Abstract
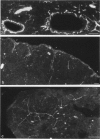
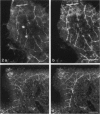
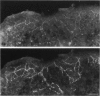
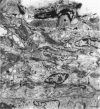
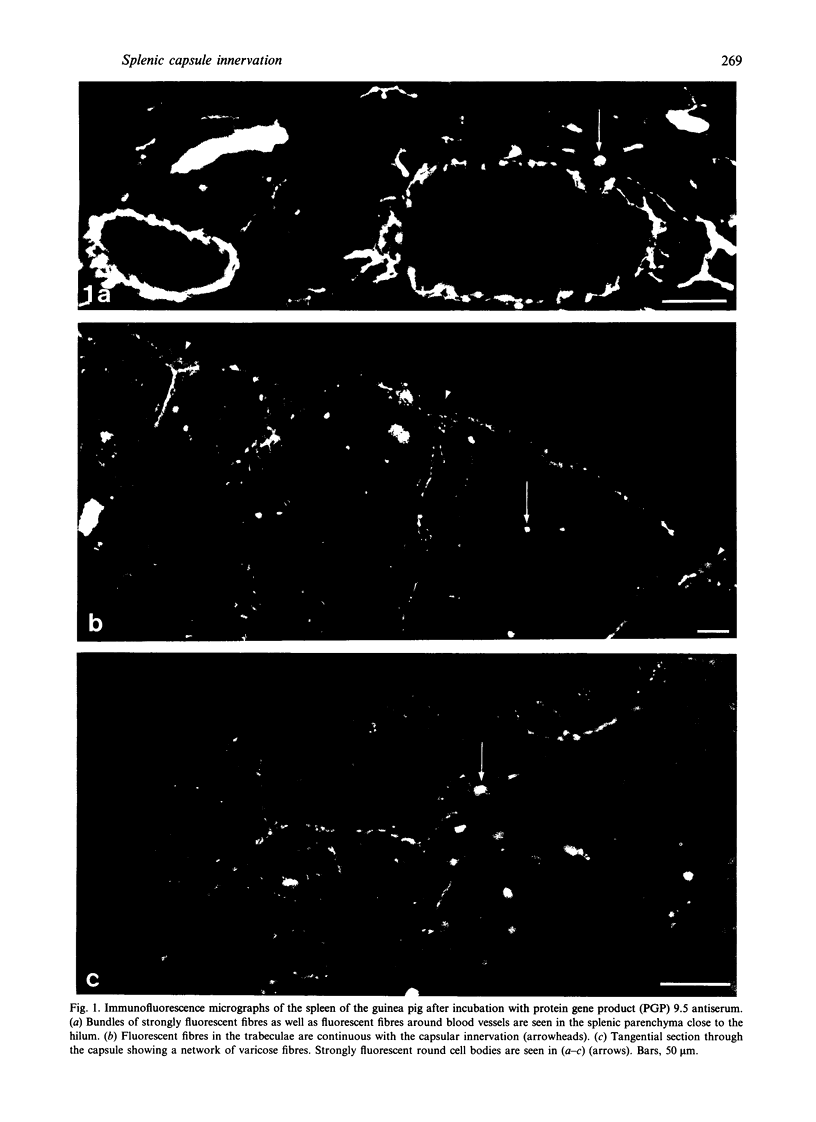
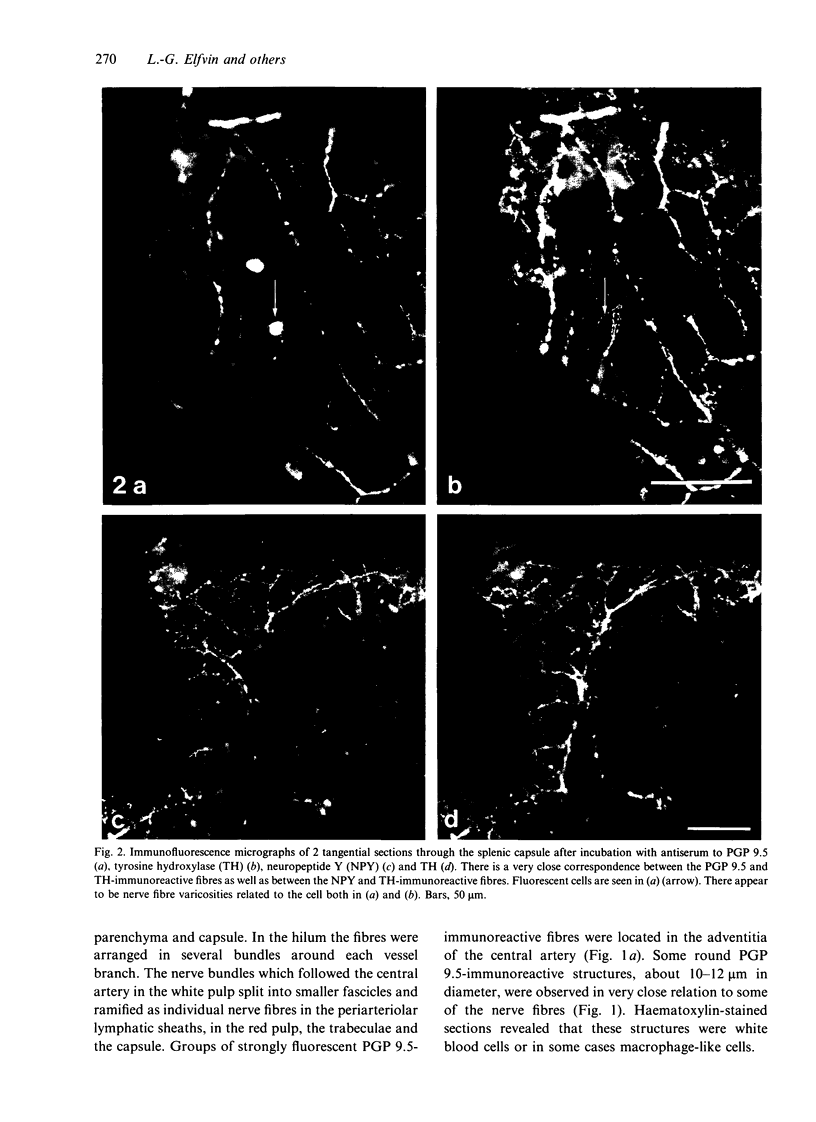
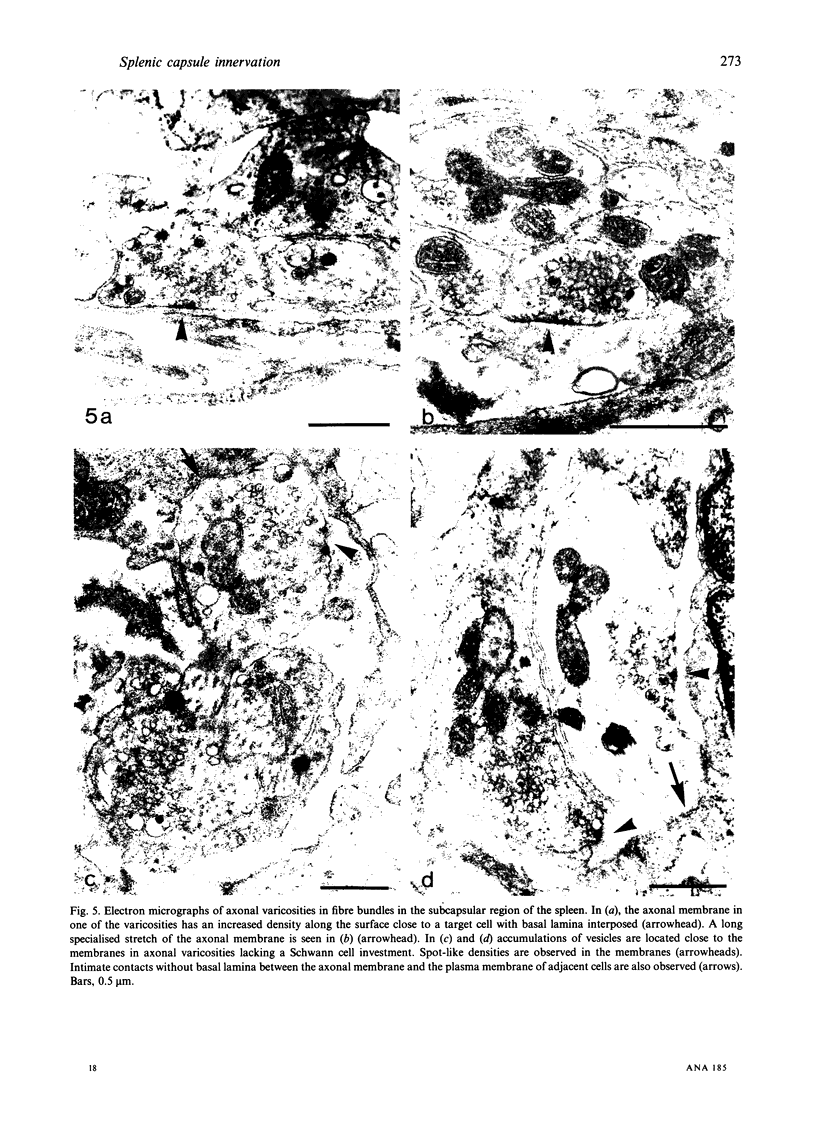
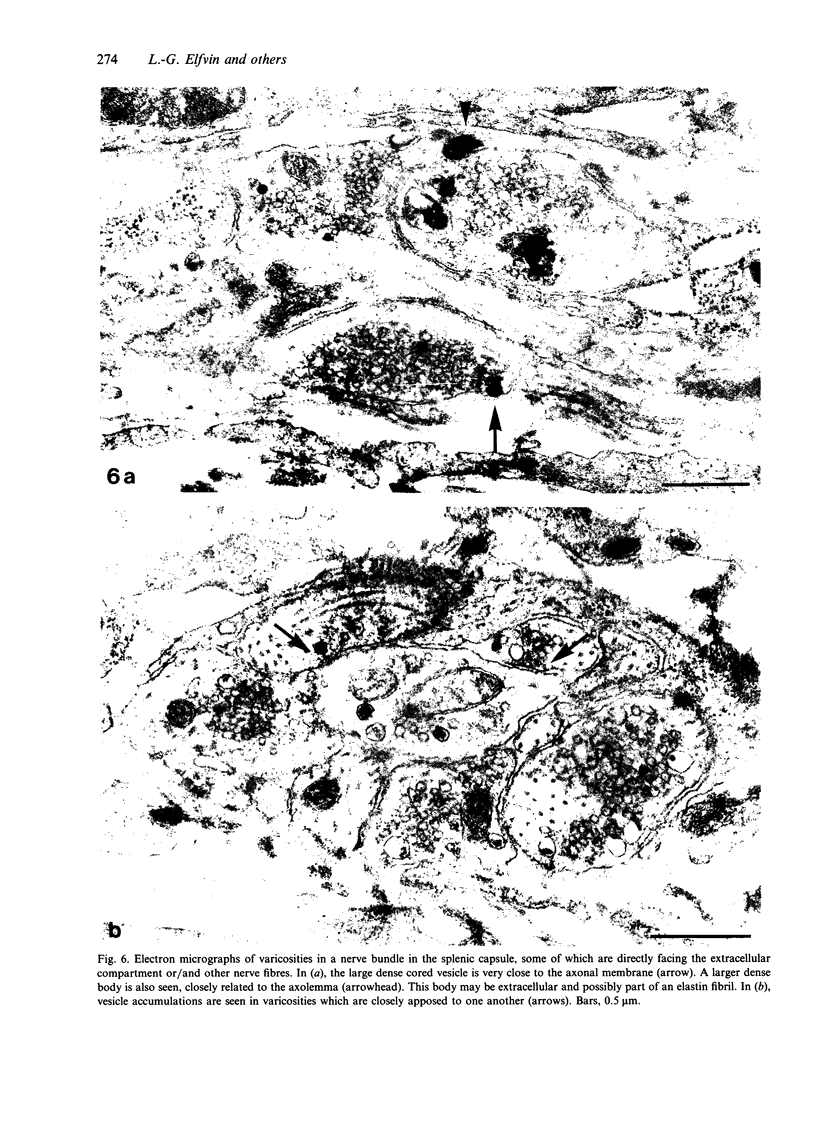
The innervation of the capsule of the guinea pig spleen was studied by light microscopy using an indirect fluorescent-labelled antibody technique, as well as by electron microscopy. A dense network of nerve fibres immunoreactive to the general neuronal marker, protein gene product 9.5 was observed in tangential sections through the capsule corresponding to the subcapsular compartment. The PGP 9.5-immunoreactivity in the fibres appeared to a large extent to be colocalised with tyrosine hydroxylase and neuropeptide Y (NPY) immunoreactivities as well as with synaptophysin immunoreactivity. Only very occasional fibres with substance P or calcitonin-gene-related peptide immunoreactivity were observed in tangential sections of the capsular region. By electron microscopy unmyelinated nerve fibres in the capsule were found to contain a large number of small dense-cored as well as clear vesicles and large dense-cored vesicles in varicose parts of the axons. The axolemma of the varicose regions was often naked, devoid of Schwann cells, and sometimes appeared denser than the nonspecialised parts of the membrane. These naked regions were observed in single sections to be apposed to splenic cells with variable intervals of extracellular space and interposed basal lamina material. Another type of contact was characterised by a very close association with splenic cells with no basal lamina interposed between the plasma membranes of the axon and the splenic