Abstract
Objective
The objective is to assess the effectiveness and safety of tirzepatide, liraglutide, and SGLT2i in individuals diagnosed with type 2 diabetes.
Methods
An inquiry was undertaken within the electronic database spanning from its inception to February 11th, 2024, aimed at identifying randomized controlled trials that assess the efficacy and safety of tirzepatide, liraglutide, canagliflozin, ertugliflozin, empagliflozin, dapagliflozin, and henagliflozin. Perform a network meta-analysis to examine the distinctions among them (PROSPERO registration number: CRD42024537006).
Results
Twenty-eight RCTs were included, involving 8499 participants. Compared with placebo, all treatments improved HbA1c levels: tirzepatide 15 mg reduced HbA1c the most (MD [95% CI], -2.24% [-2.52, -1.96]%), followed by tirzepatide 10 mg (MD [95% CI], -1.99% [-2.29, -1.69]%), tirzepatide 5 mg (MD [95% CI], -1.82% [-2.11, -1.53]%), and liraglutide 1.2 mg (MD [95% CI], -1.23% [-1.41, -1.05]%). Canagliflozin 300 mg also showed a significant reduction in HbA1c (MD [95% CI], -1.00% [-1.18, -0.82]). Tirzepatide was also the most effective in promoting weight loss, with the following results compared with placebo: tirzepatide 15 mg (MD [95% CI], -8.74 kg [-9.83, -7.66] kg), tirzepatide 10 mg (MD [95% CI], -7.13 kg [-8.40, -5.88] kg), tirzepatide 5 mg (MD [95% CI], -5.38 kg [-6.65, -4.11] kg), canagliflozin 300 mg (MD [95% CI], -2.31 kg [-2.79, -1.83] kg), and empagliflozin 10 mg (MD [95% CI], -2.00 kg [-2.44, -1.55] kg). In reducing systolic blood pressure (SBP), canagliflozin 300 mg showed the greatest effect (MD [95% CI], -5.96% [-7.96, -3.96] %). For diastolic blood pressure (DBP), henagliflozin 5 mg demonstrated the most significant reduction compared to placebo (MD [95% CI], -2.46% [-3.82, -1.10] %). Liraglutide 1.8 mg was most likely to cause adverse events (AE) (OR [95% CI], 2.57 [1.78, 3.70]), but there was no significant difference in serious adverse events (SAEs) between the interventions (including placebo).
Conclusion
Out of the seven medications examined in this study, tirzepatide demonstrates the most effective antidiabetic and weight-reducing effects. Furthermore, the dosage of Liraglutide at 1.2 mg and above demonstrates a more pronounced hypoglycemic effect in comparison to SGLT2 inhibitors. SGLT2 inhibitors exhibit a distinct hypotensive effect and are suitable for diabetic individuals experiencing hypertension.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12902-024-01805-z.
Keywords: Tirzepatide, Liraglutide, SGLT2i, Type 2 diabetes mellitus, Meta-analysis
Introduction
Diabetes affects approximately 537 million individuals worldwide, leading to chronic hyperglycemia that progressively damages multiple organs, including the retina, kidneys, nerves, blood vessels, and heart, with the potential to result in organ failure [1]. Moreover, a growing body of evidence indicates a close association between diabetes and an increased risk of various cancers [2]. The 10th IDF Diabetes Map illustrates that diabetes represents one of the most rapidly escalating global health crises of the twenty-first century. Projections indicate that the population of individuals with diabetes is expected to rise to 643 million by the year 2030 and further escalate to 783 million by 2045 [3]. Due to the high prevalence of diabetes and its detrimental effects on several bodily systems, treating diabetic patients has consistently posed a challenging issue for physicians across various fields. In recent 30 years, a series of new hypoglycemic drugs have emerged, such as GIP/GLP-1 double receptor agonists [4, 5], SGLT-2 inhibitors [6, 7] and GLP1 receptor agonists [8]. In contrast to certain conventional hypoglycemic medications, these alternatives show enhanced safety, greater efficacy, and increased simplicity, while also considering the protective benefits for cardiac and renal health. SGLT-2 inhibitors [6, 7] and GLP1 receptor agonists have been widely used in clinics. In May 2022, the first dual agonist of glucose-dependent insulinotropic peptide (GIP) and GLP-1 receptor, tizepatide, was approved for marketing by FDA. The half-life is about 5 days, allowing for administration weekly, which enhances its practicality of use. Current research indicates that teixeptide has the potential to significantly enhance blood sugar regulation and facilitate weight loss in individuals diagnosed with type 2 diabetes [4, 5].
The SGLT-2 inhibitor is a novel oral hypoglycemic medication that has garnered significant interest in recent times. It can inhibit the reabsorption of glucose by the kidney, lower the renal glucose threshold, thus promoting the excretion of urine glucose, and can significantly reduce the occurrence of cardiovascular adverse events and end-stage renal diseases [9, 10]. Liraglutide is a prominent medication belonging to the class of GLP1 receptor agonists. The mechanism operates by enhancing insulin secretion to reduce blood glucose levels while concurrently inhibiting glucagon secretion to increase blood sugar levels, all of which is contingent upon insulin's role. This medication proficiently lowers blood sugar levels by suppressing the appetite center, prolonging gastric emptying, and diminishing food consumption. Additionally, it has a minimal risk of causing hypoglycemia. At the same time, it also has a protective effect on the heart and kidney [8, 11].
While prior studies, including those by Ding et al. [5], Guan et al. [12], and Thomas et al. [13], have analyzed specific hypoglycemic agents or focused on single drug classes, our study offers a comprehensive network meta-analysis that compares three major classes of antidiabetic medications—GLP-1 receptor agonists, GIP/GLP-1 dual receptor agonists, and SGLT-2 inhibitors—within a unified analytical framework. This approach enables both direct and indirect comparisons of efficacy and safety outcomes, including HbA1c reduction, weight loss, blood pressure control, and adverse events, across a broad spectrum of drug dosages. Unlike previous studies, which often emphasize pharmacokinetic or mechanistic insights, our analysis is guided by clinical relevance, addressing the practical application of these therapies in managing Type 2 diabetes, particularly in patients with comorbid conditions such as obesity and hypertension. Through the evaluation of these therapies according to their efficacy and safety profiles, our findings offer critical insights to evidence-based guidelines, equipping clinicians with a more nuanced understanding of optimal therapy selections and potential combinations for tailored patient management.
The comparative effectiveness and safety of these three drug classes have not been thoroughly assessed in relation to each other, given their distinct mechanisms of action. Network meta-analysis (NMA) serves as an ideal statistical approach to address this gap. As a method that allows for the simultaneous comparison and ranking of multiple interventions through both direct and indirect evidence, NMAs consolidate findings from multiple comparators into a single pooled analysis [14]. Consequently, our study employs NMA to evaluate and compare the efficacy and safety of the GIP/GLP-1 dual receptor agonist tirzepatide (TIR), the GLP-1 receptor agonist liraglutide (LIR), and various SGLT-2 inhibitors, including canagliflozin (Can), ertugliflozin (Ert), empagliflozin (Emp), dapagliflozin (Dap), and henagliflozin (Hen), in the treatment of Type 2 diabetes mellitus. Our goal is to provide evidence-based insights that can assist clinicians in formulating optimal hypoglycemic regimens.
Methods
This systematic review and network meta-analysis was conducted following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 guidelines to ensure rigorous and transparent reporting of findings. The study protocol was registered on PROSPERO (ID: CRD42024537006), and we adhered to the PRISMA checklist throughout all phases of study selection, data extraction, and analysis to meet the highest reporting standards in systematic reviews and meta-analyses [15].
