ABSTRACT
Background
Endogenous glucagon‐like peptide‐1 (GLP‐1) and glucose‐dependent insulinotropic polypeptide (GIP) regulate islet cell function. GLP‐1 receptor agonists (GLP‐1RAs) have been associated with an elevated risk of acute pancreatitis. Data on the pancreatic safety of tirzepatide (a dual GLP‐1 and GIP agonist) and its effects on islet cell function in randomized controlled trials (RCTs) are scarce. Moreover, no meta‐analysis has comprehensively examined such effects of tirzepatide.
Methods
Electronic databases were searched for RCTs with tirzepatide as the intervention and a placebo or active comparator as the control. The primary outcome was adjudication‐confirmed pancreatitis; secondary outcomes were the percent changes from baseline in serum pancreatic amylase, lipase, insulin, C‐peptide, glucagon, and homeostasis model assessment of insulin resistance (HOMA2‐IR).
Results
Seventeen RCTs with 18 published reports involving 14,645 subjects were analyzed. Over a follow‐up duration of 12–72 weeks, tirzepatide had identical risks of pancreatitis to placebo (tirzepatide 5 mg: RR 2.04, 95% CI [0.27–15.69], p = 0.49; 10 mg: RR 0.63, 95% CI [0.08–5.12], p = 0.67; and 15 mg: RR 1.26, 95% CI [0.36–4.98], p = 0.72). Tirzepatide was also associated with comparable risks of pancreatitis to insulin and GLP‐1RAs. However, tirzepatide (at all doses) caused greater increases in pancreatic amylase and lipase than placebo and insulin. Individuals on tirzepatide 15 mg and GLP‐1RAs had similar risks of having increased lipase levels. The percent reductions in fasting insulin were greater with tirzepatide 10 and 15 mg than with placebo. All doses of tirzepatide caused greater percent reductions in fasting insulin, C‐peptide, and glucagon than GLP‐1RAs. Compared to placebo and GLP‐1RAs, the percent reductions in HOMA2‐IR were greater with all doses of tirzepatide.
Conclusion
The meta‐analysis provides evidence of the safety of tirzepatide regarding pancreatitis and establishes its positive effect on islet cell functions and insulin resistance.
Keywords: insulin resistance, islet cell function, lipase, pancreatic amylase, pancreatitis, tirzepatide
1. Introduction
Tirzepatide, a 39‐amino‐acid synthetic peptide, has dual agonist activity at both the glucagon‐like peptide‐1 (GLP‐1) receptors and glucose‐dependent insulinotropic polypeptide (GIP) receptors, with a greater affinity for GIP receptors [1]. By binding to and activating their unique receptors in pancreatic β‐cells, endogenous GLP‐1 and GIP induce insulin secretion in a glucose‐dependent fashion. GLP‐1 and GIP also promote β‐cell proliferation with inhibition of cellular apoptosis, thus expanding β‐cell mass. Additionally, GIP augments postprandial glucagon secretion from α‐cells during euglycemic but not during hyperglycemic periods. In contrast, GLP‐1 tends to suppress glucagon secretion in hyperglycemic states, although it loses such an inhibitory effect at hypoglycemic levels [2]. In addition to pancreatic islet cells, GLP‐1 receptors are expressed in the exocrine duct cells of the pancreas. It is postulated that stimulating these receptors with incretin therapies may lead to the overgrowth of the cells covering the smaller ducts [3]. This results in hyperplasia and chronic low‐grade or acute inflammation, potentially causing acute pancreatitis [3]. Moreover, GLP‐1 inhibits pancreatic exocrine secretion, a putative mechanism associated with the pathogenesis of pancreatitis [4]. Exendin‐4 peptide, a stable analog of GLP‐1, regulates the pancreatic expression of the Reg gene family. Changes in the expression of RegIIIβ have been linked to varying effects on pancreatitis susceptibility and pancreatic necrosis in vivo [5]. However, current evidence does not suggest a direct role for GIP receptor activation in causing pancreatitis [2]. Early clinical trials evaluating the efficacy and safety of GLP‐1RAs reported heightened risks of developing acute pancreatitis with GLP‐1RAs compared with placebo or active comparators [6]. Subsequently, post‐marketing and observational reports using the FDA database raised concerns about the risk of developing acute pancreatitis, particularly recurrent acute pancreatitis, with GLP‐1RAs [7].
Tirzepatide once‐weekly subcutaneous injection has been approved for use in adults by the FDA for glycemic management with type 2 diabetes (T2D) and for weight reduction in obesity or overweight [8, 9]. Tirzepatide has been proven in randomized‐controlled trials (RCTs) to be effective in reducing glycemic hemoglobin (HbA1c) in T2D and substantially reducing weight in patients with obesity with/without T2D [10, 11]. As tirzepatide activates both GLP‐1 and GIP receptors, the drug may be associated with pancreatitis, at least theoretically. However, in a meta‐analysis by Zeng et al. that included nine RCTs, the increased risk of pancreatitis was not significantly associated with tirzepatide compared with all control groups consisting of basal insulin, selective GLP1‐RAs, and placebo [12].
Exploring the effects of the therapeutic doses of tirzepatide on islet cell function and glucose homeostasis may help better understand its glycemic and weight‐lowering capacity. Although a post hoc exploratory study of biomarkers linked with pancreatic beta‐cell function and insulin sensitivity in a Phase 2 trial has been published, no RCTs conducted, especially for such effects of tirzepatide, are yet available [13]. Moreover, no systematic review and meta‐analysis (SRM) exist in its effects on islet cell function. Additionally, Zeng et al.’s SRM on the risk of pancreatitis with tirzepatide, the most important pancreatic safety concern, did not include recently published RCTs [8]. Hence, it was imperative to conduct an updated SRM, including all relevant RCTs of tirzepatide, reporting the pancreatic safety of tirzepatide and its effects on islet cell function to inform better clinical practice decisions by healthcare professionals.
2. Methods
2.1. Search Strategy
Several databases and registers, including MEDLINE (via PubMed), Scopus, Cochrane Central Register, and ClinicalTrials.gov, were systematically searched. The search covered these sources from their commencement to June 30, 2024. The search terms were applied to titles only; the search technique followed a Boolean approach using the terms “tirzepatide” OR “LY34 37943.” Every recently published or unpublished clinical study in English was searched exhaustively and carefully. This search involved looking through pertinent publications and references found in the clinical trials included in the present work.
2.2. Study Selection
Population, Intervention, Comparison, Outcomes and Study (PICOS) design was used as a framework to formulate eligibility criteria for the clinical trials in this SRM. The patient population (P) consisted of study subjects undergoing a therapeutic trial of tirzepatide for any clinical indication; the intervention (I) was tirzepatide once‐weekly subcutaneous injections at any dose; the comparison or control (C) included individuals receiving a placebo or active comparator; the outcomes (O) included tirzepatide's pancreatic safety and its effects on islet cell functions; and the randomized controlled trials (RCTs) were considered as the study type (S) for inclusion. This study comprised RCTs spanning a minimum 12‐week duration with study individuals aged at least 18. The trials had at least two treatment arms/groups, with one receiving tirzepatide as monotherapy or as an add‐on to other drugs and the other receiving a placebo or any other active comparator either alone or as an add‐on to other medications. Excluded from consideration were nonrandomized trials, retrospective studies, pooled analyses of clinical trials, conference proceedings, letters to editors, case reports, and articles that did not provide data on outcomes of interest. Also excluded were clinical trials involving animals or healthy humans, as well as randomized controlled trials (RCTs) with a duration of less than 12 weeks.