cell. An intimate ultrastructural relationship was often also seen between varicose vesicle-containing axons and neighbouring axons in the nerve fibre bundles. The results show that the splenic capsule and its immediate neighbouring regions are innervated by catecholaminergic, NPY-containing fibres, which appear to establish different types of relations with the splenic cells as well as with one another.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baron R., Jänig W. Sympathetic and afferent neurons projecting in the splenic nerve of the cat. Neurosci Lett. 1988 Nov 22;94(1-2):109–113. doi: 10.1016/0304-3940(88)90279-0. [DOI] [PubMed] [Google Scholar]
- Bellinger D. L., Felten S. Y., Lorton D., Felten D. L. Origin of noradrenergic innervation of the spleen in rats. Brain Behav Immun. 1989 Dec;3(4):291–311. doi: 10.1016/0889-1591(89)90029-9. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Chevendra V., Weaver L. C. Distribution of splenic, mesenteric and renal neurons in sympathetic ganglia in rats. J Auton Nerv Syst. 1991 Apr;33(1):47–53. doi: 10.1016/0165-1838(91)90017-w. [DOI] [PubMed] [Google Scholar]
- Dalsgaard C. J., Rydh M., Haegerstrand A. Cutaneous innervation in man visualized with protein gene product 9.5 (PGP 9.5) antibodies. Histochemistry. 1989;92(5):385–390. doi: 10.1007/BF00492495. [DOI] [PubMed] [Google Scholar]
- ELFVIN L. G. The ultrastructure of unmyelinated fibers in the splenic nerve of the cat. J Ultrastruct Res. 1958 Aug;1(4):428–454. doi: 10.1016/s0022-5320(58)90012-1. [DOI] [PubMed] [Google Scholar]
- Elfvin L. G., Aldskogius H., Johansson J. Splenic primary sensory afferents in the guinea pig demonstrated with anterogradely transported wheat-germ agglutinin conjugated to horseradish peroxidase. Cell Tissue Res. 1992 Aug;269(2):229–234. doi: 10.1007/BF00319613. [DOI] [PubMed] [Google Scholar]
