Abstract
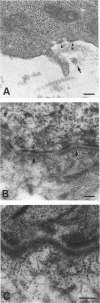
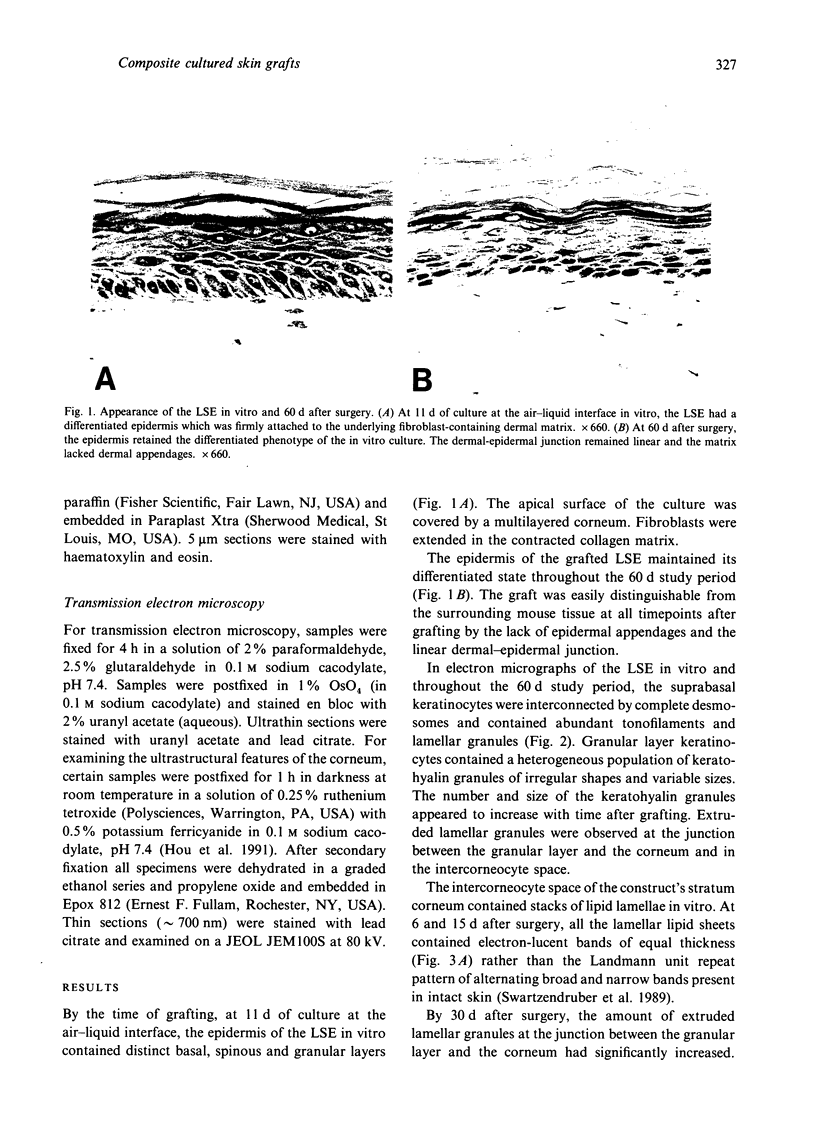
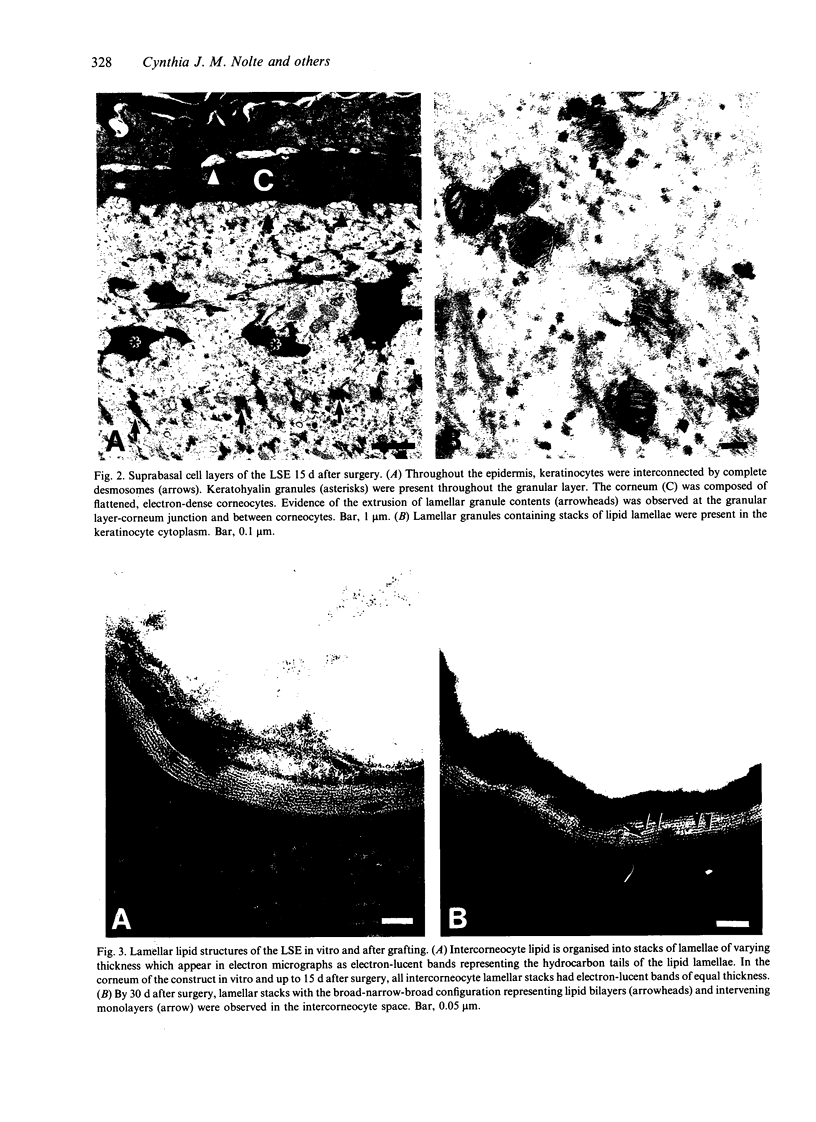
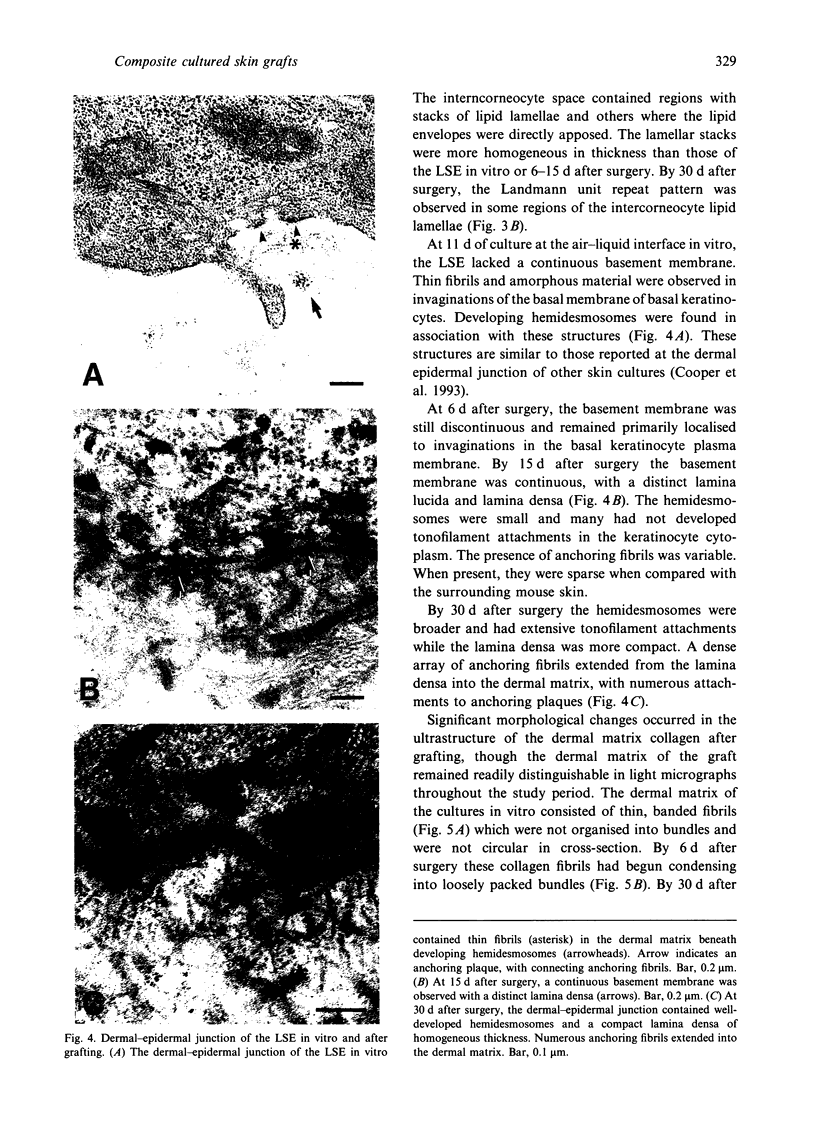
Skin substitutes composed of cultured keratinocytes with or without a dermal substrate are now being used in the treatment of burns and other cutaneous wounds. Composite skin cultures (Graftskin, LSE), consisting of epidermal keratinocytes seeded on a fibroblast-containing collagen matrix and maintained at the air-liquid interface, develop a well differentiated epidermis in vitro with many of the morphological and biochemical features of intact skin. Basement membrane-associated antigens, developing hemidesmosomes and short segments of lamina densa are present at the dermal-epidermal junction in vitro, although the LSE lacks a continuous basement membrane. As epidermal differentiation proceeds, the culture develops a stratum corneum composed of electron-dense corneocytes surrounded by extracellular lipid. However, the intercorneocyte lipid lamellae do not exhibit the repeating pattern of broad and narrow electron lucent bands observed in electron micrographs of the stratum corneum of intact skin. In this study, LSE were grafted onto full thickness wounds in athymic mice. Animals were killed 6, 15, 30 and 60 d after surgery for examination by light and electron microscopy to identify any ultrastructural changes which occurred in the culture in response to the host environment. The grafted LSE integrated well into the host tissue and remained intact throughout the 60 d study period. At the dermal-epidermal junction, a continuous basement membrane with a well defined lamina densa was established by 15 d after surgery. An extensive network of anchoring fibrils was present by 30 d after surgery. Collagen fibrils within the dermal matrix condensed by 6 d after surgery and began organising into loosely packed bundles by 15 d after surgery.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., REYNOLDS J. Transplantation studies on sheets of pure epidermal epithelium and on epidermal cell suspensions. Br J Plast Surg. 1952 Apr;5(1):25–36. doi: 10.1016/s0007-1226(52)80004-9. [DOI] [PubMed] [Google Scholar]