2.3. Outcomes Analyzed
The primary outcome of interest was the percentage of participants in the tirzepatide group and the control group who were confirmed to have pancreatitis through adjudication. Additional outcomes included the percent changes from the initial values (CFB) in pancreatic amylase, lipase, fasting insulin, and homeostasis model assessment of insulin resistance (HOMA2‐IR). Subgroup analyses were conducted according to the type of comparison groups and the tirzepatide dose.
2.4. Data Handling and Risk of Bias Assessment
Data extraction was independently conducted by four authors using standardized forms, with additional information available elsewhere [14]. The method for handling missing data has also been expanded upon in the same source [14]. The risk of bias (RoB) assessment was independently carried out by four authors using the Cochrane risk‐of‐bias tool for randomized trials, version 2 (RoB 2) in the Review Manager (RevMan) computer program, version 7.2.0 [15, 16]. The specific and overall risks of biases have been outlined in the same source [14]. Publication bias was assessed using funnel plots in the same software, with a minimum of 10 studies included in a forest plot [16, 17, 18].
2.5. Statistical Analysis
The results of the outcomes were reported using standardized mean differences (SMDs) for continuous variables and risk ratios (RRs) for dichotomous variables, together with 95% confidence intervals (CIs). The RevMan‐generated forest plots portrayed the SMD or RR for the outcomes; the left side of the forest plot favored tirzepatide, and the right side favored the control group(s) [16]. Random effects analysis models were chosen to address the anticipated heterogeneity resulting from variations in population characteristics and trial lengths. The inverse variance statistical method was applied for all instances. The SRM encompassed forest plots that integrated data from a minimum of two trials. A significance level of p < 0.05 was used.
2.6. Assessment of Heterogeneity
The assessment of heterogeneity was initially conducted by studying forest plots. Subsequently, a Chi2 test was performed using N‐1 degrees of freedom and a significance level of 0.05 to determine the statistical significance. The I 2 test was also employed in the subsequent analysis [19]. The specifics of understanding I 2 values have already been explained in depth elsewhere [14].
2.7. Grading of the Results
The Grading of Recommendations Assessment, Development and Evaluation (GRADE) methodology was used to determine the quality of evidence about each meta‐analysis outcome [20]. The details of generating the summary of findings (SoF) table and judging the quality of evidence as “high,” “moderate,” “low,” or “very low” have been previously reported [14].
2.8. Ethical Statement
This SRM followed the guidelines outlined in the Cochrane Handbook for Systematic Reviews of Interventions [21] and is reported in compliance with the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses (PRISMA) checklists [22]. The SRM was registered with PROSPERO (CRD42024568848), and the protocol summary is accessible online.
3. Results
3.1. Search Results
The steps of selecting studies are depicted in Supporting Information S1: Figure S1. The initial search identified 1092 articles; the number was narrowed to 30 after screening titles and abstracts and a subsequent full‐text review. Finally, 17 RCTs with 18 published reports involving 14,645 subjects meeting all the prespecified criteria were included in this SRM [23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39]. The trial NCT03131687 had two reports, both included in this SRM [13, 25]. Twelve studies were excluded; eight were substudies or post hoc analyses of an included trial [40, 41, 42, 43, 44, 45, 46, 47], and the other four did not report the outcomes of interest [48, 49, 50, 51].
3.2. Characteristics of Included Studies
Out of the 17 RCTs included in this SRM, one was a phase 1 [24], three were phase 2 [13, 23, 25, 39], and the other 13 were phase 3 trials [26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38]. Twelve trials included individuals with T2D [23, 24, 25, 27, 31, 32, 33, 34, 35, 36, 37, 38], four included obese/overweight subjects without diabetes [26, 28, 29, 30], and one included those with biopsy‐confirmed metabolic dysfunction associated steatohepatitis (MASH) and stage F2 or F3 fibrosis, with or without diabetes [39], as the study population. Ten RCTs used matching placebos [23, 24, 26, 27, 28, 29, 30, 31, 35, 39], four used insulin [33, 34, 36, 37], two used GLP‐1 receptor agonists (GLP‐1RA) [32, 38], and two trials used both placebo and GLP‐1RA in the control groups [13, 24, 25]. Insulin degludec was used in one trial [33], glargine in two trials [34, 37], and lispro was used in one trial as an active comparator [36]. Dulaglutide was used in two trials [13, 25, 38], and semaglutide was used in two trials in the control group [24, 32]. Most of the RCTs had three tirzepatide (5, 10, and 15 mg) [26, 31, 32, 33, 34, 35, 36, 37, 38, 39], one had an additional arm of 1 mg [13, 25], two had two arms of 10 and 15 mg [27, 29], one had a single arm of 15 mg [24], and one trial had only single tirzepatide arm of maximum tolerated dose (MTD, 10 or 15 mg) [28]. Frias 2020 had one tirzepatide arm of 12 mg (which was analyzed as tirzepatide 10 mg arm) and two arms of tirzepatide 15 mg with different dose‐escalation patterns (outcome results were polled to analyze in a single tirzepatide 15 mg arm) [23]. SURMOUNT‐OSA had two different trial populations, each with tirzepatide MTD (10 or 15 mg) and placebo arms; outcome results of tirzepatide MTD and placebo groups in Trials 1 and 2 were pooled into single groups of tirzepatide MTD and placebo [30]. All tirzepatide MTD arms were analyzed as tirzepatide 15 mg. One trial had a 12‐week duration [23], one had a 26‐week duration [13, 25], one had a 28‐week duration [24], four had 40‐week durations [31, 32, 35, 37], seven had 52‐week durations [29, 30, 33, 34, 36, 38, 39], and the other three spanned 72 weeks [26, 27, 28]. The baseline characteristics of the included RCTs were matched throughout the trial arms. The specifics of the included and excluded studies are shown in Supporting Information S1: Tables S1 and S2, respectively.
3.3. Risk of Bias in the Included Studies
Supporting Information S1: Figure S2 illustrates the specific and overall RoB in the 17 included RCTs. The overall RoB was low in most (67.7%) trials. One study (SURMOUNT‐3) had “some concerns” about attrition bias resulting from missing outcome data. Five (29.4%) studies had high risks for overall bias, which reflected the bias due to deviations from intended interventions. Publication bias was evaluated using funnel plots, which are presented in Supporting Information S1: Figure S3.
3.4. Grading of the Results
The SoF table (Supporting Information S1: Table S3) presents the grades for the certainty of the evidence supporting the primary outcome of this SRM.
3.5. Primary Outcome: Risk of Pancreatitis (Adjudication‐Confirmed)
The risks of pancreatitis were similar among the study subjects receiving any dose of tirzepatide and placebo (for tirzepatide 5 mg: RR 2.04, 95% CI [0.27–15.69], I 2 = 0%, p = 0.49, high certainty of the evidence; 10 mg: RR 0.63, 95% CI [0.08–5.12], I 2 = 0%, p = 0.67, high certainty of the evidence; and 15 mg: RR 1.26, 95% CI [0.36–4.98], I 2 = 0%, p = 0.72, moderate certainty of the evidence) (Figure 1). Such risks were also similar with all doses of tirzepatide versus insulin (for tirzepatide 5 mg: RR 2.08, 95% CI [0.08–54.32], I 2 = 65%, p = 0.66; 10 mg: RR 1.71, 95% CI [0.10–29.71], I 2 = 52%, p = 0.71; and 15 mg: RR 1.13, 95% CI [0.13–9.79], I 2 = 6%, p = 0.91) (Figure 2A), and GLP‐1RA (for tirzepatide 5 mg: RR 0.83, 95% CI [0.03–26.54], I 2 = 63%, p = 0.91; 10 mg: RR 0.67, 95% CI [0.11–3.97], I 2 = NA, p = 0.66; and 15 mg: RR 0.95, 95% CI [0.20–4.91], I 2 = 0%, p = 0.95) (Figure 2B).