Search strategy
Systematic searches were conducted across PubMed, EMBASE, and the Cochrane Library from database inception to February 11, 2024, using a defined set of keywords and Boolean operators to identify randomized clinical trials (RCTs) evaluating the efficacy and safety of tirzepatide, liraglutide, canagliflozin, ertugliflozin, empagliflozin, dapagliflozin, and henagliflozin, detailed search strategies tailored to each database are provided in Supplementary File 1. Duplicate records were removed through Endnote 20.
Study selection criteria
Studies were included in this meta-analysis based on the following PICO (Population, Intervention, Comparator, Outcomes) criteria: Population – individuals diagnosed with type 2 diabetes, aged 18 years or older; Intervention – treatment with different doses of tirzepatide (Tir), liraglutide (Lir), canagliflozin (Can), ertugliflozin (Ert), empagliflozin (Emp), dapagliflozin (Dap), or henagliflozin (Hen); Comparator – placebo or any one or more of the included intervention drugs; Outcomes – efficacy outcomes, such as reductions in HbA1c, changes in body weight, and blood pressure, as well as safety outcomes, including adverse events (AEs) and serious adverse events (SAEs).
Studies were excluded if they met any of the following criteria: secondary analysis of published RCTs, ongoing or completed experiments with unpublished results, conference papers, review articles, animal or in vitro studies, editorials, letters, statements, or if the data were incomplete or could not be extracted.
Data collection and quality assessment
Two researchers (X.P.Y and X.F.) independently conducted literature screening, data extraction, and quality assessment. Extracted data included the first author, publication year, intervention measures, sample size, baseline characteristics, changes in monitored indicators, intervention duration, and adverse events. For quality assessment, the RevMan5.4 program and the Cochrane risk of bias tool were used to evaluate each study across seven domains, categorizing each domain as low risk, high risk, or unclear risk [16]. Discrepancies were resolved through discussion between the two researchers (X.P.Y and X.F.), with a third author (Y.J.T), consulted when necessary to reach a consensus.
Data analysis
The statistical analysis was performed utilizing Stata 17.0. Continuous variables employ the mean difference (MD) to gauge the extent of the effect, whereas binary variables utilize the odds ratio (OR) to assess the size of the effect. The confidence interval (CI) is set to 95% CI. Chi-square (χ2) was used to test the statistical heterogeneity between the evaluation results, and I2 was used to quantitatively judge the heterogeneity. P > 0.05, or I2 < 50% means that there is no heterogeneity, and the fixed effect model is used. P ≤ 0.05, or I2 ≥ 50% is heterogeneity. The random effect model and sensitivity analysis are employed to establish the origin of heterogeneity. Determine the cumulative ranking curve (SUCRA) to evaluate the therapeutic effects of different therapies and arrange them in order of effectiveness (Table 2).
Table 2.
SUCRA sorting summary
| Treatment | Outcomes | |||||
|---|---|---|---|---|---|---|
| HbA1c | Weight | SBP | DBP | AE | SAE | |
| Placebo | 0 | 9.5 | 2.5 | 8.3 | 31.1 | 60.8 |
| Tir5mg | 89.6 | 88.9 | NA | NA | 74.4 | 68.6 |
| Tir10mg | 94 | 94.4 | NA | NA | 76.6 | 58.3 |
| Tir15mg | 99.7 | 100 | NA | NA | 79.3 | 31.4 |
| Lir0.6mg | 43.8 | 0.9 | NA | NA | NA | 41.2 |
| Lir1.2mg | 80.6 | 6.5 | NA | NA | NA | 48.5 |
| Lir1.8mg | 79.7 | 16.9 | NA | NA | 98.6 | 60.9 |
| Can100mg | 54 | 48.8 | 55.1 | 69.7 | 56.1 | 49.1 |
| Can200mg | 59.4 | 52.5 | 66.1 | 65.3 | 66.7 | 44.3 |
| Can300mg | 67.5 | 78.4 | 82.1 | 72.6 | 56.7 | 34.2 |
| Ert5mg | 24.9 | 60.2 | 68.1 | 46.8 | 19.9 | 82.9 |
| Ert15mg | 42 | 60.8 | 46.3 | 46.1 | 32.7 | 65.6 |
| Emp5mg | 20.6 | 41.3 | 12.3 | 15.3 | 10.7 | 25.5 |
| Emp10mg | 30.2 | 65.6 | 28.1 | 28.4 | 6.8 | 66.2 |
| Emp25mg | 44.2 | 61.8 | 33 | 46.6 | 11.4 | 47.5 |
| Dap5mg | 9.4 | 39.6 | 76.1 | NA | 43.4 | 36.3 |
| Dap10mg | 14 | 60.7 | 50.3 | NA | 58.3 | 28.8 |
| Hen5mg | 45.9 | 30.8 | 59.8 | 78.5 | 56.8 | 65.2 |
| Hen10mg | 50.5 | 32.3 | 70 | 72.5 | 70.4 | 34.7 |
Results
Study selection and characteristics
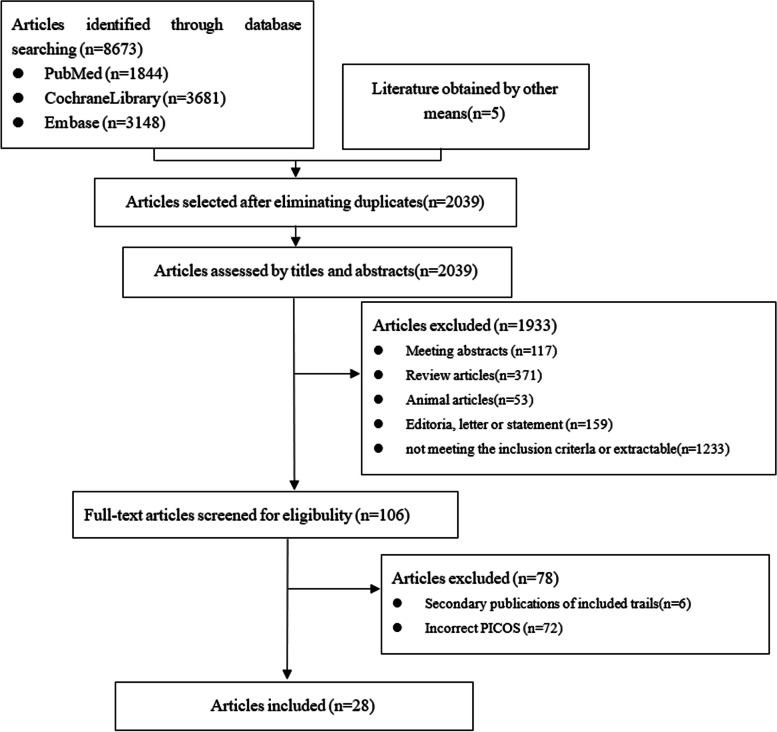
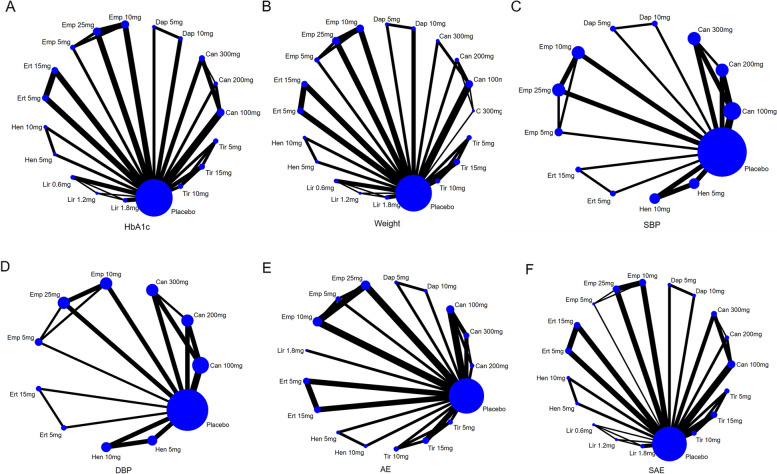
Some doses of drugs in the RCTs were seldom used in clinical settings, or the number of subjects was too small. Two researchers (X.P.Y and X.F.) decided to exclude the following intervention measures: tirzepatide 1 mg, tirzepatide 12 mg, liraglutide 0.1 mg, liraglutide 0.3 mg, liraglutide 0.9 mg, canagliflozin 50 mg, empagliflozin 50 mg, and dapagliflozin 2.5 mg. According to the retrieval strategy, a total of 8673 articles were retrieved, and 28 articles [17–44] remained after de-duplication, primary screening, and re-screening, involving 8499 patients. See Fig. 1 for the process and results of literature screening, Fig. 2 for literature quality evaluation, and Table 1 for baseline characteristics. The intervention duration ranged from 4 to 52 weeks, including 4-week RCT1, 8-week RCT1, 5-week RCT1, 14-week RCT1, 24-week RCT8, 26-week RCT8, 28-week RCT1, 40-week RCT1, and 52-week RCT2. The network diagram presented below illustrates all outcome indicators. Each node (blue dot) represents an intervention; the size of each node reflects the number of participants in the intervention, with larger nodes indicating more participants. The connecting line between two points signifies a direct comparison between the two interventions; the thicker the line, the more studies available to compare the two interventions. Out of the 28 documents analyzed, one presented a significant risk in terms of potential blindness and the reliability of the data obtained [31]. Another document [32] included a single-blind component in the experimental process, allowing researchers to adjust medications. See Fig. 3 for the specific evaluation structure.