- Banks-Schlegel S., Green H. Formation of epidermis by serially cultivated human epidermal cells transplanted as an epithelium to athymic mice. Transplantation. 1980 Apr;29(4):308–313. doi: 10.1097/00007890-198004000-00010. [DOI] [PubMed] [Google Scholar]
- Bell E., Rosenberg M., Kemp P., Gay R., Green G. D., Muthukumaran N., Nolte C. Recipes for reconstituting skin. J Biomech Eng. 1991 May;113(2):113–119. doi: 10.1115/1.2891224. [DOI] [PubMed] [Google Scholar]
- Bell E., Sher S., Hull B., Merrill C., Rosen S., Chamson A., Asselineau D., Dubertret L., Coulomb B., Lapiere C. The reconstitution of living skin. J Invest Dermatol. 1983 Jul;81(1 Suppl):2s–10s. doi: 10.1111/1523-1747.ep12539993. [DOI] [PubMed] [Google Scholar]
- Boddé H. E., Holman B., Spies F., Weerheim A., Kempenaar J., Mommaas M., Ponec M. Freeze-fracture electron microscopy of in vitro reconstructed human epidermis. J Invest Dermatol. 1990 Jul;95(1):108–116. doi: 10.1111/1523-1747.ep12874082. [DOI] [PubMed] [Google Scholar]
- Bosca A. R., Tinois E., Faure M., Kanitakis J., Roche P., Thivolet J. Epithelial differentiation of human skin equivalents after grafting onto nude mice. J Invest Dermatol. 1988 Aug;91(2):136–141. doi: 10.1111/1523-1747.ep12464379. [DOI] [PubMed] [Google Scholar]
- Boyce S. T., Hansbrough J. F. Biologic attachment, growth, and differentiation of cultured human epidermal keratinocytes on a graftable collagen and chondroitin-6-sulfate substrate. Surgery. 1988 Apr;103(4):421–431. [PubMed] [Google Scholar]
- Carver N., Leigh I. M. Keratinocyte grafts and skin equivalents. Int J Dermatol. 1991 Aug;30(8):540–551. doi: 10.1111/j.1365-4362.1991.tb02636.x. [DOI] [PubMed] [Google Scholar]
- Compton C. C., Gill J. M., Bradford D. A., Regauer S., Gallico G. G., O'Connor N. E. Skin regenerated from cultured epithelial autografts on full-thickness burn wounds from 6 days to 5 years after grafting. A light, electron microscopic and immunohistochemical study. Lab Invest. 1989 May;60(5):600–612. [PubMed] [Google Scholar]
- Contard P., Bartel R. L., Jacobs L., 2nd, Perlish J. S., MacDonald E. D., 2nd, Handler L., Cone D., Fleischmajer R. Culturing keratinocytes and fibroblasts in a three-dimensional mesh results in epidermal differentiation and formation of a basal lamina-anchoring zone. J Invest Dermatol. 1993 Jan;100(1):35–39. doi: 10.1111/1523-1747.ep12349952. [DOI] [PubMed] [Google Scholar]
