Abstract
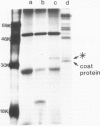
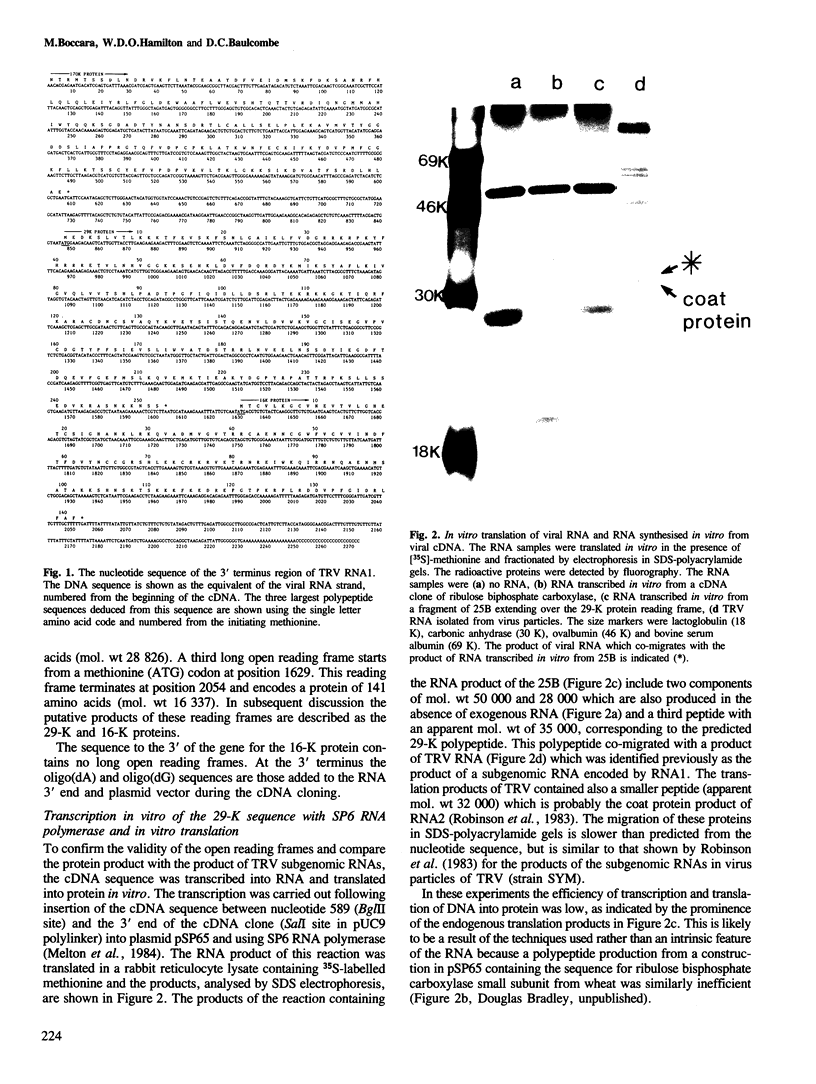
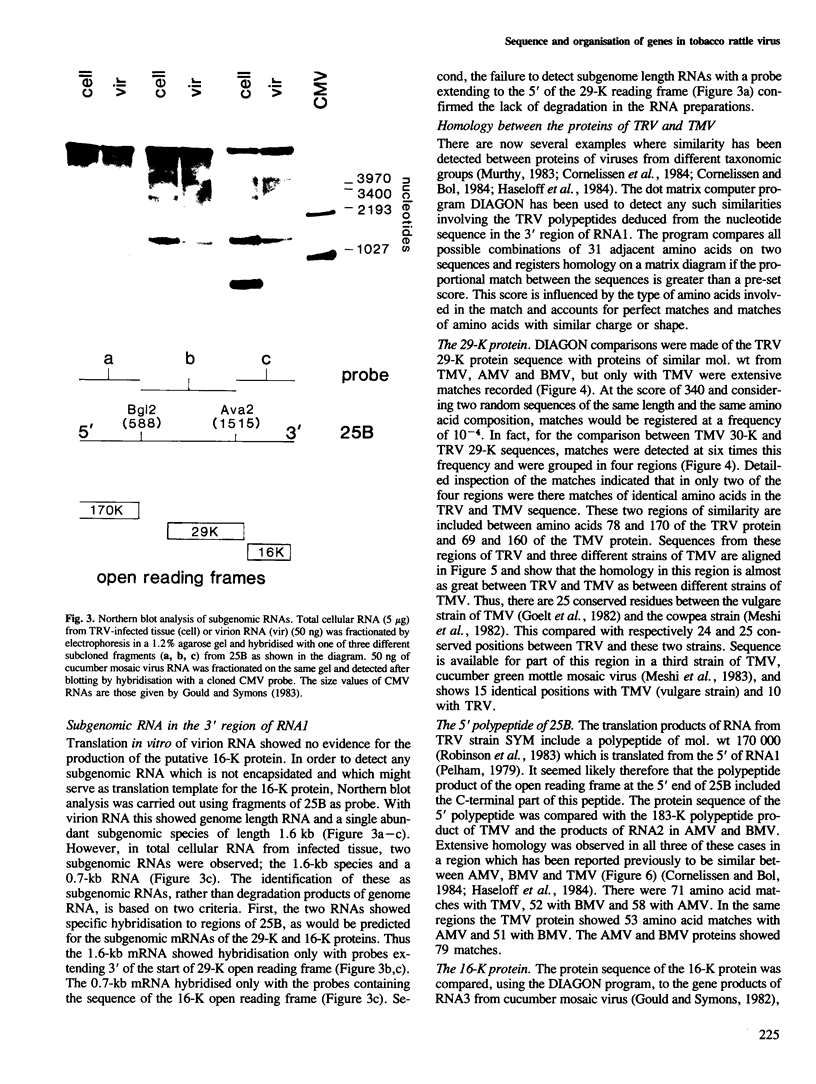
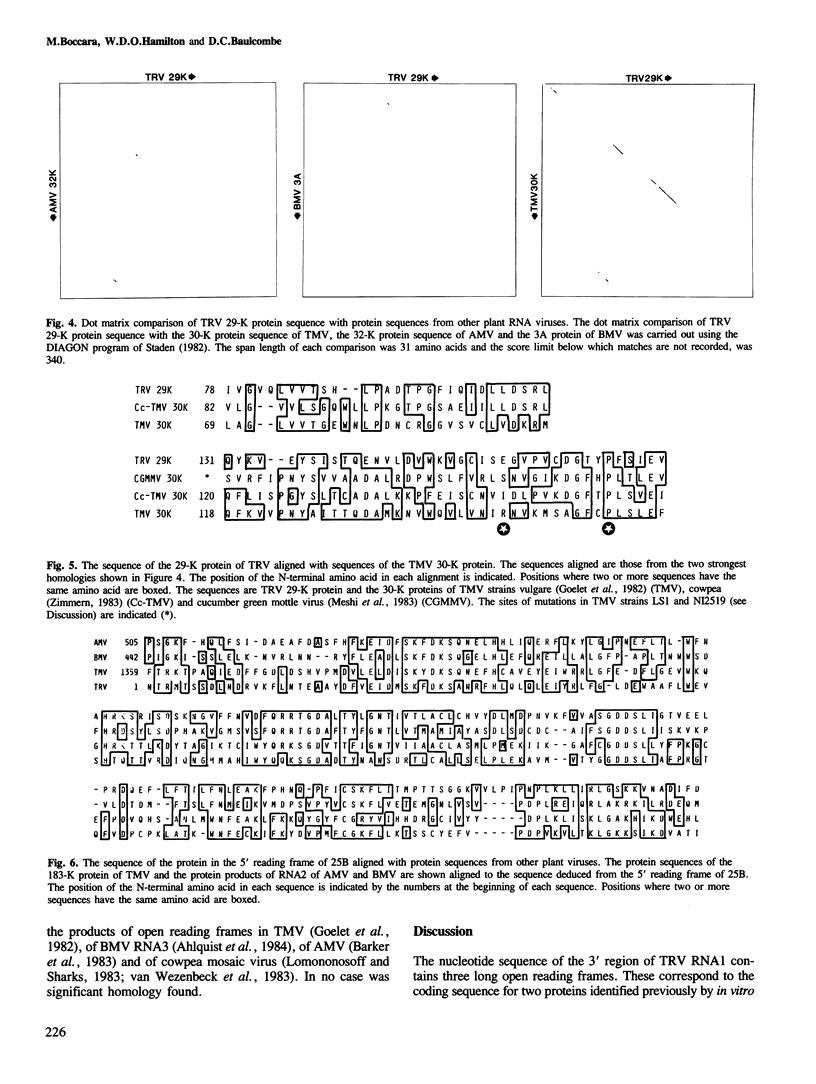
The RNA1 of tobacco rattle virus (TRV) has been cloned as cDNA and the nucleotide sequence determined of 2 kb from the 3'-terminal region. The sequence contains three long open reading frames. One of these starts 5' of the cDNA and probably corresponds to the carboxy-terminal sequence of a 170-K protein encoded on RNA1. The deduced protein sequence from this reading frame shows homology with the putative replicases of tobacco mosaic virus (TMV) and tricornaviruses. The location of the second open reading frame, which encodes a 29-K polypeptide, was shown by Northern blot analysis to coincide with a 1.6-kb subgenomic RNA. The validity of this reading frame was confirmed by showing that the cDNA extending over this region could be transcribed and translated in vitro to produce a polypeptide of the predicted size which co-migrates in electrophoresis with a translation product of authentic viral RNA. The sequence of this 29-K polypeptide showed homology with two regions in the 30-K protein of TMV. This homology includes positions in the TMV 30-K protein where mutations have been identified which affect the transport of virus between cells. The third open reading frame encodes a potential 16-K protein and was shown by Northern blot hybridisation to be contained within the region of a 0.7-kb subgenomic RNA which is found in cellular RNA of infected cells but not virus particles. The many similarities between TRV and TMV in viral morphology, gene organisation and sequence suggest that these two viral groups may share a common viral ancestor.
Keywords: subgenomic RNA, tobacco mosaic virus, tobacco rattle virus, transport protein, viral evolution
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlquist P., Dasgupta R., Kaesberg P. Nucleotide sequence of the brome mosaic virus genome and its implications for viral replication. J Mol Biol. 1984 Feb 5;172(4):369–383. doi: 10.1016/s0022-2836(84)80012-1. [DOI] [PubMed] [Google Scholar]