FIGURE 1.
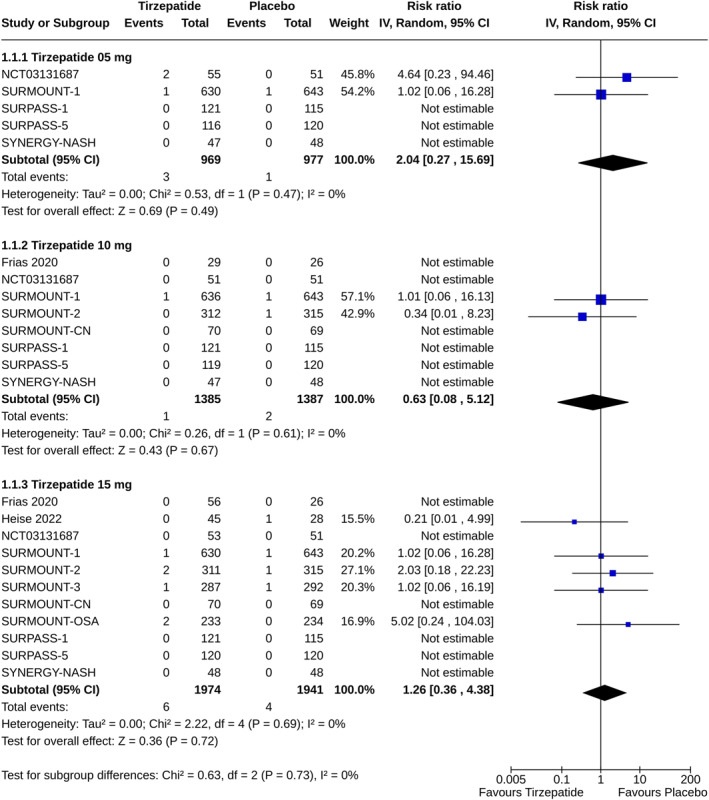
Forest plot highlighting the proportions of study subjects with pancreatitis: tirzepatide versus placebo.
FIGURE 2.
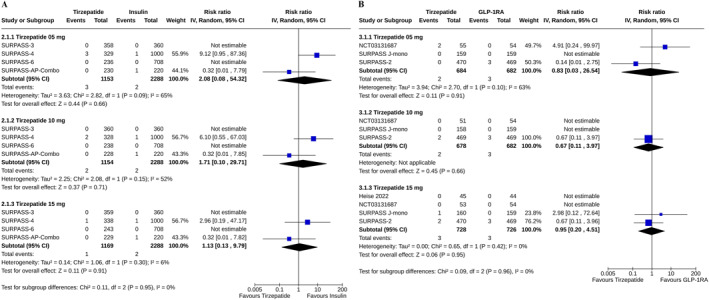
Forest plot highlighting the proportions of study subjects with pancreatitis (A) tirzepatide versus insulin, (B) tirzepatide versus GLP‐1RA.
3.6. Pancreatic Amylase and Lipase
Greater rises (measured as percent CFB) in serum pancreatic amylase were found in all groups of tirzepatide than in the placebo (for tirzepatide 5 mg: SMD 19.58%, 95% CI [13.03–26.12], I 2 = 60%, p < 0.00001, moderate certainty of the evidence; 10 mg: SMD 23.16%, 95% CI [17.13–29.19], I 2 = 68%, p < 0.00001, moderate certainty of the evidence; and 15 mg: SMD 23.69%, 95% CI [18.78–28.61], I 2 = 65%, p < 0.00001, moderate certainty of the evidence) (Figure 3A), and insulin (for tirzepatide 5 mg: SMD 12.99%, 95% CI [2.35–23.63], I 2 = 89%, p = 0.02; 10 mg: SMD 16.00%, 95% CI [1.02–30.98], I 2 = 94%, p = 0.04; and 15 mg: SMD 17.42%, 95% CI [1.85–32.99], I 2 = 95%, p = 0.03) (Figure 3B) groups.
FIGURE 3.
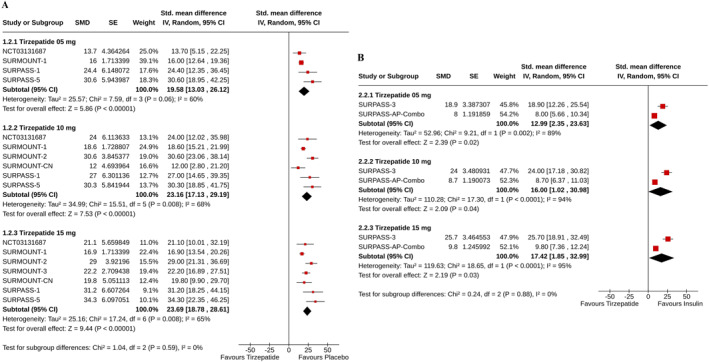
Forest plot highlighting the percent changes from baseline in pancreatic amylase: (A) tirzepatide versus Placebo, (B) tirzepatide versus insulin.
Tirzepatide groups also demonstrated higher increments in serum lipase than the placebo (for tirzepatide 5 mg: SMD 25.46%, 95% CI [19.49–31.43], I 2 = 13%, p < 0.00001, high certainty of the evidence; 10 mg: SMD 27.99%, 95% CI [23.91–32.07], I 2 = 2%, p < 0.00001, high certainty of the evidence; and 15 mg: SMD 32.58%, 95% CI [27.17–38.00], I 2 = 41%, p < 0.00001, high certainty of the evidence) (Figure 4A), and insulin (for tirzepatide 5 mg: SMD 18.68%, 95% CI [2.08–35.28], I 2 = 93%, p = 0.03; 10 mg: SMD 21.05%, 95% CI [6.24–35.86], I 2 = 91%, p = 0.005; and 15 mg: SMD 25.20%, 95% CI [7.48–42.91], I 2 = 92%, p = 0.005) (Figure 4B) groups. The risks of the study subjects to have an increased lipase with tirzepatide 15 mg and GLP‐1RA were identical (RR 1.14, 95% CI [0.24–5.28], I 2 = 44%, p = 0.87) (Figure 4C).
FIGURE 4.
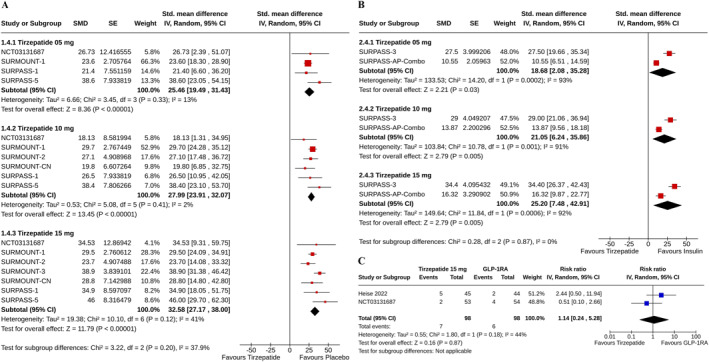
Forest plot highlighting the (A) percent changes from baseline in lipase: tirzepatide versus placebo, (B) percent changes from baseline in lipase: tirzepatide versus insulin, (C) proportions of study subjects with increased lipase: tirzepatide versus GLP‐1RA.