- Cooper M. L., Hansbrough J. F. Use of a composite skin graft composed of cultured human keratinocytes and fibroblasts and a collagen-GAG matrix to cover full-thickness wounds on athymic mice. Surgery. 1991 Feb;109(2):198–207. [PubMed] [Google Scholar]
- Cumpstone M. B., Kennedy A. H., Harmon C. S., Potts R. O. The water permeability of primary mouse keratinocyte cultures grown at the air-liquid interface. J Invest Dermatol. 1989 Apr;92(4):598–600. doi: 10.1111/1523-1747.ep12712043. [DOI] [PubMed] [Google Scholar]
- Demarchez M., Hartmann D. J., Regnier M., Asselineau D. The role of fibroblasts in dermal vascularization and remodeling of reconstructed human skin after transplantation onto the nude mouse. Transplantation. 1992 Aug;54(2):317–326. doi: 10.1097/00007890-199208000-00023. [DOI] [PubMed] [Google Scholar]
- Fartasch M., Bassukas I. D., Diepgen T. L. Structural relationship between epidermal lipid lamellae, lamellar bodies and desmosomes in human epidermis: an ultrastructural study. Br J Dermatol. 1993 Jan;128(1):1–9. doi: 10.1111/j.1365-2133.1993.tb00138.x. [DOI] [PubMed] [Google Scholar]
- Faure M., Mauduit G., Schmitt D., Kanitakis J., Demidem A., Thivolet J. Growth and differentiation of human epidermal cultures used as auto- and allografts in humans. Br J Dermatol. 1987 Feb;116(2):161–170. doi: 10.1111/j.1365-2133.1987.tb05807.x. [DOI] [PubMed] [Google Scholar]
- Gallico G. G., 3rd, O'Connor N. E., Compton C. C., Kehinde O., Green H. Permanent coverage of large burn wounds with autologous cultured human epithelium. N Engl J Med. 1984 Aug 16;311(7):448–451. doi: 10.1056/NEJM198408163110706. [DOI] [PubMed] [Google Scholar]
- Grafting of burns with cultured epithelium prepared from autologous epidermal cells. Lancet. 1981 Jan 10;1(8211):75–78. [PubMed] [Google Scholar]
- Hansbrough J. F., Boyce S. T., Cooper M. L., Foreman T. J. Burn wound closure with cultured autologous keratinocytes and fibroblasts attached to a collagen-glycosaminoglycan substrate. JAMA. 1989 Oct 20;262(15):2125–2130. [PubMed] [Google Scholar]
- Higounenc I., Démarchez M., Régnier M., Schmidt R., Ponec M., Shroot B. Improvement of epidermal differentiation and barrier function in reconstructed human skin after grafting onto athymic nude mice. Arch Dermatol Res. 1994;286(2):107–114. doi: 10.1007/BF00370736. [DOI] [PubMed] [Google Scholar]