Fig. 1.
The process and results of literature screening
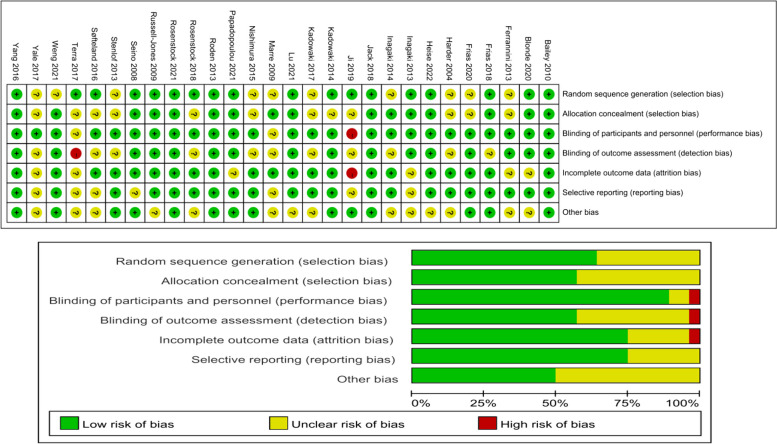
Fig. 2.
Literature quality evaluation
Table 1.
Baseline data
| Study | Gender(M/F) | Age | N | Treatment | Duration (weeks) | Outcomes |
|---|---|---|---|---|---|---|
| Rosenstock, et al. (2021) [17] | 56/59 | 53.6 ± 12.8 | 115 | Placebo | 40 | HbA1c,Weight,AE, SAE |
| 56/65 | 54.1 ± 11.9 | 121 | Tir 5mg | |||
| 72/49 | 55.8 ± 10.4 | 121 | Tir 10mg | |||
| 63/58 | 52.9 ± 12.3 | 121 | Tir 15mg | |||
| Frias, et al. (2020) [18] | 12/14 | 56.0 ± 10.13 | 26 | Placebo | 12 | HbA1c,Weight,AE, SAE |
| 16/13 | 55.5 ± 8.54 | 29 | Tir 15mg | |||
| Frias, et al. (2018) [19] | 29/22 | 56.6 ± 8.9 | 51 | Placebo | 26 | HbA1c,Weight,AE, SAE |
| 34/21 | 57.9 ± 8.2 | 55 | Tir 5mg | |||
| 30/21 | 56.5 ± 9.9 | 51 | Tir 10mg | |||
| 22/31 | 56.0 ± 7.6 | 53 | Tir 15mg | |||
| Heise, et al. (2022) [20] | 21/7 | 60.4 ± 7.6 | 28 | Placebo | 28 | Weight,AE, SAE |
| 31/14 | 61.1 ± 7.1 | 45 | Tir 15mg | |||
| Blonde, et al. (2020) [21] | 58/42 | 56.0 ± 9.9 | 100 | Placebo | 26 | HbA1c,Weight,AE, SAE |
| 125/78 | 54.7 ± 10.1 | 203 | Lir 1.8mg | |||
| Harder, et al. (2004) [22] | 1/11 | 60.1 ± 6.7 | 12 | Placebo | 8 | HbA1c,Weight |
| 11/10 | 59.9 ± 11.0 | 21 | Lir 0.6mg | |||
| Seino, et al. (2008) [23] | 29/17 | 57.5 ± 8.7 | 46 | Placebo | 14 | HbA1c,Weight |
| 28/17 | 60 ± 7.0 | 45 | Lir 0.6mg | |||
| Marre, et al. (2009) [24] | 54/60 | 54.7 ± 10.0 | 114 | Placebo | 26 | HbA1c,Weight, SAE |
| 126/107 | 55.7 ± 9.9 | 233 | Lir 0.6mg | |||
| 102/126 | 57.7 ± 9.0 | 228 | Lir 1.2mg | |||
| 124/110 | 55.6 ± 10.0 | 234 | Lir 1.8mg | |||
| Russell-Jones, et al. (2009) [25] | 56/59 | 57.5 ± 9.6 | 115 | Placebo | 26 | HbA1c,Weight,AE, SAE |
| 132/100 | 57.6 ± 9.5 | 232 | Lir 1.8mg | |||
| Yale, et al. (2017) [26] | 41/28 | 64.3 ± 7.76 | 69 | Placebo | 52 | HbA1c,Weight,AE, SAE |
| 37/37 | 64.3 ± 8.49 | 74 | Can 100mg | |||
| 42/30 | 65.8 ± 7.88 | 72 | Can 300mg | |||
| Kadowaki, et al. (2017) [27] | 53/15 | 56.0 ± 9.5 | 68 | Placebo | 24 | HbA1c,Weight,AE, SAE |
| 54/16 | 58.4 ± 8.9 | 70 | Can 100mg | |||
| Inagaki, et al. (2013) [28] | 54/21 | 57.7 ± 11.0 | 75 | Placebo | 12 | HbA1c,Weight,SBP,DBP,AE, SAE |
| 52/22 | 57.7 ± 10.5 | 74 | Can 100mg | |||
| 49/27 | 57.0 ± 10.7 | 76 | Can 200mg | |||
| 55/20 | 57.1 ± 10.1 | 75 | Can 300mg | |||
| Inagaki, et al. (2014) [29] | 60/33 | 58.2 ± 11.0 | 93 | Placebo | 24 | HbA1c,Weight,SBP,DBP,AE, SAE |
| 60/33 | 58.4 ± 10.4 | 93 | Can 100mg | |||
| 72/16 | 57.4 ± 11.1 | 88 | Can 200mg | |||
| Stenlof, et al. (2013) [30] | 88/104 | 55.7 ± 10.9 | 192 | Placebo | 26 | HbA1c,Weight,SBP,DBP,AE, SAE |
| 81/114 | 55.1 ± 10.8 | 195 | Can 100mg | |||
| 89/108 | 55.3 ± 10.2 | 197 | Can 300mg | |||
| Ji, et al. (2019) [31] | 88/79 | 56.9 ± 9.0 | 167 | Placebo | 26 | HbA1c,Weight,SBP,DBP,AE, SAE |
| 95/65 | 56.1 ± 9.0 | 160 | Ert 5mg | |||
| 98/71 | 56.3 ± 9.3 | 169 | Ert 15mg | |||
| Terra, et al. (2017) [32] | 82/71 | 56.1 ± 10.9 | 153 | Placebo | 26 | HbA1c,Weight,AE, SAE |
| 89/67 | 56.8 ± 11.4 | 156 | Ert 5mg | |||