3.7. Fasting Insulin
The percent reductions in fasting serum insulin were greater with tirzepatide 10 mg (SMD −27.76%, 95% CI [−45.88–9.64], I 2 = 89%, p = 0.003, low certainty of the evidence), and 15 mg (SMD −37.0%, 95% CI [−44.32–29.69], I 2 = 73%, p < 0.00001, moderate certainty of the evidence) than with placebo. The CFB in fasting insulin was similar in tirzepatide 5 mg and placebo groups (SMD −22.39%, 95% CI [−49.55–4.77], I 2 = 67%, p = 0.11, moderate certainty of the evidence) (Figure 5A). Compared to GLP‐1RA, the percent reductions in fasting insulin were greater with all does of tirzepatide (for tirzepatide 5 mg: SMD −22.45%, 95% CI [−31.53–13.38], I 2 = 0%, p < 0.00001; for 10 mg: SMD −30.62%, 95% CI [−39.01–22.24], I 2 = 0%, p < 0.00001; and for 15 mg: SMD −22.6%, 95% CI [−41.39–3.81], I 2 = 77%, p = 0.02) (Figure 5B).
FIGURE 5.
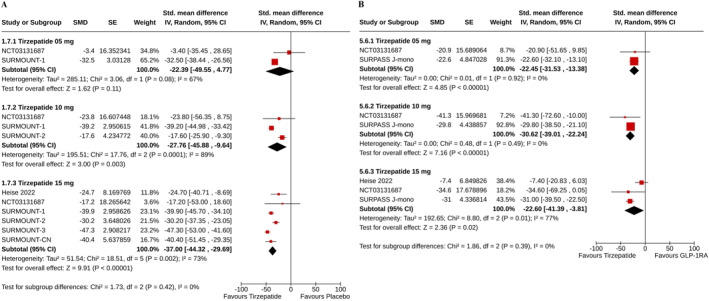
Forest plot highlighting the changes from baseline in fasting insulin: (A) tirzepatide versus placebo, (B) tirzepatide versus GLP‐1RA.
3.8. Fasting C‐Peptide
Compared to GLP‐1RA, the percent reductions in fasting C‐peptide were greater with all does of tirzepatide (for tirzepatide 5 mg: SMD −14.07%, 95% CI [−20.43–7.72], I 2 = 0%, p < 0.0001; for 10 mg: SMD −21.98%, 95% CI [−27.88–16.09], I 2 = 0%, p < 0.00001; and for 15 mg: SMD −15.83%, 95% CI [−30.32 to −1.33], I 2 = 53%, p = 0.03) (Figure 6A).
FIGURE 6.
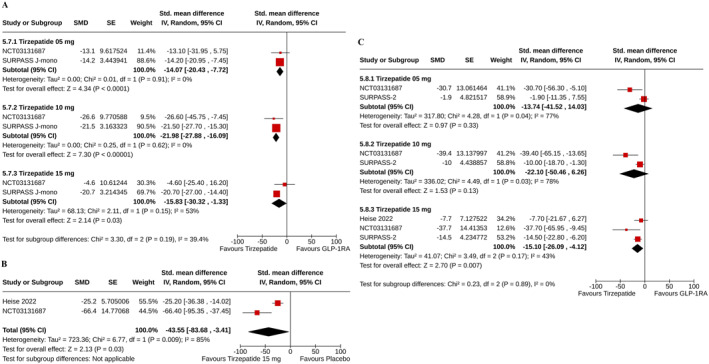
Forest plot highlighting the changes from baseline in (A) fasting C‐peptide in tirzepatide versus GLP‐1RA, (B) fasting glucagon in tirzepatide versus placebo, (C) fasting glucagon in tirzepatide versus GLP‐1RA.
3.9. Fasting Glucagon
Compared with placebo, the percent reduction in fasting glucagon was greater with tirzepatide 15 mg (SMD −43.55%, 95% CI [−83.68 to −3.41], I 2 = 85%, p = 0.03) (Figure 6B). The reductions in glucagon were comparable between tirzepatide 5 mg (SMD −13.74%, 95% CI [−41.52–14.03], I 2 = 77%, p = 0.33) and 10 mg (SMD −22.10%, 95% CI [−50.46–6.26], I 2 = 78%, p = 0.13) groups with GLP‐1RA group; such reduction was greater with tirzepatide 15 mg than GLP‐1RA (SMD −15.10%, 95% CI [−26.09–4.12], I 2 = 43%, p = 0.007) (Figure 6C).
3.10. HOMA2‐IR
The percent reductions in HOMA2‐IR were greater with all doses of tirzepatide than with placebo (for tirzepatide 5 mg: SMD −16.70%, 95% CI [−31.78–1.61], I 2 = 15%, p = 0.03; 10 mg: SMD −20.21%, 95% CI [−32.14–8.29], I 2 = 0%, p = 0.0009; and 15 mg: SMD −31.81%, 95% CI [−42.47–21.15], I 2 = 0%, p < 0.00001) (Supporting Information S1: Figure S4A), and GLP‐1RA (for tirzepatide 5 mg: SMD −11.32%, 95% CI [−17.92–4.72], I 2 = 0%, p = 0.0008; 10 mg: SMD −20.92%, 95% CI [−40.29–1.55], I 2 = 46%, p = 0.03; and 15 mg: SMD −20.34%, 95% CI [−26.35–14.33], I 2 = 0%, p < 0.00001) (Supporting Information S1: Figure S4B).
4. Discussion
The current SRM summarized the effects of tirzepatide on the pancreas based on the results of 17 RCTs with tirzepatide in individuals with overweight/obesity with and without T2D. Tirzepatide was compared against a placebo or different active comparators, like insulin or GLP‐1RAs, in the included RCTs. Although greater rises in pancreatic amylase and lipase were observed in the tirzepatide group, the risk of pancreatitis with tirzepatide was identical to the control group. Improvements in islet cell functions and insulin resistance were also more robust with tirzepatide.
It was reassuring to note that tirzepatide use was not associated with a statistically significant excess risk of pancreatitis compared with placebo, GLP‐1RA, or insulin. Zeng et al., in their meta‐analysis, also found that tirzepatide use was not associated with an increased risk for clinically, biochemically, and imaging‐confirmed pancreatitis [12]. Although clinically evident pancreatitis risk was similar, greater increases in pancreatic enzymes like amylase and lipase levels were found with all doses of tirzepatide compared with placebo or insulin. A single study (SURPASS J‐mono) reported greater rises in pancreatic amylase with all doses of tirzepatide than GLP‐1RA. However, such rises in lipase levels were only observed with higher doses of tirzepatide (10 and 15 mg) [38]. However, none of the study participants had markedly increased (≥ 3 times the upper limit of normal range) pancreatic amylase levels in either the tirzepatide (all doses) or the GLP‐1RA group in another study, NCT03131687 [13, 25]. In the same study, identical proportions of participants had increased lipase levels in the tirzepatide 5 and 10 mg and the GLP‐1RA group [13, 25]. In this meta‐analysis, patients receiving tirzepatide 15 mg and GLP‐1RA had a comparable risk of an increase in lipase. Thus, while tirzepatide offers a greater reduction in body weight and metabolic parameters than GLP‐1RAs, current data suggest that the agent is as safe as GLP1‐RAs in terms of pancreatic safety. GLP‐1RAs are known to directly increase pancreatic enzyme secretion by acting on the GLP‐1 receptors expressed on pancreatic acinar cells, and there is an elevation of circulating pancreatic enzymes in some people treated with GLP‐1RA [52]. Elevated levels of amylase and lipase may also be seen in T2D, even without clinically overt pancreatitis [53]. However, the initial concerns about a possible link between GLP‐1RA use and the development of pancreatitis or pancreatic cancer have not been substantiated in RCTs and real‐world data [52, 54, 55]. Different dosages of tirzepatide have been studied in the RCTs included in our meta‐analysis, and there were no significant differences in the rates of pancreatitis between them. Whether or not the degree of pancreatic enzyme elevation seen with tirzepatide is clinically relevant remains unknown.
