000;20:7654–7661. doi: 10.1128/mcb.20.20.7654-7661.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maresova L., Urbankova E., Gaskova D., Sychrova H. Measurements of plasma membrane potential changes in Saccharomyces cerevisiae cells reveal the importance of the Tok1 channel in membrane potential maintenance. FEMS Yeast Res. 2006;6:1039–1046. doi: 10.1111/j.1567-1364.2006.00140.x. [DOI] [PubMed] [Google Scholar]
- 20.Zahumenský J., Jančíková I., Drietomská A., Švenkrtová A., Hlaváček O., Hendrych T., Plášek J., Sigler K., Gášková D. Yeast Tok1p channel is a major contributor to membrane potential maintenance under chemical stress. Biochim. Biophys. Acta Biomembr. 2017;1859:1974–1985. doi: 10.1016/j.bbamem.2017.06.019. [DOI] [PubMed] [Google Scholar]
- 21.Prasad R., Höfer M. Tetraphenylphosphonium is an indicator of negative membrane potential in Candida albicans. Biochim. Biophys. Acta Biomembr. 1986;861:377–380. doi: 10.1016/0005-2736(86)90442-6. [DOI] [PubMed] [Google Scholar]
- 22.Vacata V., Kotyk A., Sigler K. Membrane potential in yeast cells measured by direct and indirect methods. Biochim. Biophys. Acta. 1981;643:265–268. doi: 10.1016/0005-2736(81)90241-8. [DOI] [PubMed] [Google Scholar]
- 23.Shapiro R.S., Robbins N., Cowen L.E. Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol. Mol. Biol. Rev. 2011;75:213–267. doi: 10.1128/mmbr.00045-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klengel T., Liang W.J., Chaloupka J., Ruoff C., Schröppel K., Naglik J.R., Eckert S.E., Mogensen E.G., Haynes K., Tuite M.F., et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr. Biol. 2005;15:2021–2026C. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrevens S., Van Zeebroeck G., Riedelberger M., Tournu H., Kuchler K., Van Dijck P. Methionine is required for cAMP-PKA-mediated morphogenesis and virulence of Candida albicans. Mol. Microbiol. 2018;108:258–275. doi: 10.1111/mmi.13933. [DOI] [PubMed] [Google Scholar]
- 26.Lo H.J., Köhler J.R., DiDomenico B., Loebenberg D., Cacciapuoti A., Fink G.R. Nonfilamentous C. albicans Mutants Are Avirulent. Cell. 1997;90:939–949. doi: 10.1016/s0092-8674(00)80358-x. [DOI] [PubMed] [Google Scholar]
- 27.Gow N.A.R., van de Veerdonk F.L., Brown A.J.P., Netea M.G. Candida albicans morphogenesis and host defence: discriminating invasion from colonization. Nat. Rev. Microbiol. 2011;10:112–122. doi: 10.1038/nrmicro2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Biswas S., Van Dijck P., Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol. Mol. Biol. Rev. 2007;71:348–376. doi: 10.1128/MMBR.00009-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes C.M., Gallagher P.G., Buck M.E., Butler M.H., Goldstein S.A. Proton block and voltage gating are potassium-dependent in the cardiac leak channel KCNK3. J. Biol. Chem. 2000;275:16969–16978. doi: 10.1074/jbc.m001948200. [DOI] [PubMed] [Google Scholar]
- 30.Lopes C.M., Zilberberg N., Goldstein S.A. Block of KCNK3 by protons. Evidence that 2-P-domain potassium channel subunits function as homodimers. J. Biol. Chem. 2001;276:24449–24452. doi: 10.1074/jbc.c100184200. [DOI] [PubMed] [Google Scholar]
- 31.Ma L., Zhang X., Zhou M., Chen H. Acid-sensitive TWIK and TASK Two-pore Domain Potassium Channels Change Ion Selectivity and Become Permeable to Sodium in Extracellular Acidification. J. Biol. Chem. 2012;287:37145–37153. doi: 10.1074/jbc.m112.398164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Patel A.J., Honoré E., Maingret F., Lesage F., Fink M., Duprat F., Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maingret F., Patel A.J., Lesage F., Lazdunski M., Honoré E. Mechano- or acid stimulation, two interactive modes of activation of the TREK-1 potassium channel. J. Biol. Chem. 1999;274:26691–26696. doi: 10.1074/jbc.274.38.26691. [DOI] [PubMed] [Google Scholar]
- 34.Kim Y., Gnatenco C., Bang H., Kim D. Localization of TREK-2 K + channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pH i. Pflügers Archiv. 2001;442:952–960. doi: 10.1007/s004240100626. [DOI] [PubMed] [Google Scholar]
- 35.Maingret F., Lauritzen I., Patel A.J., Heurteaux C., Reyes R., Lesage F., Lazdunski M., Honoré E. TREK-1 is a heat-activated background K+ channel. EMBO J. 2000;19:2483–2491. doi: 10.1093/emboj/19.11.2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kang D., Choe C., Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J. Physiol. 2005;564:103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sandoz G., Douguet D., Chatelain F., Lazdunski M., Lesage F. Extracellular acidification exerts opposite actions on TREK1 and TREK2 potassium channels via a single conserved histidine residue. Proc. Natl. Acad. Sci. USA. 2009;106:14628–14633. doi: 10.1073/pnas.0906267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen A., Ben-Abu Y., Hen S., Zilberberg N. A Novel Mechanism for Human K2P2.1 Channel Gating. J. Biol. Chem. 2008;283:19448–19455. doi: 10.1074/jbc.m801273200. [DOI] [PubMed] [Google Scholar]
- 39.Bagriantsev S.N., Peyronnet R., Clark K.A., Honoré E., Minor D.L. Multiple modalities converge on a common gate to control K2Pchannel function. EMBO J. 2011;30:3594–3606. doi: 10.1038/emboj.2011.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ke R., Ingram P.J., Haynes K. An integrative model of ion regulation in yeast. PLoS Comput. Biol. 2013;9 doi: 10.1371/journal.pcbi.1002879. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
-
•
Electrophysiology source data have been deposited at Dryad and are publicly available as of the date of publication: Accession numbers are listed in the key resources table.
-
•
There are no applicable codes.
-
•
There are no other applicable items.