| 90/62 | 56.2 ± 10.8 | 152 | Ert 15mg | |||
| Rosenstock, et al. (2018) [44] | 98/111 | 56.5 ± 8.7 | 209 | Placebo | 26 | HbA1c,Weight,AE, SAE |
| 97/110 | 56.6 ± 8.1 | 207 | Ert 5mg | |||
| 93/112 | 56.9 ± 9.4 | 205 | Ert 15mg | |||
| Dagogo-Jack S, et al. (2018) [33] | 100/53 | 58.3 ± 9.2 | 153 | Placebo | 52 | HbA1c,Weight,AE, SAE |
| 81/75 | 59.2 ± 9.3 | 156 | Ert 5mg | |||
| 82/71 | 59.7 ± 8.6 | 153 | Ert 15mg | |||
| Kadowaki, et al. (2014) [34] | 80/29 | 58.7 ± 8.7 | 109 | Placebo | 12 | HbA1c,Weight,SBP,DBP,AE, SAE |
| 84/26 | 57.3 ± 11.2 | 110 | Emp 5mg | |||
| 77/32 | 57.9 ± 9.4 | 109 | Emp 10mg | |||
| 84/25 | 57.2 ± 9.7 | 109 | Emp 25mg | |||
| Søfteland, et al. (2017) [35] | 60/48 | 55.9 ± 9.7 | 108 | Placebo | 24 | HbA1c,Weight,AE, SAE |
| 66/43 | 54.3 ± 9.6 | 109 | Emp 10mg | |||
| 71/39 | 55.4 ± 9.9 | 110 | Emp 25mg | |||
| Nishimura, et al. (2015) [36] | 17/4 | 60.7 ± 10.8 | 21 | Placebo | 4 | AE, SAE |
| 14/6 | 64.8 ± 5.9 | 20 | Emp 10mg | |||
| 16/3 | 62.6 ± 7.8 | 19 | Emp 25mg | |||
| Ferrannini, et al. (2013) [37] | 45/37 | 58.0(28–80) | 82 | Placebo | 12 | HbA1c,Weight,AE |
| 46/35 | 59.0(37–78) | 81 | Emp 5mg | |||
| 40/41 | 58.0(30–76) | 81 | Emp 10mg | |||
| 41/41 | 57.0(30–79) | 82 | Emp 25mg | |||
| Roden, et al. (2013) [38] | 123/105 | 54.9 ± 10.9 | 228 | Placebo | 24 | HbA1c,Weight,SBP,DBP,AE, SAE |
| 142/82 | 56.2 ± 11.6 | 224 | Emp 10mg | |||
| 145/79 | 53.8 ± 11.6 | 224 | Emp 25mg | |||
| Papadopoulou, et al. (2021) [39] | 21/21 | 60.6 ± 9.4 | 42 | Placebo | 12 | HbA1c,Weight |
| 23/20 | 61.7 ± 6.7 | 43 | Dap 10mg | |||
| Yang, et al. (2016) [40] | 86/59 | 53.5 ± 9.2 | 145 | Placebo | 24 | HbA1c,Weight,SBP,AE, SAE |
| 67/80 | 53.1 ± 9.1 | 147 | Dap 5mg | |||
| 88/64 | 54.6 ± 9.5 | 152 | Dap 10mg | |||
| Bailey, et al. (2010) [41] | 76/67 | 53.7 ± 10.3 | 143 | Placebo | 24 | HbA1c,Weight,AE, SAE |
| 69/68 | 54.3 ± 9.4 | 137 | Dap 5mg | |||
| 77/58 | 52.7 ± 9.9 | 135 | Dap 10mg | |||
| Weng, et al. (2021) [42] | 93/68 | 55.3 ± 9.5 | 161 | Placebo | 24 | HbA1c,Weight,SBP,DBP,AE, SAE |
| 103/59 | 54.3 ± 9.5 | 162 | Hen 5mg | |||
| 101/59 | 54.7 ± 10.7 | 160 | Hen 10mg | |||
| Lu, et al. (2021) [43] | 100/51 | 52.4 ± 10.2 | 151 | Placebo | 24 | HbA1c,Weight,SBP,DBP,AE, SAE |
| 88/62 | 53.3 ± 9.6 | 150 | Hen 5mg | |||
| 115/36 | 52.2 ± 9.4 | 151 | Hen 10mg |
Fig. 3.
The specific evaluation structure
Efficacy outcomes
HbA1c (main outcome indicator)
In the network meta-analysis of HbA1c, 19 interventions across 26 studies were included, involving different doses of seven hypoglycemic drugs: tirzepatide (5 mg, 10 mg, 15 mg), liraglutide (0.6 mg, 1.2 mg, 1.8 mg), canagliflozin (100 mg, 200 mg, 300 mg), ertugliflozin (5 mg, 15 mg), empagliflozin (5 mg, 10 mg, 25 mg), dapagliflozin (5 mg, 10 mg), and henagliflozin (5 mg, 10 mg), as well as placebo. The dot diagram is shown in Fig. 3A. The heterogeneity test results showed χ2 = 4.52, I2 = 0%, P = 0.95 (P > 0.05), indicating no significant heterogeneity among the included studies; thus, a fixed-effects model was used for the combined effect.