Obesity is the key driver in the pathogenesis of T2D. Current guidelines recommend 5–15% weight loss in individuals with obesity to improve glycemic and metabolic parameters and prevent complications [56]. The obesity‐centric approach to managing T2D has led to the popularity of the GLP‐1RAs and subsequently the “dual” or “triple” agonists, which work on other receptors besides GLP‐1 receptors. The glucose‐lowering efficacy of tirzepatide results from concurrent improvements in multiple key components of the pathophysiology of T2D, including β‐cell function, insulin sensitivity, and glucagon secretion [57]. We found that tirzepatide reduced fasting insulin and HOMA‐IR significantly more than placebo and GLP‐1RA, indicating its favorable effect on insulin resistance. The reduction in fasting C‐peptide level was also greater with tirzepatide than GLP‐1RA. In addition, there were more reductions in fasting glucagon levels with tirzepatide than with placebo and GLP‐1RA. The incretin hormones GLP‐1 and GIP are known to increase glucose‐stimulated insulin secretion and increase beta cell proliferation and regeneration while reducing their apoptosis. By inducing significant weight loss, they can also improve insulin sensitivity. GLP‐1 has additional effects such as increased lipolysis and increased satiety. While GLP‐1 and GIP both increase insulin levels, the effects on glucagon are contradictory since GLP‐1 decreases glucagon, but GIP can increase its levels [58]. In non‐diabetics, GIP stimulates insulin secretion but does not alter glucagon release during hyperglycemia, whereas during hypoglycemia, it increases glucagon release without affecting insulin secretion [59]. Notably, in subjects with T2D, there was increased glucagon secretion in response to GIP, even during hyperglycemia. In preclinical studies, tirzepatide has significantly improved β‐cell function and insulin sensitivity and reduced glucagon secretion in people with T2D [24]. In vitro studies have shown that the two incretins, GIP and GLP‐1, administered together, can exhibit synergistic action on both insulin synthesis and secretion and also on the genes associated with β‐cell differentiation and survival [60]. However, the insulin‐sensitizing effects of tirzepatide were not explained by the greater weight loss alone, suggesting additional effects mediated via dual receptor agonism [13].
Several studies have substantiated the greater weight loss and cardiometabolic benefits of tirzepatide compared with GLP‐1RAs. One proposed hypothesis behind this is that there is facilitation of the effects of GIP on re‐sensitized β‐cells. However, similar results have not been seen with other GLP‐1/GIP dual agonists that have not produced the same results as tirzepatide [61]. Tirzepatide has a higher potency at the GIP than at the GLP‐1 receptor. Additionally, it has unique post‐receptor signaling properties at the GLP‐1 receptor with a bias toward cyclic AMP production than β‐arrestin recruitment. This is evident from in vitro experiments with islet cells, which revealed that β‐arrestin1 limits the insulin response to GLP‐1 but not to tirzepatide [62]. This, in turn, leads to reduced GLP‐1 receptor internalization and enhancement of insulin secretion.
To our knowledge, this is the first SRM to study the pancreatic safety of tirzepatide and its effects on islet cell function. While there have been prior meta‐analyses on the therapeutic efficacy or the overall safety profile of tirzepatide, we focused on its pancreatic effects. Meta‐analyses of their safety profile concentrated mostly on adverse gastrointestinal effects and hypoglycemia [63, 64]. We included data on adjudication‐confirmed pancreatitis events and elevation of pancreatic enzymes to provide more specific information limited to pancreatic adverse effects. One important limitation of our analysis was the small number of RCTs regarding the changes in C‐peptide and glucagon levels, which could be a key player in explaining the differences in efficacy between tirzepatide and GLP‐1RA. Due to a very small number of events, pancreatic cancers were not included as an outcome in our meta‐analysis. The included RCTs were not designed to evaluate the safety profile of tirzepatide, particularly concerning amylase and lipase elevation as the primary outcomes. Also, the follow‐up time for patients in the RCTs treated with tirzepatide was relatively short, and therefore, longer‐term follow‐up is required for more robust evidence in favor of its safety and reassurance regarding the sustainability of improvement in insulin resistance.
5. Conclusion
This SRM suggests that the dual GLP‐1/GIP receptor agonist tirzepatide improves beta cell functioning and insulin sensitivity while having a good safety profile for pancreatic adverse events. While elevation of pancreatic enzymes could be seen, its clinical significance is unclear, and clinically manifested pancreatitis is rare. Further longer‐term and larger studies are required to make clearer recommendations regarding routine monitoring of pancreatic enzymes after initiation or dose up‐titration of tirzepatide, as well as studies comparing the effects of tirzepatide to available GLP‐1RAs on the pancreatic islet cell hormones.
Author Contributions
A. B. M. Kamrul‐Hasan and Joseph M. Pappachan conceptualized the meta‐analysis. The literature search was done by Sunetra Mondal, Deep Dutta, Lakshmi Nagendra, and Mohammed Ruhul Kabir. A. B. M. Kamrul‐Hasan and Joseph M. Pappachan did detailed reviews of the articles. Data was entered by A. B. M. Kamrul‐Hasan, Deep Dutta, and Mohammed Ruhul Kabir. A. B. M. Kamrul‐Hasan, Sunetra Mondal, and Lakshmi Nagendra did the statistical analysis. All authors contributed equally to the manuscript preparation and approval for submission. The manuscript has been read and approved by all the authors for submission to this journal for consideration for publication.
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting information
Supporting Information S1
Acknowledgments
The authors have nothing to report.
Funding: The authors received no specific funding for this work.