- Hou S. Y., Mitra A. K., White S. H., Menon G. K., Ghadially R., Elias P. M. Membrane structures in normal and essential fatty acid-deficient stratum corneum: characterization by ruthenium tetroxide staining and x-ray diffraction. J Invest Dermatol. 1991 Feb;96(2):215–223. doi: 10.1111/1523-1747.ep12461361. [DOI] [PubMed] [Google Scholar]
- Johnson E. W., Meunier S. F., Roy C. J., Parenteau N. L. Serial cultivation of normal human keratinocytes: a defined system for studying the regulation of growth and differentiation. In Vitro Cell Dev Biol. 1992 Jun;28A(6):429–435. doi: 10.1007/BF02634047. [DOI] [PubMed] [Google Scholar]
- Kanitakis J., Mauduit G., Faure M., Schmitt D., Thivolet J. Ultrastructural studies of cultured human epithelial sheets used as skin allografts. Virchows Arch A Pathol Anat Histopathol. 1987;410(6):523–530. doi: 10.1007/BF00781688. [DOI] [PubMed] [Google Scholar]
- Langdon R. C., Cuono C. B., Birchall N., Madri J. A., Kuklinska E., McGuire J., Moellmann G. E. Reconstitution of structure and cell function in human skin grafts derived from cryopreserved allogeneic dermis and autologous cultured keratinocytes. J Invest Dermatol. 1988 Nov;91(5):478–485. doi: 10.1111/1523-1747.ep12476623. [DOI] [PubMed] [Google Scholar]
- Madden M. R., Finkelstein J. L., Staiano-Coico L., Goodwin C. W., Shires G. T., Nolan E. E., Hefton J. M. Grafting of cultured allogeneic epidermis on second- and third-degree burn wounds on 26 patients. J Trauma. 1986 Nov;26(11):955–962. doi: 10.1097/00005373-198611000-00001. [DOI] [PubMed] [Google Scholar]
- Madison K. C., Swartzendruber D. C., Wertz P. W., Downing D. T. Murine keratinocyte cultures grown at the air/medium interface synthesize stratum corneum lipids and "recycle" linoleate during differentiation. J Invest Dermatol. 1989 Jul;93(1):10–17. doi: 10.1111/1523-1747.ep12277335. [DOI] [PubMed] [Google Scholar]
- Mak V. H., Cumpstone M. B., Kennedy A. H., Harmon C. S., Guy R. H., Potts R. O. Barrier function of human keratinocyte cultures grown at the air-liquid interface. J Invest Dermatol. 1991 Mar;96(3):323–327. doi: 10.1111/1523-1747.ep12465212. [DOI] [PubMed] [Google Scholar]
