Abstract
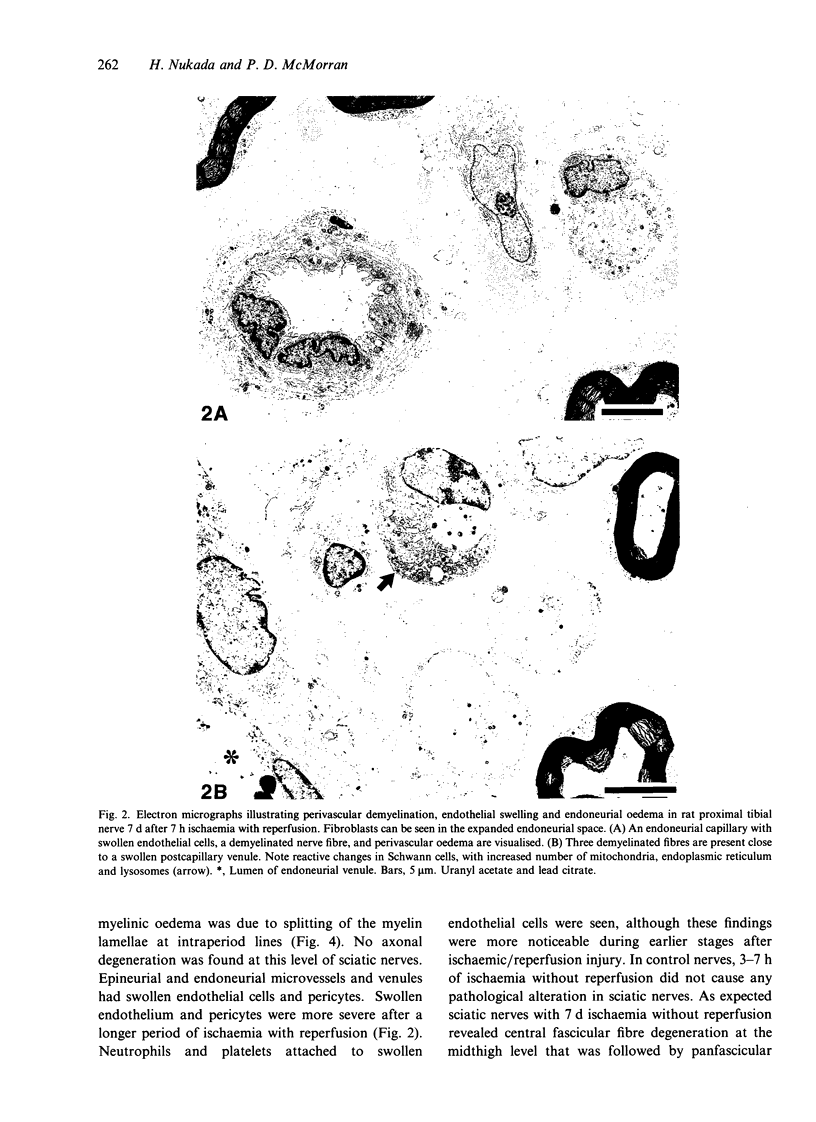
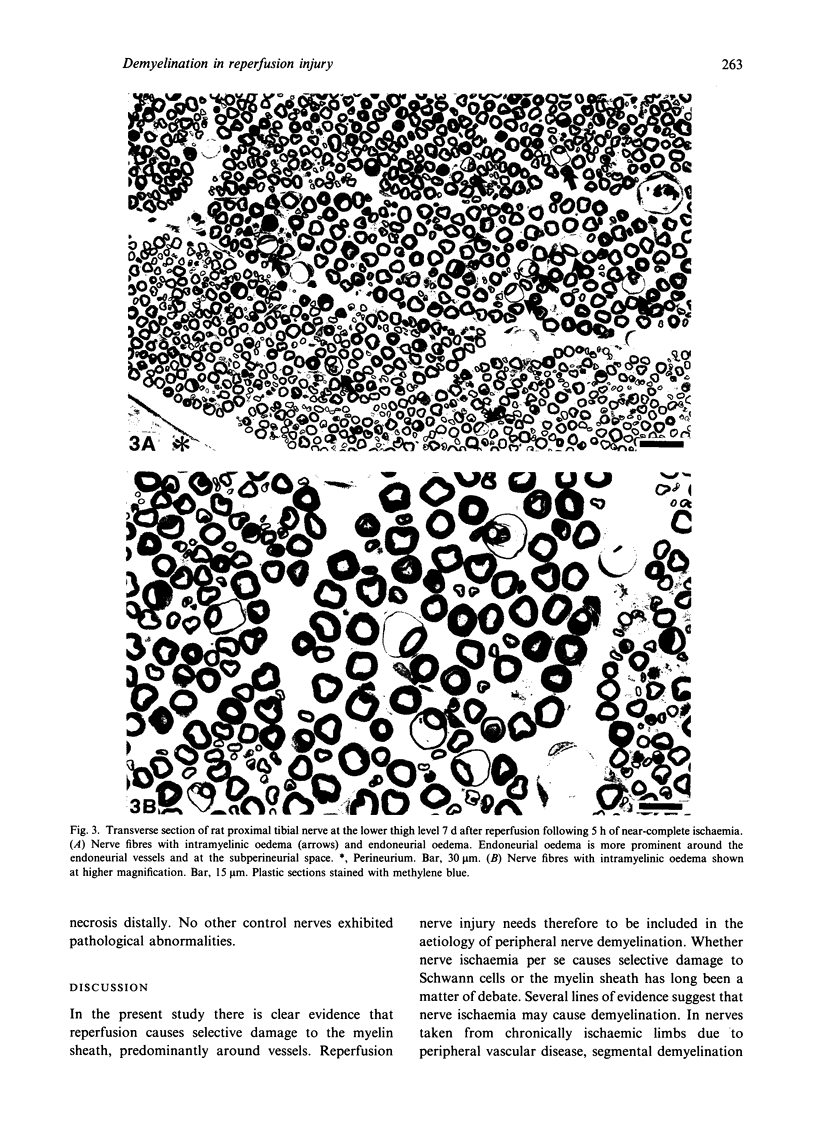
Nerve ischaemia plays a major role in the development of pathological alterations in various neuropathies, and the effects of ischaemia are amplified by reperfusion in various tissues. While pathological alterations in acutely ischaemic nerve have been established, nerve pathology resulting from reperfusion injury has never been elucidated. To evaluate what cell type in peripheral nerve is affected by reoxygenation following a hypoxic episode, we developed an animal model of transient severe limb ischaemia. Near-complete ischaemia, confirmed by the measurement of nerve blood flow, was achieved by clamping multiple arteries of supply to rat hindlimb. After 3, 5 or 7 h of limb ischaemia, vascular clips were released to reperfuse blood flow. Pathology in sciatic, tibial and peroneal nerves at the lower thigh level was examined at 7 d after reperfusion. All reperfused nerves developed demyelinated nerve fibres, particularly in perivascular regions. Although 3 h of ischaemia followed by reperfusion caused demyelination, perivascular demyelination was more prominent after a longer period of ischaemia with reperfusion. Two types of nerve oedema were observed; endoneurial oedema especially in perivascular and subperineurial spaces, and intramyelinic oedema. Nerve fibres with intramyelinic oedema were not confined to the perivascular region. Swollen endothelial cells in endoneurial vessels were also invariably observed. Nerve ischaemia per se, without reperfusion, did not induce these pathological changes. Because myelin appears to be particularly susceptible to activated free radicals, oxidative stress, activated neutrophils, and cytokine formation seem to be important underlying mechanisms in the development of perivascular demyelination and intramyelinic oedema in ischaemic/reperfused nerves.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asbury A. K., Aldredge H., Hershberg R., Fisher C. M. Oculomotor palsy in diabetes mellitus: a clinico-pathological study. Brain. 1970;93(3):555–566. doi: 10.1093/brain/93.3.555. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S. Ischaemic injury mediator. Nature. 1990 May 3;345(6270):27–28. doi: 10.1038/345027b0. [DOI] [PubMed] [Google Scholar]
- Chopra J. S., Hurwitz L. J. Internodal length of sural nerve fibres in chronic occlusive vascular disease. J Neurol Neurosurg Psychiatry. 1967 Jun;30(3):207–214. doi: 10.1136/jnnp.30.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amour M. L., Lebrun L. H., Rabbat A., Trudel J., Daneault N. Peripheral neurological complications of aortoiliac vascular disease. Can J Neurol Sci. 1987 May;14(2):127–130. doi: 10.1017/s0317167100026238. [DOI] [PubMed] [Google Scholar]