References
- 1. Coskun T., Sloop K. W., Loghin C., et al., “LY3298176, a Novel Dual GIP and GLP‐1 Receptor Agonist for the Treatment of Type 2 Diabetes Mellitus: From Discovery to Clinical Proof of Concept,” Molecular Metabolism 18 (2018): 3–14, 10.1016/j.molmet.2018.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Seino Y., Fukushima M., and Yabe D., “GIP, and GLP‐1, the Two Incretin Hormones: Similarities and Differences,” Journal of Diabetes Investigation 1, no. 1–2 (2010): 8–23, 10.1111/j.2040-1124.2010.00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gier B., Matveyenko A. V., Kirakossian D., Dawson D., Dry S. M., and Butler P. C., “Chronic GLP‐1 Receptor Activation by Exendin‐4 Induces Expansion of Pancreatic Duct Glands in Rats and Accelerates Formation of Dysplastic Lesions and Chronic Pancreatitis in the Kras(G12D) Mouse Model,” Diabetes 61, no. 5 (2012): 1250–1262, 10.2337/db11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wettergren A., Wøjdemann M., and Holst J. J., “Glucagon‐Like Peptide‐1 Inhibits Gastropancreatic Function by Inhibiting Central Parasympathetic Outflow,” American Journal of Physiology 275, no. 5 (1998): G984–G992, 10.1152/ajpgi.1998.275.5.G984. [DOI] [PubMed] [Google Scholar]
- 5. Koehler J. A., Baggio L. L., Lamont B. J., Ali S., and Drucker D. J., “Glucagon‐Like Peptide‐1 Receptor Activation Modulates Pancreatitis‐Associated Gene Expression But Does Not Modify the Susceptibility to Experimental Pancreatitis in Mice,” Diabetes 58, no. 9 (2009): 2148–2161, 10.2337/db09-0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Saisho Y., “Incretin‐Based Therapy and Pancreatitis: Accumulating Evidence and Unresolved Questions,” Annals of Translational Medicine 6, no. 7 (2018): 131, 10.21037/atm.2018.02.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Liu L., Chen J., Wang L., Chen C., and Chen L., “Association Between Different GLP‐1 Receptor Agonists and Gastrointestinal Adverse Reactions: A Real‐World Disproportionality Study Based on FDA Adverse Event Reporting System Database,” Frontiers in Endocrinology 13 (2022): 1043789, 10.3389/fendo.2022.1043789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. New Drug Therapy Approvals , Advancing Health through Innovation, United States Food and Drug Administration; (2022), https://www.fda.gov/drugs/novel‐drug‐approvals‐fda/new‐drug‐therapy‐approvals‐2022. [Google Scholar]
- 9. FDA News Release , FDA Approves New Medication for Chronic Weight Management, United States Food and Drug Administration; (2024), https://www.fda.gov/news‐events/press‐announcements/fda‐approves‐new‐medication‐chronic‐weight‐management. [Google Scholar]
- 10. Permana H., Yanto T. A., and Hariyanto T. I., “Efficacy and Safety of Tirzepatide as Novel Treatment for Type 2 Diabetes: A Systematic Review and Meta‐Analysis of Randomized Clinical Trials,” Diabetes & Metabolic Syndrome 16, no. 11 (2022): 102640, 10.1016/j.dsx.2022.102640. [DOI] [PubMed] [Google Scholar]
- 11. Dutta D., Kamrul‐Hasan A. B. M., Nagendra L., and Bhattacharya S., “Efficacy and Safety of Novel Twincretin Tirzepatide, a Dual GIP/GLP‐1 Receptor Agonist, as an Anti‐Obesity Medicine in Individuals Without Diabetes: A Systematic Review and Meta‐Analysis,” touchREV Endocrinology 20, no. 2 (2024): 72–80, 10.17925/EE.2024.20.2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zeng Q., Xu J., Mu X., Shi Y., Fan H., and Li S., “Safety Issues of Tirzepatide (Pancreatitis and Gallbladder or Biliary Disease) in Type 2 Diabetes and Obesity: A Systematic Review and Meta‐Analysis,” Frontiers in Endocrinology 14 (2023): 1214334, 10.3389/fendo.2023.1214334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Thomas M. K., Nikooienejad A., Bray R., et al., “Dual GIP and GLP‐1 Receptor Agonist Tirzepatide Improves Beta‐Cell Function and Insulin Sensitivity in Type 2 Diabetes,” Journal of Clinical Endocrinology and Metabolism 106, no. 2 (2021): 388–396, 10.1210/clinem/dgaa863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamrul‐Hasan A. B. M., Alam M. S., Talukder S. K., Dutta D., and Selim S., “Efficacy and Safety of Omarigliptin, a Novel Once‐Weekly Dipeptidyl Peptidase‐4 Inhibitor, in Type 2 Diabetes Mellitus: A Systematic Review and Meta‐Analysis,” Endocrinology and Metabolism Seoul Korea 39, no. 1 (2024): 109–126, 10.3803/EnM.2023.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Higgins J. P. T., Savović J., Page M. J., Elbers R. G., and Sterne J. A. C., “Chapter 8: Assessing Risk of Bias in a Randomized Trial,” in Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023), eds. Higgins J. P. T., Thomas J., Chandler J., et al. (Cochrane, 2023), www.training.cochrane.org/handbook. [Google Scholar]
- 16. Review Manager (RevMan) [Computer Program], Version 7.2.0 (Cochrane Collaboration, 2024), https://revman.cochrane.org/. [Google Scholar]
- 17. Song F., Eastwood A. J., Gilbody S., Duley L., and Sutton A. J., “Publication and Related Biases,” Health Technology Assessment 4, no. 10 (2000): 1–115, 10.3310/hta4100. [DOI] [PubMed] [Google Scholar]
- 18. Debray T. P. A., Moons K. G. M., and Riley R. D., “Detecting Small‐Study Effects and Funnel Plot Asymmetry in Meta‐Analysis of Survival Data: A Comparison of New and Existing Tests,” Research Synthesis Methods 9, no. 1 (2018): 41–50, 10.1002/jrsm.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins J. P., Altman D. G., Gøtzsche P. C., et al., “The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials,” BMJ 343, no. 2 (2011): d5928, 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Guyatt G., Oxman A. D., Akl E. A., et al., “GRADE Guidelines: 1. Introduction‐GRADE Evidence Profiles and Summary of Findings Tables,” Journal of Clinical Epidemiology 64, no. 4 (2011): 383–394, 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 21. Higgins J. P. T., Thomas J., Chandler J., et al., eds., Cochrane Handbook for Systematic Reviews of Interventions Version 6.4 (Updated August 2023) (Cochrane, 2023), www.training.cochrane.org/handbook. [Google Scholar]