- Mao-Qiang M., Feingold K. R., Elias P. M. Inhibition of cholesterol and sphingolipid synthesis causes paradoxical effects on permeability barrier homeostasis. J Invest Dermatol. 1993 Aug;101(2):185–190. doi: 10.1111/1523-1747.ep12363729. [DOI] [PubMed] [Google Scholar]
- Nanchahal J., Otto W. R., Dover R., Dhital S. K. Cultured composite skin grafts: biological skin equivalents permitting massive expansion. Lancet. 1989 Jul 22;2(8656):191–193. doi: 10.1016/s0140-6736(89)90374-7. [DOI] [PubMed] [Google Scholar]
- Parenteau N. L., Bilbo P., Nolte C. J., Mason V. S., Rosenberg M. The organotypic culture of human skin keratinocytes and fibroblasts to achieve form and function. Cytotechnology. 1992;9(1-3):163–171. doi: 10.1007/BF02521744. [DOI] [PubMed] [Google Scholar]
- Parenteau N. L., Nolte C. M., Bilbo P., Rosenberg M., Wilkins L. M., Johnson E. W., Watson S., Mason V. S., Bell E. Epidermis generated in vitro: practical considerations and applications. J Cell Biochem. 1991 Mar;45(3):245–251. doi: 10.1002/jcb.240450304. [DOI] [PubMed] [Google Scholar]
- Ponec M., Weerheim A., Kempenaar J., Mommaas A. M., Nugteren D. H. Lipid composition of cultured human keratinocytes in relation to their differentiation. J Lipid Res. 1988 Jul;29(7):949–961. [PubMed] [Google Scholar]
- Rygaard J. Skin grafts in nude mice. 3. Fate of grafts from man and donors of other taxonomic classes. Acta Pathol Microbiol Scand A. 1974 Jan;82(1):105–112. [PubMed] [Google Scholar]
- Régnier M., Caron D., Reichert U., Schaefer H. Reconstructed human epidermis: a model to study in vitro the barrier function of the skin. Skin Pharmacol. 1992;5(1):49–56. doi: 10.1159/000211017. [DOI] [PubMed] [Google Scholar]
- Swartzendruber D. C., Wertz P. W., Kitko D. J., Madison K. C., Downing D. T. Molecular models of the intercellular lipid lamellae in mammalian stratum corneum. J Invest Dermatol. 1989 Feb;92(2):251–257. doi: 10.1111/1523-1747.ep12276794. [DOI] [PubMed] [Google Scholar]
- Woodley D. T., Briggaman R. A., Herzog S. R., Meyers A. A., Peterson H. D., O'Keefe E. J. Characterization of "neo-dermis" formation beneath cultured human epidermal autografts transplanted on muscle fascia. J Invest Dermatol. 1990 Jul;95(1):20–26. doi: 10.1111/1523-1747.ep12872722. [DOI] [PubMed] [Google Scholar]