- Elfvin L. G., Lindh B., Hökfelt T. The chemical neuroanatomy of sympathetic ganglia. Annu Rev Neurosci. 1993;16:471–507. doi: 10.1146/annurev.ne.16.030193.002351. [DOI] [PubMed] [Google Scholar]
- Felten D. L., Felten S. Y., Carlson S. L., Olschowka J. A., Livnat S. Noradrenergic and peptidergic innervation of lymphoid tissue. J Immunol. 1985 Aug;135(2 Suppl):755s–765s. [PubMed] [Google Scholar]
- Felten S. Y., Olschowka J. Noradrenergic sympathetic innervation of the spleen: II. Tyrosine hydroxylase (TH)-positive nerve terminals form synapticlike contacts on lymphocytes in the splenic white pulp. J Neurosci Res. 1987;18(1):37–48. doi: 10.1002/jnr.490180108. [DOI] [PubMed] [Google Scholar]
- Fillenz M. The innervation of the cat spleen. Proc R Soc Lond B Biol Sci. 1970 Jan 20;174(1037):459–468. doi: 10.1098/rspb.1970.0005. [DOI] [PubMed] [Google Scholar]
- Fried G., Terenius L., Brodin E., Efendic S., Dockray G., Fahrenkrug J., Goldstein M., Hökfelt T. Neuropeptide Y, enkephalin and noradrenaline coexist in sympathetic neurons innervating the bovine spleen. Biochemical and immunohistochemical evidence. Cell Tissue Res. 1986;243(3):495–508. doi: 10.1007/BF00218056. [DOI] [PubMed] [Google Scholar]
- Gulbenkian S., Wharton J., Polak J. M. The visualisation of cardiovascular innervation in the guinea pig using an antiserum to protein gene product 9.5 (PGP 9.5). J Auton Nerv Syst. 1987 Mar;18(3):235–247. doi: 10.1016/0165-1838(87)90122-6. [DOI] [PubMed] [Google Scholar]
- Heusermann U., Stutte H. J. Electron microscopic studies of the innervation of the human spleen. Cell Tissue Res. 1977 Oct 26;184(2):225–236. doi: 10.1007/BF00223070. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Bramich N. J., Edwards F. R., Klemm M. Transmission at autonomic neuroeffector junctions. Trends Neurosci. 1992 Feb;15(2):40–46. doi: 10.1016/0166-2236(92)90024-3. [DOI] [PubMed] [Google Scholar]