- 22. Page M. J., McKenzie J. E., Bossuyt P. M., et al., “The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews,” BMJ 372 (2021): n71, 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Frias J. P., Nauck M. A., Van J., et al., “Efficacy and Tolerability of Tirzepatide, a Dual Glucose‐Dependent Insulinotropic Peptide and Glucagon‐Like Peptide‐1 Receptor Agonist in Patients With Type 2 Diabetes: A 12‐Week, Randomized, Double‐Blind, Placebo‐Controlled Study to Evaluate Different Dose‐Escalation Regimens,” Diabetes, Obesity and Metabolism 22, no. 6 (2020): 938–946, 10.1111/dom.13979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heise T., Mari A., DeVries J. H., et al., “Effects of Subcutaneous Tirzepatide Versus Placebo or Semaglutide on Pancreatic Islet Function and Insulin Sensitivity in Adults With Type 2 Diabetes: A Multicentre, Randomised, Double‐Blind, Parallel‐Arm, Phase 1 Clinical Trial,” Lancet Diabetes & Endocrinology 10, no. 6 (2022): 418–429, 10.1016/S2213-8587(22)00085-7. [DOI] [PubMed] [Google Scholar]
- 25. Frias J. P., Nauck M. A., Van J., et al., “Efficacy and Safety of LY3298176, a Novel Dual GIP and GLP‐1 Receptor Agonist, in Patients With Type 2 Diabetes: A Randomised, Placebo‐Controlled and Active Comparator‐Controlled Phase 2 Trial,” Lancet 392, no. 10160 (2018): 2180–2193, 10.1016/S0140-6736(18)32260-8. [DOI] [PubMed] [Google Scholar]
- 26. Jastreboff A. M., Aronne L. J., Ahmad N. N., et al., “SURMOUNT‐1 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity,” New England Journal of Medicine 387, no. 3 (2022): 205–216, 10.1056/NEJMoa2206038. [DOI] [PubMed] [Google Scholar]
- 27. Garvey W. T., Frias J. P., Jastreboff A. M., et al., “SURMOUNT‐2 Investigators. Tirzepatide Once Weekly for the Treatment of Obesity in People With Type 2 Diabetes (SURMOUNT‐2): A Double‐Blind, Randomised, Multicentre, Placebo‐Controlled, Phase 3 Trial,” Lancet 402, no. 10402 (2023): 613–626, 10.1016/S0140-6736(23)01200-X. [DOI] [PubMed] [Google Scholar]
- 28. Wadden T. A., Chao A. M., Machineni S., et al., “Tirzepatide After Intensive Lifestyle Intervention in Adults With Overweight or Obesity: The SURMOUNT‐3 Phase 3 Trial,” Nature Medicine 29, no. 11 (2023): 2909–2918, 10.1038/s41591-023-02597-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zhao L., Cheng Z., Lu Y., et al., “Tirzepatide for Weight Reduction in Chinese Adults With Obesity: The SURMOUNT‐CN Randomized Clinical Trial,” JAMA 332, no. 7 (2024): 551–560, 10.1001/jama.2024.9217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Malhotra A., Grunstein R. R., Fietze I., et al., “SURMOUNT‐OSA Investigators. Tirzepatide for the Treatment of Obstructive Sleep Apnea and Obesity,” New England Journal of Medicine 391, no. 13 (2024): 1193–1205, 10.1056/NEJMoa2404881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Rosenstock J., Wysham C., Frías J. P., et al., “Efficacy and Safety of a Novel Dual GIP and GLP‐1 Receptor Agonist Tirzepatide in Patients With Type 2 Diabetes (SURPASS‐1): A Double‐Blind, Randomised, Phase 3 Trial,” Lancet 398, no. 10295 (2021): 143–155, 10.1016/S0140-6736(21)01324-6. [DOI] [PubMed] [Google Scholar]
- 32. Frías J. P., Davies M. J., Rosenstock J., et al., SURPASS‐2 Investigators , “Tirzepatide Versus Semaglutide Once Weekly in Patients With Type 2 Diabetes,” New England Journal of Medicine 385, no. 6 (2021): 503–515, 10.1056/NEJMoa2107519. [DOI] [PubMed] [Google Scholar]
- 33. Ludvik B., Giorgino F., Jódar E., et al., “Once‐Weekly Tirzepatide Versus Once‐Daily Insulin Degludec as Add‐On to Metformin With or Without SGLT2 Inhibitors in Patients With Type 2 Diabetes (SURPASS‐3): A Randomised, Open‐Label, Parallel‐Group, Phase 3 Trial,” Lancet 398, no. 10300 (2021): 583–598, 10.1016/S0140-6736(21)01443-4. [DOI] [PubMed] [Google Scholar]
- 34. Del P. S., Kahn S. E., Pavo I., and, et al. SURPASS‐4 Investigators , “Tirzepatide Versus Insulin Glargine in Type 2 Diabetes and Increased Cardiovascular Risk (SURPASS‐4): A Randomised, Open‐Label, Parallel‐Group, Multicentre, Phase 3 Trial,” Lancet 398, no. 10313 (2021): 1811–1824, 10.1016/S0140-6736(21)02188-7. [DOI] [PubMed] [Google Scholar]
- 35. Dahl D., Onishi Y., Norwood P., et al., “Effect of Subcutaneous Tirzepatide vs Placebo Added to Titrated Insulin Glargine on Glycemic Control in Patients With Type 2 Diabetes: The SURPASS‐5 Randomized Clinical Trial,” JAMA 327, no. 6 (2022): 534–545, 10.1001/jama.2022.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rosenstock J., Frías J. P., Rodbard H. W., et al., “Tirzepatide vs Insulin Lispro Added to Basal Insulin in Type 2 Diabetes: The SURPASS‐6 Randomized Clinical Trial,” JAMA 330, no. 17 (2023): 1631–1640, 10.1001/jama.2023.20294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gao L., Lee B. W., Chawla M., et al., “Tirzepatide Versus Insulin Glargine as Second‐Line or Third‐Line Therapy in Type 2 Diabetes in the Asia‐Pacific Region: The SURPASS‐AP‐Combo Trial,” Nature Medicine 29, no. 6 (2023): 1500–1510, 10.1038/s41591-023-02344-1. [DOI] [PubMed] [Google Scholar]
- 38. Inagaki N., Takeuchi M., Oura T., Imaoka T., and Seino Y., “Efficacy and Safety of Tirzepatide Monotherapy Compared With Dulaglutide in Japanese Patients With Type 2 Diabetes (SURPASS J‐Mono): A Double‐Blind, Multicentre, Randomised, Phase 3 Trial,” Lancet Diabetes & Endocrinology 10, no. 9 (2022): 623–633, 10.1016/S2213-8587(22)00188-7. [DOI] [PubMed] [Google Scholar]
- 39. Loomba R., Hartman M. L., Lawitz E. J., et al., “SYNERGY‐NASH Investigators. Tirzepatide for Metabolic Dysfunction‐Associated Steatohepatitis With Liver Fibrosis,” New England Journal of Medicine 391, no. 4 (2024): 299–310, 10.1056/NEJMoa2401943. [DOI] [PubMed] [Google Scholar]
- 40. Battelino T., Bergenstal R. M., Rodríguez A., et al., “Efficacy of Once‐Weekly Tirzepatide Versus Once‐Daily Insulin Degludec on Glycaemic Control Measured by Continuous Glucose Monitoring in Adults With Type 2 Diabetes (SURPASS‐3 CGM): A Substudy of the Randomised, Open‐Label, Parallel‐Group, Phase 3 SURPASS‐3 Trial,” Lancet Diabetes & Endocrinology 10, no. 6 (2022): 407–417, 10.1016/S2213-8587(22)00077-8. [DOI] [PubMed] [Google Scholar]
- 41. Cariou B., Linge J., Neeland I. J., et al., “Effect of Tirzepatide on Body Fat Distribution Pattern in People With Type 2 Diabetes,” Diabetes, Obesity and Metabolism 26, no. 6 (2024): 2446–2455, 10.1111/dom.15566. [DOI] [PubMed] [Google Scholar]
- 42. Gastaldelli A., Cusi K., Fernández Landó L., Bray R., Brouwers B., and Rodríguez Á, “Effect of Tirzepatide Versus Insulin Degludec on Liver Fat Content and Abdominal Adipose Tissue in People With Type 2 Diabetes (SURPASS‐3 MRI): A Substudy of the Randomised, Open‐Label, Parallel‐Group, Phase 3 SURPASS‐3 Trial,” Lancet Diabetes & Endocrinology 10, no. 6 (2022): 393–406, 10.1016/S2213-8587(22)00070-5. [DOI] [PubMed] [Google Scholar]