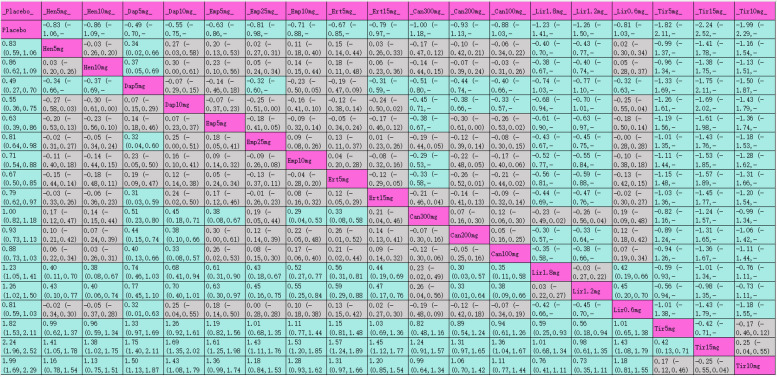
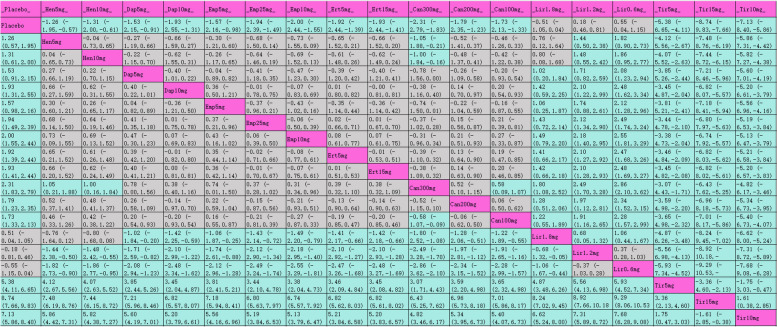
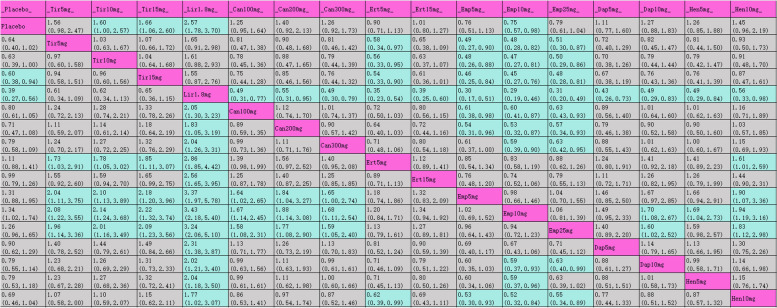
The league table (Table 3) presents pairwise comparisons between various interventions: all interventions achieved statistically significant improvements compared with placebo. Tirzepatide 15 mg had the most substantial HbA1c reduction (MD [95% CI], −2.24% [−2.52%, −1.96%]), followed by tirzepatide 10 mg (MD [95% CI], −1.99% [−2.29%, −1.69%]), tirzepatide 5 mg (MD [95% CI], −1.82% [−2.11%, −1.53%]), and liraglutide 1.2 mg (MD [95% CI], −1.23% [−1.41%, −1.05%]). The SUCRA rankings for hypoglycemic efficacy are shown in Table 3 and Fig. 4.1 with tirzepatide 15 mg (99.7%) ranked highest, followed by tirzepatide 10 mg (94%), tirzepatide 5 mg (89.6%), liraglutide 1.2 mg (80.6%), and liraglutide 1.8 mg (79.7%).
Table 3.
League table for HbA1c
Purple indicates interventions; green signifies statistically significant differences in pairwise comparisons; gray indicates no statistical significance
Fig. 4.
SUCRA plots for each outcome indicator
Weight (secondary outcome indicator)
The network meta-analysis of body weight included 19 interventions from 27 studies with various doses of seven hypoglycemic drugs: tirzepatide (5 mg, 10 mg, 15 mg), liraglutide (0.6 mg, 1.2 mg, 1.8 mg), canagliflozin (100 mg, 200 mg, 300 mg), ertugliflozin (5 mg, 15 mg), empagliflozin (5 mg, 10 mg, 25 mg), dapagliflozin (5 mg, 10 mg), and henagliflozin (5 mg, 10 mg). Placebo was also included in the analysis. The dot diagram is shown in Fig. 3B. Heterogeneity test results for this analysis were χ2 = 10.09, I2 = 0%, P = 0.523 (P > 0.05), suggesting homogeneity among studies, supporting the use of a fixed-effects model.
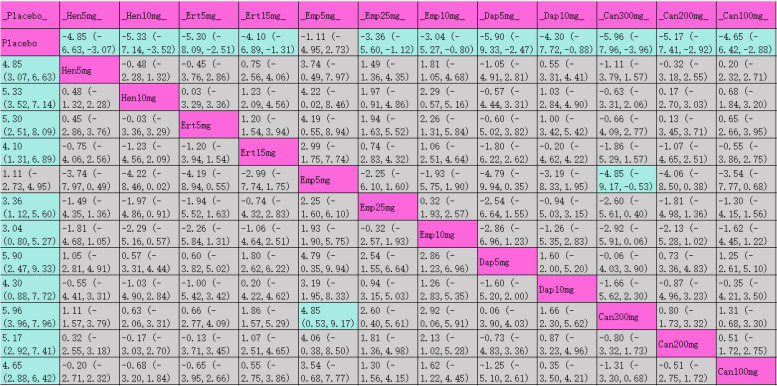
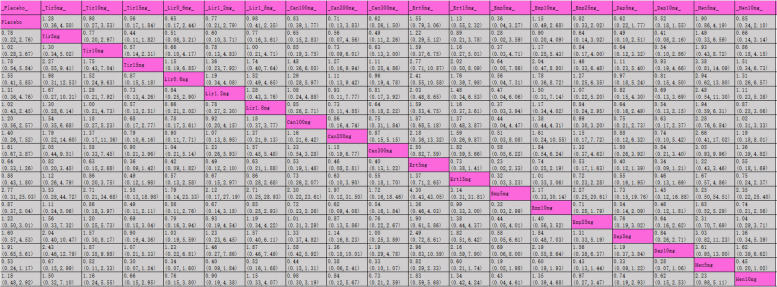
The league table (Table 4) shows the effectiveness of each intervention in weight reduction compared to placebo. Tirzepatide 15 mg exhibited the most significant weight reduction (MD [95% CI], −8.74 kg [−9.83 kg, −7.66 kg]), followed by tirzepatide 10 mg (MD [95% CI], −7.13 kg [−8.40 kg, −5.86 kg]). SUCRA rankings for weight loss are indicated in Table 4 and Fig. 4.2, with tirzepatide 15 mg at the top (100%), followed by tirzepatide 10 mg (94.4%), tirzepatide 5 mg (88.9%), canagliflozin 300 mg (78.4%), and empagliflozin 10 mg (65.6%).
Table 4.
League table for weight
Purple indicates interventions; green signifies statistically significant differences in pairwise comparisons; gray indicates no statistical significance
SBP (secondary outcome indicator)
The network meta-analysis of systolic blood pressure (SBP) included 13 interventions from nine studies. These interventions consisted of various doses of five hypoglycemic drugs. The heterogeneity test results indicated χ2 = 5.04, I2 = 0%, P = 0.538 (P > 0.05), suggesting no significant heterogeneity, and a fixed-effects model was applied.
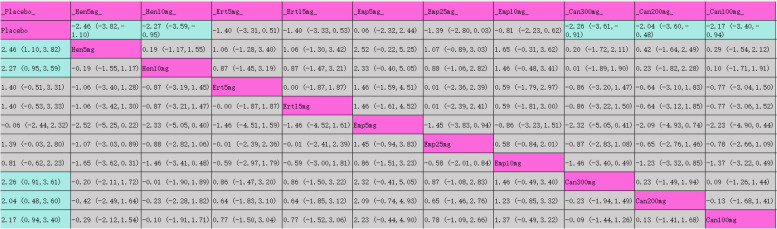
The league table (Table 5) displays each intervention’s impact on reducing SBP compared to placebo. Canagliflozin 300 mg showed the most substantial SBP reduction (MD [95% CI], −5.96 mmHg [−7.96 mmHg, −3.96 mmHg]). SUCRA results in Table 5 and Fig. 4.3, rank canagliflozin 300 mg highest (82.1%), followed by dapagliflozin 5 mg (76.1%) and henagliflozin 10 mg (70%).
Table 5.
League table for SBP
Purple indicates interventions; green signifies statistically significant differences in pairwise comparisons; gray indicates no statistical significance
DBP (secondary outcome indicator)
In the network meta-analysis of diastolic blood pressure (DBP), 11 interventions from eight studies were included, comprising various doses of four hypoglycemic drugs. The heterogeneity test showed χ2 = 5.6, I2 = 0%, P = 0.47 (P > 0.05), indicating no heterogeneity among studies, supporting a fixed-effects model.
The league table (Table 6) shows the DBP reduction effectiveness of each intervention compared to placebo, with henagliflozin 5 mg achieving the best DBP reduction (MD [95% CI], −2.46 mmHg [−3.82 mmHg, −1.10 mmHg]). Table 6 and Fig. 4.4 provides SUCRA results, with henagliflozin 5 mg at the top (78.5%), followed by canagliflozin 300 mg (72.6%) and henagliflozin 10 mg (72.5%).