- Dyck P. J., Conn D. L., Okazaki H. Necrotizing angiopathic neuropathy. Three-dimensional morphology of fiber degeneration related to sites of occluded vessels. Mayo Clin Proc. 1972 Jul;47(7):461–475. [PubMed] [Google Scholar]
- Eames R. A., Lange L. S. Clinical and pathological study of ischaemic neuropathy. J Neurol Neurosurg Psychiatry. 1967 Jun;30(3):215–226. doi: 10.1136/jnnp.30.3.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler R. L., Schmid-Schönbein G. W., Pavelec R. S. Leukocyte capillary plugging in myocardial ischemia and reperfusion in the dog. Am J Pathol. 1983 Apr;111(1):98–111. [PMC free article] [PubMed] [Google Scholar]
- Freischlag J. A., Hanna D. Neutrophil (PMN) phagocytosis and chemotaxis after reperfusion injury. J Surg Res. 1992 Feb;52(2):152–156. doi: 10.1016/0022-4804(92)90297-d. [DOI] [PubMed] [Google Scholar]
- Granger D. N., Benoit J. N., Suzuki M., Grisham M. B. Leukocyte adherence to venular endothelium during ischemia-reperfusion. Am J Physiol. 1989 Nov;257(5 Pt 1):G683–G688. doi: 10.1152/ajpgi.1989.257.5.G683. [DOI] [PubMed] [Google Scholar]
- HUTCHINSON E. C., LIVERSEDGE L. A. Neuropathy in peripheral vascular disease; it bearing on diabetic neuropathy. Q J Med. 1956 Apr;25(98):267–274. [PubMed] [Google Scholar]
- Hernandez L. A., Grisham M. B., Twohig B., Arfors K. E., Harlan J. M., Granger D. N. Role of neutrophils in ischemia-reperfusion-induced microvascular injury. Am J Physiol. 1987 Sep;253(3 Pt 2):H699–H703. doi: 10.1152/ajpheart.1987.253.3.H699. [DOI] [PubMed] [Google Scholar]
- Hess K., Eames R. A., Darveniza P., Gilliatt R. W. Acute ischaemic neuropathy in the rabbit. J Neurol Sci. 1979 Dec;44(1):19–43. doi: 10.1016/0022-510x(79)90220-x. [DOI] [PubMed] [Google Scholar]
- Jaeschke H., Farhood A., Smith C. W. Neutrophils contribute to ischemia/reperfusion injury in rat liver in vivo. FASEB J. 1990 Dec;4(15):3355–3359. [PubMed] [Google Scholar]
- Lampert P. W. Autoimmune and virus-induced demyelinating diseases. A review. Am J Pathol. 1978 Apr;91(1):176–208. [PMC free article] [PubMed] [Google Scholar]
- Levin K. H. AAEE case report #19: ischemic monomelic neuropathy. Muscle Nerve. 1989 Oct;12(10):791–795. doi: 10.1002/mus.880121002. [DOI] [PubMed] [Google Scholar]
- Low P. A., Nukada H., Schmelzer J. D., Tuck R. R., Dyck P. J. Endoneurial oxygen tension and radial topography in nerve edema. Brain Res. 1985 Aug 19;341(1):147–154. doi: 10.1016/0006-8993(85)91482-9. [DOI] [PubMed] [Google Scholar]
- Matheis G., Sherman M. P., Buckberg G. D., Haybron D. M., Young H. H., Ignarro L. J. Role of L-arginine-nitric oxide pathway in myocardial reoxygenation injury. Am J Physiol. 1992 Feb;262(2 Pt 2):H616–H620. doi: 10.1152/ajpheart.1992.262.2.H616. [DOI] [PubMed] [Google Scholar]