- 43. Heerspink H. J. L., Sattar N., Pavo I., et al., “Effects of Tirzepatide Versus Insulin Glargine on Kidney Outcomes in Type 2 Diabetes in the SURPASS‐4 Trial: Post‐Hoc Analysis of an Open‐Label, Randomised, Phase 3 Trial,” Lancet Diabetes & Endocrinology 10, no. 11 (2022): 774–785, 10.1016/S2213-8587(22)00243-1. [DOI] [PubMed] [Google Scholar]
- 44. Hartman M. L., Sanyal A. J., Loomba R., et al., “Effects of Novel Dual GIP and GLP‐1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes,” Diabetes Care 43, no. 6 (2020): 1352–1355, 10.2337/dc19-1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pirro V., Roth K. D., Lin Y., et al., “Effects of Tirzepatide, a Dual GIP and GLP‐1 RA, on Lipid and Metabolite Profiles in Subjects With Type 2 Diabetes,” Journal of Clinical Endocrinology and Metabolism 107, no. 2 (2022): 363–378, 10.1210/clinem/dgab722. [DOI] [PubMed] [Google Scholar]
- 46. Wilson J. M., Nikooienejad A., Robins D. A., et al., “The Dual Glucose‐Dependent Insulinotropic Peptide and Glucagon‐like Peptide‐1 Receptor Agonist, Tirzepatide, Improves Lipoprotein Biomarkers Associated With Insulin Resistance and Cardiovascular Risk in Patients With Type 2 Diabetes,” Diabetes, Obesity and Metabolism 22, no. 12 (2020): 2451–2459, 10.1111/dom.14174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson J. M., Lin Y., Luo M. J., et al., “The Dual Glucose‐Dependent Insulinotropic Polypeptide and Glucagon‐Like Peptide‐1 Receptor Agonist Tirzepatide Improves Cardiovascular Risk Biomarkers in Patients With Type 2 Diabetes: A Post Hoc Analysis,” Diabetes, Obesity and Metabolism 24, no. 1 (2022): 148–153, 10.1111/dom.14553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Feng P., Sheng X., Ji Y., et al., “A Phase 1 Multiple Dose Study of Tirzepatide in Chinese Patients With Type 2 Diabetes,” Advances in Therapy 40, no. 8 (2023): 3434–3445, 10.1007/s12325-023-02536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Furihata K., Mimura H., Urva S., Oura T., Ohwaki K., and Imaoka T., “A Phase 1 Multiple‐Ascending Dose Study of Tirzepatide in Japanese Participants With Type 2 Diabetes,” Diabetes, Obesity and Metabolism 24, no. 2 (2022): 239–246, 10.1111/dom.14572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Kadowaki T., Chin R., Ozeki A., Imaoka T., and Ogawa Y., “Safety and Efficacy of Tirzepatide as an Add‐On to Single Oral Antihyperglycaemic Medication in Patients With Type 2 Diabetes in Japan (SURPASS J‐Combo): A Multicentre, Randomised, Open‐Label, Parallel‐Group, Phase 3 Trial,” Lancet Diabetes & Endocrinology 10, no. 9 (2022): 634–644, 10.1016/S2213-8587(22)00187-5. [DOI] [PubMed] [Google Scholar]
- 51. Urva S., Coskun T., Loh M. T., et al., “LY3437943, a Novel Triple GIP, GLP‐1, and Glucagon Receptor Agonist in People With Type 2 Diabetes: A Phase 1b, Multicentre, Double‐Blind, Placebo‐Controlled, Randomised, Multiple‐Ascending Dose Trial,” Lancet 400, no. 10366 (2022): 1869–1881, 10.1016/S0140-6736(22)02033-5. [DOI] [PubMed] [Google Scholar]
- 52. Abd El Aziz M., Cahyadi O., Meier J. J., Schmidt W. E., and Nauck M. A., “Incretin‐Based Glucose‐Lowering Medications and the Risk of Acute Pancreatitis and Malignancies: A Meta‐Analysis Based on Cardiovascular Outcomes Trials,” Diabetes, Obesity and Metabolism 22, no. 4 (2020): 699–704, 10.1111/dom.13924. [DOI] [PubMed] [Google Scholar]
- 53. Steinberg W. M., Nauck M. A., Zinman B., et al., “LEADER Trial Investigators. LEADER 3‐Lipase and Amylase Activity in Subjects With Type 2 Diabetes: Baseline Data From Over 9000 Subjects in the LEADER Trial,” Pancreas 43, no. 8 (2014): 1223–1231, 10.1097/MPA.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nauck M. A., Frossard J. L., Barkin J. S., et al., “Assessment of Pancreas Safety in the Development Program of Once‐Weekly GLP‐1 Receptor Agonist Dulaglutide,” Diabetes Care 40, no. 5 (2017): 647–654, 10.2337/dc16-0984. [DOI] [PubMed] [Google Scholar]
- 55. Azoulay L., Filion K. B., Platt R. W., et al., “Association Between Incretin‐Based Drugs and the Risk of Acute Pancreatitis,” JAMA Internal Medicine 176, no. 10 (2016): 1464–1473, 10.1001/jamainternmed.2016.1522. [DOI] [PubMed] [Google Scholar]
- 56. Lingvay I., Sumithran P., Cohen R. V., and le Roux C. W., “Obesity Management as a Primary Treatment Goal for Type 2 Diabetes: Time to Reframe the Conversation,” Lancet 399, no. 10322 (2022): 394–405, 10.1016/S0140-6736(21)01919-X. [DOI] [PubMed] [Google Scholar]
- 57. Forzano I., Varzideh F., Avvisato R., Jankauskas S. S., Mone P., and Santulli G., “Tirzepatide: A Systematic Update,” International Journal of Molecular Sciences 23, no. 23 (2022): 14631, 10.3390/ijms232314631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Baggio L. L. and Drucker D. J., “Biology of Incretins: GLP‐1 and GIP,” Gastroenterology 132, no. 6 (2007): 2131–2157, 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 59. Meier J. J., Gallwitz B., Siepmann N., et al., “Gastric Inhibitory Polypeptide (GIP) Dose‐Dependently Stimulates Glucagon Secretion in Healthy Human Subjects at Euglycaemia,” Diabetologia 46, no. 6 (2003): 798–801, 10.1007/s00125-003-1103-y. [DOI] [PubMed] [Google Scholar]
- 60. Lupi R., Del Guerra S., D'Aleo V., Boggi U., Filipponi F., and Marchetti P., “The Direct Effects of GLP‐1 and GIP, Alone or in Combination, on Human Pancreatic Islets,” Regulatory Peptides 165, no. 2–3 (2010): 129–132, 10.1016/j.regpep.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 61. Holst J. J. and Rosenkilde M. M., “GIP as a Therapeutic Target in Diabetes and Obesity: Insight From Incretin Co‐agonists,” Journal of Cinical Endocrinology and Metabolism 105, no. 8 (2020): e2710–e2716, 10.1210/clinem/dgaa327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Willard F. S., Douros J. D., Gabe M. B., et al., “Tirzepatide Is an Imbalanced and Biased Dual GIP and GLP‐1 Receptor Agonist,” JCI Insight 5, no. 17 (2020): e140532, 10.1172/jci.insight.140532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mishra R., Raj R., Elshimy G., et al., “Adverse Events Related to Tirzepatide,” Journal of the Endocrine Society 7, no. 4 (2023): bvad016, 10.1210/jendso/bvad016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Tang Y., Zhang L., Zeng Y., Wang X., and Zhang M., “Efficacy and Safety of Tirzepatide in Patients With Type 2 Diabetes: A Systematic Review and Meta‐Analysis,” Frontiers in Pharmacology 13 (2022): 1016639, 10.3389/fphar.2022.1016639. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information S1