- Ahlquist P., Janda M. cDNA cloning and in vitro transcription of the complete brome mosaic virus genome. Mol Cell Biol. 1984 Dec;4(12):2876–2882. doi: 10.1128/mcb.4.12.2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S. Shotgun DNA sequencing using cloned DNase I-generated fragments. Nucleic Acids Res. 1981 Jul 10;9(13):3015–3027. doi: 10.1093/nar/9.13.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apel K., Kloppstech K. The plastid membranes of barley (Hordeum vulgare). Light-induced appearance of mRNA coding for the apoprotein of the light-harvesting chlorophyll a/b protein. Eur J Biochem. 1978 Apr 17;85(2):581–588. doi: 10.1111/j.1432-1033.1978.tb12273.x. [DOI] [PubMed] [Google Scholar]
- Barker R. F., Jarvis N. P., Thompson D. V., Loesch-Fries L. S., Hall T. C. Complete nucleotide sequence of alfalfa mosaic virus RNA3. Nucleic Acids Res. 1983 May 11;11(9):2881–2891. doi: 10.1093/nar/11.9.2881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Cohen S. N. Analysis of gene control signals by DNA fusion and cloning in Escherichia coli. J Mol Biol. 1980 Apr;138(2):179–207. doi: 10.1016/0022-2836(80)90283-1. [DOI] [PubMed] [Google Scholar]
- Cornelissen B. J., Janssen H., Zuidema D., Bol J. F. Complete nucleotide sequence of tobacco streak virus RNA 3. Nucleic Acids Res. 1984 Mar 12;12(5):2427–2437. doi: 10.1093/nar/12.5.2427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritsch C., Mayo M. A., Hirth L. Further studies on the translation products of tobacco rattle virus RNA in vitro. Virology. 1977 Apr;77(2):722–732. doi: 10.1016/0042-6822(77)90494-9. [DOI] [PubMed] [Google Scholar]
- Goelet P., Karn J. Tobacco mosaic virus induces the synthesis of a family of 3' coterminal messenger RNAs and their complements. J Mol Biol. 1982 Jan 25;154(3):541–550. doi: 10.1016/s0022-2836(82)80013-2. [DOI] [PubMed] [Google Scholar]