- McCord J. M. Oxygen-derived free radicals in postischemic tissue injury. N Engl J Med. 1985 Jan 17;312(3):159–163. doi: 10.1056/NEJM198501173120305. [DOI] [PubMed] [Google Scholar]
- Menger M. D., Lehr H. A., Messmer K. Role of oxygen radicals in the microcirculatory manifestations of postischemic injury. Klin Wochenschr. 1991 Dec 15;69(21-23):1050–1055. doi: 10.1007/BF01645157. [DOI] [PubMed] [Google Scholar]
- Myers R. R., Heckman H. M., Galbraith J. A., Powell H. C. Subperineurial demyelination associated with reduced nerve blood flow and oxygen tension after epineurial vascular stripping. Lab Invest. 1991 Jul;65(1):41–50. [PubMed] [Google Scholar]
- Myers R. R., Powell H. C. Galactose neuropathy: impact of chronic endoneurial edema on nerve blood flow. Ann Neurol. 1984 Nov;16(5):587–594. doi: 10.1002/ana.410160510. [DOI] [PubMed] [Google Scholar]
- Newrick P. G., Wilson A. J., Jakubowski J., Boulton A. J., Ward J. D. Sural nerve oxygen tension in diabetes. Br Med J (Clin Res Ed) 1986 Oct 25;293(6554):1053–1054. doi: 10.1136/bmj.293.6554.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nukada H., Dyck P. J. Acute ischemia causes axonal stasis, swelling, attenuation, and secondary demyelination. Ann Neurol. 1987 Sep;22(3):311–318. doi: 10.1002/ana.410220306. [DOI] [PubMed] [Google Scholar]
- Nukada H., Dyck P. J. Microsphere embolization of nerve capillaries and fiber degeneration. Am J Pathol. 1984 May;115(2):275–287. [PMC free article] [PubMed] [Google Scholar]
- Nukada H. Increased susceptibility to ischemic damage in streptozocin-diabetic nerve. Diabetes. 1986 Sep;35(9):1058–1061. doi: 10.2337/diab.35.9.1058. [DOI] [PubMed] [Google Scholar]
- Nukada H., Powell H. C., Myers R. R. Perineurial window: demyelination in nonherniated endoneurium with reduced nerve blood flow. J Neuropathol Exp Neurol. 1992 Sep;51(5):523–530. doi: 10.1097/00005072-199209000-00007. [DOI] [PubMed] [Google Scholar]
- Nukada H., Powell H. C., Myers R. R. Spatial distribution of nerve injury after occlusion of individual major vessels in rat sciatic nerves. J Neuropathol Exp Neurol. 1993 Sep;52(5):452–459. doi: 10.1097/00005072-199309000-00003. [DOI] [PubMed] [Google Scholar]
- Ohnishi A., Harada M., Tateishi J., Ogata J., Kawanami S. Segmental demyelination and remyelination in lumbar spinal roots of patients dying with diabetes mellitus. Ann Neurol. 1983 May;13(5):541–548. doi: 10.1002/ana.410130512. [DOI] [PubMed] [Google Scholar]