- Jackson P., Thompson R. J. The demonstration of new human brain-specific proteins by high-resolution two-dimensional polyacrylamide gel electrophoresis. J Neurol Sci. 1981 Mar;49(3):429–438. doi: 10.1016/0022-510x(81)90032-0. [DOI] [PubMed] [Google Scholar]
- Lorton D., Bellinger D. L., Felten S. Y., Felten D. L. Substance P innervation of spleen in rats: nerve fibers associate with lymphocytes and macrophages in specific compartments of the spleen. Brain Behav Immun. 1991 Mar;5(1):29–40. doi: 10.1016/0889-1591(91)90005-u. [DOI] [PubMed] [Google Scholar]
- Luff S. E., Hengstberger S. G., McLachlan E. M., Anderson W. P. Distribution of sympathetic neuroeffector junctions in the juxtaglomerular region of the rabbit kidney. J Auton Nerv Syst. 1992 Oct;40(3):239–253. doi: 10.1016/0165-1838(92)90206-v. [DOI] [PubMed] [Google Scholar]
- Luff S. E., McLachlan E. M. The form of sympathetic postganglionic axons at clustered neuromuscular junctions near branch points of arterioles in the submucosa of the guinea pig ileum. J Neurocytol. 1988 Aug;17(4):451–463. doi: 10.1007/BF01189802. [DOI] [PubMed] [Google Scholar]
- Lundberg J. M., Anggård A., Pernow J., Hökfelt T. Neuropeptide Y-, substance P- and VIP-immunoreactive nerves in cat spleen in relation to autonomic vascular and volume control. Cell Tissue Res. 1985;239(1):9–18. doi: 10.1007/BF00214896. [DOI] [PubMed] [Google Scholar]
- Lundberg L. M., Alm P., Wharton J., Polak J. M. Protein gene product 9.5 (PGP 9.5). A new neuronal marker visualizing the whole uterine innervation and pregnancy-induced and developmental changes in the guinea pig. Histochemistry. 1988;90(1):9–17. doi: 10.1007/BF00495700. [DOI] [PubMed] [Google Scholar]