- Goelet P., Lomonossoff G. P., Butler P. J., Akam M. E., Gait M. J., Karn J. Nucleotide sequence of tobacco mosaic virus RNA. Proc Natl Acad Sci U S A. 1982 Oct;79(19):5818–5822. doi: 10.1073/pnas.79.19.5818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould A. R., Symons R. H. Cucumber mosaic virus RNA 3. Determination of the nucleotide sequence provides the amino acid sequences of protein 3A and viral coat protein. Eur J Biochem. 1982 Aug;126(2):217–226. [PubMed] [Google Scholar]
- Hansen J. B., Olsen R. H. Isolation of large bacterial plasmids and characterization of the P2 incompatibility group plasmids pMG1 and pMG5. J Bacteriol. 1978 Jul;135(1):227–238. doi: 10.1128/jb.135.1.227-238.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison B. D., Robinson D. J. The tobraviruses. Adv Virus Res. 1978;23:25–77. doi: 10.1016/s0065-3527(08)60097-4. [DOI] [PubMed] [Google Scholar]
- Haseloff J., Goelet P., Zimmern D., Ahlquist P., Dasgupta R., Kaesberg P. Striking similarities in amino acid sequence among nonstructural proteins encoded by RNA viruses that have dissimilar genomic organization. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4358–4362. doi: 10.1073/pnas.81.14.4358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T., Jackson R., Zimmern D. Multiple proteins and subgenomic mRNAs may be derived from a single open reading frame on tobacco mosaic virus RNA. Nucleic Acids Res. 1983 Feb 11;11(3):801–821. doi: 10.1093/nar/11.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. J., Hunt T. Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods Enzymol. 1983;96:50–74. doi: 10.1016/s0076-6879(83)96008-1. [DOI] [PubMed] [Google Scholar]
- King A. M., McCahon D., Slade W. R., Newman J. W. Recombination in RNA. Cell. 1982 Jul;29(3):921–928. doi: 10.1016/0092-8674(82)90454-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomonossoff G. P., Shanks M. The nucleotide sequence of cowpea mosaic virus B RNA. EMBO J. 1983;2(12):2253–2258. doi: 10.1002/j.1460-2075.1983.tb01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ohno T., Okada Y. Nucleotide sequence of the 30K protein cistron of cowpea strain of tobacco mosaic virus. Nucleic Acids Res. 1982 Oct 11;10(19):6111–6117. doi: 10.1093/nar/10.19.6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Murthy M. R. Comparison of the nucleotide sequences of cucumber mosaic virus and brome mosaic virus. J Mol Biol. 1983 Aug 15;168(3):469–475. doi: 10.1016/s0022-2836(83)80296-4. [DOI] [PubMed] [Google Scholar]