Table 6.
League table for DBP
Purple indicates interventions; green signifies statistically significant differences in pairwise comparisons; gray indicates no statistical significance
AE (secondary outcome indicator)
The network meta-analysis of adverse events (AEs) included 19 interventions from 24 studies. The heterogeneity test results for AEs showed χ2 = 4.4, I2 = 0%, P = 0.819 (P > 0.05), indicating homogeneity.
The league table (Table 7) demonstrated liraglutide 1.8 mg with the highest AE risk (OR [95% CI]: 2.57 [1.78, 3.70]), while empagliflozin 10 mg showed a lower AE risk compared to placebo. SUCRA rankings, provided in Table 7 and Fig. 4.5, indicate liraglutide 1.8 mg at the highest AE risk (98.6%), followed by tirzepatide 15 mg (79.3%), tirzepatide 10 mg (76.6%), tirzepatide 5 mg (74.4%), and henagliflozin 10 mg (70.4%).
Table 7.
League table for AE
Purple indicates interventions; green signifies statistically significant differences in pairwise comparisons; gray indicates no statistical significance
SAE (secondary outcome indicator)
The network meta-analysis for serious adverse events (SAE) included 19 interventions in 24 studies, with heterogeneity test results χ2 = 2.90, I2 = 0%, P = 0.968 (P > 0.05), suggesting homogeneity across studies and justifying a fixed-effects model.
The league table (Table 8) indicated no significant difference in SAE risk between any interventions and placebo. The top three SUCRA values for SAE likelihood in Table 8 and Fig. 4.6, were ertugliflozin 5 mg (82.9%), tirzepatide 5 mg (68.6%), and empagliflozin 10 mg (66.2%), while the lowest values were tirzepatide 15 mg (31.4%), dapagliflozin 10 mg (28.8%), and empagliflozin 5 mg (25.5%).
Table 8.
League table for SAE
Purple indicates interventions; green signifies statistically significant differences in pairwise comparisons; gray indicates no statistical significance
Publication bias
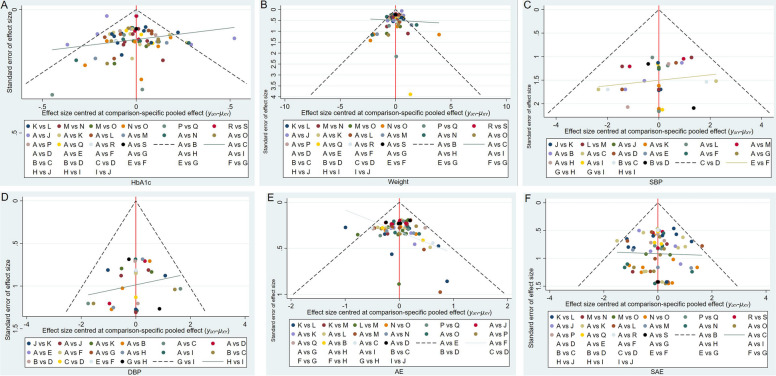
Funnel plots were utilized to analyze the differences in average changes across all assessment variables between the treatment and placebo groups. Most of the data points in all of the funnel plots were situated on either side of the vertical axis. They were fundamentally symmetric and may have some level of publication bias. Certain instances exhibit varying levels of asymmetry, indicating the potential presence of publication bias (Fig. 5).
Fig. 5.
Publication bias
Quality assessment of included studies
Of the 28 studies, the majority were judged to have low risk of bias in random sequence generation and allocation concealment, with 16 studies meeting these criteria. However, 12 studies presented unclear risk of bias in blinding of outcome assessments due to insufficient details provided on blinding procedures. Notably, one study exhibited a high risk of bias in the blinding of participants and personnel, impacting the reliability of the findings. In terms of incomplete outcome data and selective reporting, most studies were rated as low risk, with only one showing a high risk due to missing data and lack of reported outcomes. Figure 2 illustrates the quality assessment results across all domains, allowing readers to visually interpret the strengths and limitations of each study’s methodological rigor. The overall quality of the included studies supports the robustness of the findings; however, the limitations in blinding procedures should be considered when interpreting the results.
Discussion
In this meta-analysis, among the 28 trials analyzed, tirzepatide at doses of 5 mg, 10 mg, and 15 mg demonstrated the most significant HbA1c reduction and weight loss, aligning with the findings by Ding et al. [5]. As the first dual agonist of GIP and GLP-1 receptors, tirzepatide employs a dual-target mechanism that enables stronger hypoglycemic and weight-reducing effects than other existing hypoglycemic agents, with high tolerability and safety. These qualities underscore its value in comprehensive diabetes management.
Obesity and type 2 diabetes share a fundamental pathophysiological mechanism. Research indicates that a weight reduction of 15% or greater can markedly enhance blood sugar regulation in individuals with diabetes, with some attaining a state of "remission" that is not achievable through alternative hypoglycemic treatments [45]. The incretin hormones glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP-1) regulate blood sugar levels, and tirzepatide targets both GIP and GLP-1 receptors. This dual action helps regulate insulin secretion through GLP-1, delays gastric emptying to reduce appetite, and inhibits gastric acid secretion and motility via GIP, offering an appetite-suppressing effect while potentially preserving islet function.
This study indicates that tirzepatide, particularly at doses of 15 mg and 10 mg, shows a higher likelihood of adverse events (AEs) compared to other medications. Tirzepatide 5 mg, however, is associated with a higher probability of serious adverse events (SAEs). Gastrointestinal reactions, such as nausea, vomiting, and diarrhea, are the main AEs of tirzepatide and align with those observed with GLP-1 receptor agonists. In our analysis, liraglutide 1.8 mg has the highest probability of AEs, underscoring a consideration for clinicians when choosing hypoglycemic agents, as hypoglycemia risk is a significant concern. For instance, although insulin effectively lowers blood glucose, its hypoglycemic risk limits its use. In contrast, tirzepatide has a remarkably low hypoglycemia risk when used alone, as indicated by its action mechanism and existing research [17–20]. This low risk enhances confidence in tirzepatide’s use for blood sugar control, contributing to high compliance with HbA1c targets in the tirzepatide group.
Liraglutide, particularly at 1.2 mg and 1.8 mg, shows strong efficacy among GLP-1 receptor agonists, outperforming SGLT2 inhibitors in HbA1c reduction and ranking second to tirzepatide. Evidence shows that liraglutide presents unique benefits relative to other hypoglycemic agents, though its safety profile includes a high probability of AEs, particularly gastrointestinal side effects. Most studies report these adverse effects as mild to moderate, usually diminishing over time with continued treatment.
For systolic and diastolic blood pressure reduction, tirzepatide and liraglutide data were limited in the analyzed studies, leading us to focus on SGLT2 inhibitors for these outcomes. Among SGLT2 inhibitors, canagliflozin performed best in lowering both systolic and diastolic blood pressure, followed by henagliflozin. The cardioprotective effects of SGLT2 inhibitors are well-documented and provide considerable benefits for diabetic patients with hypertension [9, 10, 46]. The cardiovascular effects of SGLT2 inhibitors may be linked to osmotic diuresis, reduced renal glucose reabsorption, and inhibition of the renin–angiotensin–aldosterone system, contributing to lower blood pressure [47, 48]. For example, Zhou et al. [49] and Hussein et al. [48] showed, through meta-analyses, that SGLT2 inhibitors are associated with reduced risks of cardiovascular death and heart failure hospitalization compared to GLP-1 receptor agonists, while GLP-1 receptor agonists demonstrated stronger HbA1c and weight reduction effects.