- POOLE E. W. Ischaemic and post-ischaemic paraesthesiae; normal responses in the upper limb with special reference to the effect of age. J Neurol Neurosurg Psychiatry. 1956 May;19(2):148–154. doi: 10.1136/jnnp.19.2.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- Powell H. C., Myers R. R. Schwann cell changes and demyelination in chronic galactose neuropathy. Muscle Nerve. 1983 Mar-Apr;6(3):218–227. doi: 10.1002/mus.880060309. [DOI] [PubMed] [Google Scholar]
- Rosen G. M., Freeman B. A. Detection of superoxide generated by endothelial cells. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7269–7273. doi: 10.1073/pnas.81.23.7269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmelzer J. D., Zochodne D. W., Low P. A. Ischemic and reperfusion injury of rat peripheral nerve. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1639–1642. doi: 10.1073/pnas.86.5.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS P. K., LASCELLES R. G. SCHWANN-CELL ABNORMALITIES IN DIABETIC NEUROPATHY. Lancet. 1965 Jun 26;1(7400):1355–1357. doi: 10.1016/s0140-6736(65)92154-9. [DOI] [PubMed] [Google Scholar]
- Towfighi J., Gonatas N. K., McCree L. Hexachlorophene neuropathy in rats. Lab Invest. 1973 Oct;29(4):428–436. [PubMed] [Google Scholar]
- Tuck R. R., Schmelzer J. D., Low P. A. Endoneurial blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984 Sep;107(Pt 3):935–950. doi: 10.1093/brain/107.3.935. [DOI] [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Welbourn C. R., Goldman G., Paterson I. S., Valeri C. R., Shepro D., Hechtman H. B. Pathophysiology of ischaemia reperfusion injury: central role of the neutrophil. Br J Surg. 1991 Jun;78(6):651–655. doi: 10.1002/bjs.1800780607. [DOI] [PubMed] [Google Scholar]
- Wilbourn A. J., Furlan A. J., Hulley W., Ruschhaupt W. Ischemic monomelic neuropathy. Neurology. 1983 Apr;33(4):447–451. doi: 10.1212/wnl.33.4.447. [DOI] [PubMed] [Google Scholar]
- Wiśniewski H. M., Brostoff S. W., Carter H., Eylar E. H. Recurrent experimental allergic polyganglioradiculoneuritis. Multiple demyelinating episodes in rhesus monkey sensitized with rabbit sciatic nerve myelin. Arch Neurol. 1974 May;30(5):347–358. doi: 10.1001/archneur.1974.00490350005002. [DOI] [PubMed] [Google Scholar]
- Zweier J. L., Flaherty J. T., Weisfeldt M. L. Direct measurement of free radical generation following reperfusion of ischemic myocardium. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1404–1407. doi: 10.1073/pnas.84.5.1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zweier J. L., Kuppusamy P., Lutty G. A. Measurement of endothelial cell free radical generation: evidence for a central mechanism of free radical injury in postischemic tissues. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4046–4050. doi: 10.1073/pnas.85.11.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]