- Ohno T., Takamatsu N., Meshi T., Okada Y., Nishiguchi M., Kiho Y. Single amino acid substitution in 30K protein of TMV defective in virus transport function. Virology. 1983 Nov;131(1):255–258. doi: 10.1016/0042-6822(83)90551-2. [DOI] [PubMed] [Google Scholar]
- Rave N., Crkvenjakov R., Boedtker H. Identification of procollagen mRNAs transferred to diazobenzyloxymethyl paper from formaldehyde agarose gels. Nucleic Acids Res. 1979 Aug 10;6(11):3559–3567. doi: 10.1093/nar/6.11.3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SIEGEL A., ZAITLIN M., SEHGAL O. P. The isolation of defective tobacco mosaic virus strains. Proc Natl Acad Sci U S A. 1962 Oct 15;48:1845–1851. doi: 10.1073/pnas.48.10.1845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staden R. An interactive graphics program for comparing and aligning nucleic acid and amino acid sequences. Nucleic Acids Res. 1982 May 11;10(9):2951–2961. doi: 10.1093/nar/10.9.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmern D. Homologous proteins encoded by yeast mitochondrial introns and by a group of RNA viruses from plants. J Mol Biol. 1983 Dec 15;171(3):345–352. doi: 10.1016/0022-2836(83)90098-0. [DOI] [PubMed] [Google Scholar]
- Zimmern D., Hunter T. Point mutation in the 30-K open reading frame of TMV implicated in temperature-sensitive assembly and local lesion spreading of mutant Ni 2519. EMBO J. 1983;2(11):1893–1900. doi: 10.1002/j.1460-2075.1983.tb01676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Wezenbeek P., Verver J., Harmsen J., Vos P., van Kammen A. Primary structure and gene organization of the middle-component RNA of cowpea mosaic virus. EMBO J. 1983;2(6):941–946. doi: 10.1002/j.1460-2075.1983.tb01525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]