While tirzepatide may appeal to type 2 diabetic patients seeking weight loss, current evidence on its cardiovascular and renal outcomes is limited. Ongoing studies, expected to conclude in 2025, will provide further insights into these outcomes [50].
This network meta-analysis offers a thorough comparison of tirzepatide, liraglutide, and SGLT2 inhibitors, presenting insights into their comparative efficacy and safety profiles, particularly highlighting tirzepatide’s potential for HbA1c reduction and weight loss. Furthermore, using a network meta-analytic framework enables a robust assessment of multiple treatments within a single analysis, facilitating direct and indirect comparisons. Despite these strengths, the study has limitations. First, the number of included studies was small, potentially affecting the generalizability and power of the results. Second, non-English studies were excluded, introducing potential publication bias, and limiting comprehensiveness. Third, the included studies had varying follow-up durations, which might affect the consistency of long-term outcome assessments. Additionally, we could not assess cardiovascular outcomes for GLP-1 receptor agonists or evaluate other GLP-1RAs besides liraglutide, restricting conclusions on their cardiovascular effects.
Conclusion
Among the seven medications analyzed in this study, tirzepatide exhibits the most significant anti-diabetic and weight loss effects. This is especially beneficial for individuals experiencing obesity or excess weight who are also managing type 2 diabetes. The cardiovascular advantages of tirzepatide are now being investigated. The hypoglycemic effect of Liraglutide1.2mg dosage form above 1.2 mg is better than SGLT2i. SGLT2i has a certain antihypertensive effect and is suitable for patients with diabetes complicated with hypertension or other cardiovascular diseases. The results of this study may provide some reference for clinicians to choose new drugs for diabetes. Nonetheless, considering the limitations identified in this study, it is imperative that additional randomized controlled trials are conducted, featuring larger sample sizes, extended follow-up periods, and rigorous quality standards to substantiate the findings further.
Supplementary Information
Acknowledgements
The authors have no acknowledgments to report.
Authors’ contributions
Yunjie Teng: Data curation, Formal Analysis, Methodology, Software, Writing – original draft. Xue Fan: Data curation, Software, Writing – original draft. Rui Yu: Formal Analysis, Methodology, Writin–original draft. Xiaoping Yang: Conceptualization, Supervision, Validation, Visualization, Writing – review & editing. All authors contributed to the manuscript and approved the last version for submission.
Funding
1. Supported by the founding from Key Laboratory of Special Environment and Health Research in Xinjiang (SKL-SEHR-2024–08).
2. Special Fund Project for Youth Scientific Research in the First Affiliated Hospital of Xinjiang Medical University (2022YFY-QKQN-26).
3. Special Project of Health Care Scientific Research in Xinjiang Uygur Autonomous Region (BG202405).
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
This is a systematic review and meta-analysis, ethics approval and consent to participate are not applicable.
Consent for publication
Not applicable. This study does not involve human participants.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Arnold SV, Khunti K, Tang F, Chen H, Nicolucci A, Gomes MB, Ji L, et al. Impact of micro- and macrovascular complications of type 2 diabetes on quality of life: insights from the DISCOVER prospective cohort study. Endocrinol Diabetes Metab. 2022;5(2):e00321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pearson-Stuttard J, Papadimitriou N, Markozannes G, Cividini S, Kakourou A, Gill D, Rizos EC, et al. Type 2 diabetes and cancer: an umbrella review of observational and mendelian randomization studies. Cancer Epidemiol Biomarkers Prev. 2021;30(6):1218–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, Stein C, Basit A, Chan JCN, Mbanya JC, Pavkov ME, Ramachandaran A, Wild SH, James S, Herman WH, Zhang P, Bommer C, Kuo S, Boyko EJ, Magliano DJ. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract. 2022;183:109119. 10.1016/j.diabres.2021.109119. Epub 2021. Erratum in: Diabetes Res Clin Pract. 2023;204:110945.
- 4.Sardar MB, Nadeem ZA, Babar M. Tirzepatide: A novel cardiovascular protective agent in type 2 diabetes mellitus and obesity. Curr Probl Cardiol. 2024;49(5):102489. [DOI] [PubMed] [Google Scholar]
- 5.Ding Y, Shi Y, Guan R, Yan S, Liu H, Wang Z, Li J, et al. Evaluation and comparison of efficacy and safety of tirzepatide and semaglutide in patients with type 2 diabetes mellitus: a Bayesian network meta-analysis. Pharmacol Res. 2024;199:107031. [DOI] [PubMed] [Google Scholar]
- 6.Bechlioulis A, Markozannes G, Chionidi I, Liberopoulos E, Naka KK, Ntzani EE, Liatis S, et al. The effect of SGLT2 inhibitors, GLP1 agonists, and their sequential combination on cardiometabolic parameters: a randomized, prospective, intervention study. J Diabetes Complications. 2023;37(4):108436. [DOI] [PubMed] [Google Scholar]
- 7.Chen JY, Pan HC, Shiao CC, Chuang MH, See CY, Yeh TH, Yang Y, et al. Impact of SGLT2 inhibitors on patient outcomes: a network meta-analysis. Cardiovasc Diabetol. 2023;22(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marso SP, Daniels GH, Brown-Frandsen K, Kristensen P, Mann JF, Nauck MA, Nissen SE, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarafidis PA, Tsapas A. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2016;374(11):1092. [DOI] [PubMed] [Google Scholar]
- 10.Perkovic V, Jardine MJ, Neal B, Bompoint S, Heerspink HJL, Charytan DM, Edwards R, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med. 2019;380(24):2295–306. [DOI] [PubMed] [Google Scholar]
- 11.Imprialos KP, Stavropoulos K, Doumas M. Liraglutide and renal outcomes in type 2 diabetes. N Engl J Med. 2017;377(22):2196. [DOI] [PubMed] [Google Scholar]
- 12.Guan R, Yang Q, Yang X, Du W, Li X, Ma G. Efficacy and safety of tirzepatide in patients with type 2 diabetes mellitus: a bayesian network meta-analysis. Front Pharmacol. 2022;13:998816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karagiannis T, Malandris K, Avgerinos I, Stamati A, Kakotrichi P, Liakos A, Vasilakou D, et al. Subcutaneously administered tirzepatide vs semaglutide for adults with type 2 diabetes: a systematic review and network meta-analysis of randomised controlled trials. Diabetologia. 2024;67(7):1206–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ceriello A, Rodbard HW, Battelino T, Brosius F, Cosentino F, Green J, Ji L, et al. Data from network meta-analyses can inform clinical practice guidelines and decision-making in diabetes management: perspectives of the taskforce of the guideline workshop. Cardiovasc Diabetol. 2023;22(1):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Page MJ, Moher D, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ (Clinical research ed). 2021;372:n160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, Thomas J. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10(10):ED000142. [DOI] [PMC free article] [PubMed]
- 17.Rosenstock J, Wysham C, Frías JP, Kaneko S, Lee CJ, Fernández Landó L, Mao H, et al. Efficacy and safety of a novel dual GIP and GLP-1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS-1): a double-blind, randomised, phase 3 trial. Lancet (London, England). 2021;398(10295):143–55. [DOI] [PubMed] [Google Scholar]