- Meckler R. L., Weaver L. C. Comparison of the distributions of renal and splenic neurons in sympathetic ganglia. J Auton Nerv Syst. 1984 Sep;11(2):189–200. doi: 10.1016/0165-1838(84)90076-6. [DOI] [PubMed] [Google Scholar]
- Navone F., Jahn R., Di Gioia G., Stukenbrok H., Greengard P., De Camilli P. Protein p38: an integral membrane protein specific for small vesicles of neurons and neuroendocrine cells. J Cell Biol. 1986 Dec;103(6 Pt 1):2511–2527. doi: 10.1083/jcb.103.6.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice F. L., Kinnman E., Aldskogius H., Johansson O., Arvidsson J. The innervation of the mystacial pad of the rat as revealed by PGP 9.5 immunofluorescence. J Comp Neurol. 1993 Nov 15;337(3):366–385. doi: 10.1002/cne.903370303. [DOI] [PubMed] [Google Scholar]
- Romano T. A., Felten S. Y., Felten D. L., Olschowka J. A. Neuropeptide-Y innervation of the rat spleen: another potential immunomodulatory neuropeptide. Brain Behav Immun. 1991 Mar;5(1):116–131. doi: 10.1016/0889-1591(91)90011-x. [DOI] [PubMed] [Google Scholar]
- Saito H. Innervation of the guinea pig spleen studied by electron microscopy. Am J Anat. 1990 Nov;189(3):213–235. doi: 10.1002/aja.1001890305. [DOI] [PubMed] [Google Scholar]
- Weihe E., Nohr D., Michel S., Müller S., Zentel H. J., Fink T., Krekel J. Molecular anatomy of the neuro-immune connection. Int J Neurosci. 1991 Jul;59(1-3):1–23. doi: 10.3109/00207459108985446. [DOI] [PubMed] [Google Scholar]
- Wilkinson K. D., Lee K. M., Deshpande S., Duerksen-Hughes P., Boss J. M., Pohl J. The neuron-specific protein PGP 9.5 is a ubiquitin carboxyl-terminal hydrolase. Science. 1989 Nov 3;246(4930):670–673. doi: 10.1126/science.2530630. [DOI] [PubMed] [Google Scholar]