- 18.Frias JP, Nauck MA, Van J, Benson C, Bray R, Cui X, Milicevic Z, et al. Efficacy and tolerability of tirzepatide, a dual glucose-dependent insulinotropic peptide and glucagon-like peptide-1 receptor agonist in patients with type 2 diabetes: A 12-week, randomized, double-blind, placebo-controlled study to evaluate different dose-escalation regimens. Diabetes Obes Metab. 2020;22(6):938–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frias JP, Nauck MA, Van J, Kutner ME, Cui X, Benson C, Urva S, et al. Efficacy and safety of LY3298176, a novel dual GIP and GLP-1 receptor agonist, in patients with type 2 diabetes: a randomised, placebo-controlled and active comparator-controlled phase 2 trial. Lancet (London, England). 2018;392(10160):2180–93. [DOI] [PubMed] [Google Scholar]
- 20.Heise T, Mari A, DeVries JH, Urva S, Li J, Pratt EJ, Coskun T, et al. Effects of subcutaneous tirzepatide versus placebo or semaglutide on pancreatic islet function and insulin sensitivity in adults with type 2 diabetes: a multicentre, randomised, double-blind, parallel-arm, phase 1 clinical trial. Lancet Diabetes Endocrinol. 2022;10(6):418–29. [DOI] [PubMed] [Google Scholar]
- 21.Blonde L, Belousova L, Fainberg U, Garcia-Hernandez PA, Jain SM, Kaltoft MS, Mosenzon O, et al. Liraglutide as add-on to sodium-glucose co-transporter-2 inhibitors in patients with inadequately controlled type 2 diabetes: LIRA-ADD2SGLT2i, a 26-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2020;22(6):929–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harder H, Nielsen L, Tu DT, Astrup A. The effect of liraglutide, a long-acting glucagon-like peptide 1 derivative, on glycemic control, body composition, and 24-h energy expenditure in patients with type 2 diabetes. Diabetes Care. 2004;27(8):1915–21. [DOI] [PubMed] [Google Scholar]
- 23.Seino Y, Rasmussen MF, Zdravkovic M, Kaku K. Dose-dependent improvement in glycemia with once-daily liraglutide without hypoglycemia or weight gain: a double-blind, randomized, controlled trial in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract. 2008;81(2):161–8. [DOI] [PubMed] [Google Scholar]
- 24.Marre M, Shaw J, Brändle M, Bebakar WM, Kamaruddin NA, Strand J, Zdravkovic M, et al. Liraglutide, a once-daily human GLP-1 analogue, added to a sulphonylurea over 26 weeks produces greater improvements in glycaemic and weight control compared with adding rosiglitazone or placebo in subjects with type 2 diabetes (LEAD-1 SU). Diabet Med. 2009;26(3):268–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Russell-Jones D, Vaag A, Schmitz O, Sethi BK, Lalic N, Antic S, Zdravkovic M, et al. Liraglutide vs insulin glargine and placebo in combination with metformin and sulfonylurea therapy in type 2 diabetes mellitus (LEAD-5 met+SU): a randomised controlled trial. Diabetologia. 2009;52(10):2046–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yale JF, Xie J, Sherman SE, Garceau C. Canagliflozin in conjunction with sulfonylurea maintains glycemic control and weight loss over 52 weeks: a randomized, controlled trial in patients with type 2 diabetes mellitus. Clin Ther. 2017;39(11):2230-42.e2. [DOI] [PubMed] [Google Scholar]
- 27.Kadowaki T, Inagaki N, Kondo K, Nishimura K, Kaneko G, Maruyama N, Nakanishi N, et al. Efficacy and safety of canagliflozin as add-on therapy to teneligliptin in Japanese patients with type 2 diabetes mellitus: Results of a 24-week, randomized, double-blind, placebo-controlled trial. Diabetes Obes Metab. 2017;19(6):874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inagaki N, Kondo K, Yoshinari T, Maruyama N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin in Japanese patients with type 2 diabetes: a randomized, double-blind, placebo-controlled, 12-week study. Diabetes Obes Metab. 2013;15(12):1136–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inagaki N, Kondo K, Yoshinari T, Takahashi N, Susuta Y, Kuki H. Efficacy and safety of canagliflozin monotherapy in Japanese patients with type 2 diabetes inadequately controlled with diet and exercise: a 24-week, randomized, double-blind, placebo-controlled, Phase III study. Expert Opin Pharmacother. 2014;15(11):1501–15. [DOI] [PubMed] [Google Scholar]
- 30.Stenlöf K, Cefalu WT, Kim KA, Alba M, Usiskin K, Tong C, Canovatchel W, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15(4):372–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ji L, Liu Y, Miao H, Xie Y, Yang M, Wang W, Mu Y, et al. Safety and efficacy of ertugliflozin in Asian patients with type 2 diabetes mellitus inadequately controlled with metformin monotherapy: VERTIS Asia. Diabetes Obes Metab. 2019;21(6):1474–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Terra SG, Focht K, Davies M, Frias J, Derosa G, Darekar A, Golm G, et al. Phase III, efficacy and safety study of ertugliflozin monotherapy in people with type 2 diabetes mellitus inadequately controlled with diet and exercise alone. Diabetes Obes Metab. 2017;19(5):721–8. [DOI] [PubMed] [Google Scholar]
- 33.Dagogo-Jack S, Liu J, Eldor R, Amorin G, Johnson J, Hille D, Liao Y, et al. Efficacy and safety of the addition of ertugliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sitagliptin: The VERTIS SITA2 placebo-controlled randomized study. Diabetes Obes Metab. 2018;20(3):530–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kadowaki T, Haneda M, Inagaki N, Terauchi Y, Taniguchi A, Koiwai K, Rattunde H, et al. Empagliflozin monotherapy in Japanese patients with type 2 diabetes mellitus: a randomized, 12-week, double-blind, placebo-controlled, phase II trial. Adv Ther. 2014;31(6):621–38. [DOI] [PubMed] [Google Scholar]
- 35.Søfteland E, Meier JJ, Vangen B, Toorawa R, Maldonado-Lutomirsky M, Broedl UC. Empagliflozin as add-on therapy in patients with type 2 diabetes inadequately controlled with linagliptin and metformin: a 24-week randomized, double-blind, parallel-group trial. Diabetes Care. 2017;40(2):201–9. [DOI] [PubMed] [Google Scholar]
- 36.Nishimura R, Tanaka Y, Koiwai K, Inoue K, Hach T, Salsali A, Lund SS, et al. Effect of empagliflozin monotherapy on postprandial glucose and 24-hour glucose variability in Japanese patients with type 2 diabetes mellitus: a randomized, double-blind, placebo-controlled, 4-week study. Cardiovasc Diabetol. 2015;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ferrannini E, Seman L, Seewaldt-Becker E, Hantel S, Pinnetti S, Woerle HJ. A Phase IIb, randomized, placebo-controlled study of the SGLT2 inhibitor empagliflozin in patients with type 2 diabetes. Diabetes Obes Metab. 2013;15(8):721–8. [DOI] [PubMed] [Google Scholar]
- 38.Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ, Broedl UC. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1(3):208–19. [DOI] [PubMed] [Google Scholar]
- 39.Papadopoulou E, Loutradis C, Tzatzagou G, Kotsa K, Zografou I, Minopoulou I, Theodorakopoulou MP, et al. Dapagliflozin decreases ambulatory central blood pressure and pulse wave velocity in patients with type 2 diabetes: a randomized, double-blind, placebo-controlled clinical trial. J Hypertens. 2021;39(4):749–58. [DOI] [PubMed] [Google Scholar]
- 40.Yang W, Han P, Min KW, Wang B, Mansfield T, T’Joen C, Iqbal N, et al. Efficacy and safety of dapagliflozin in Asian patients with type 2 diabetes after metformin failure: a randomized controlled trial. J Diabetes. 2016;8(6):796–808. [DOI] [PubMed] [Google Scholar]
- 41.Bailey CJ, Gross JL, Pieters A, Bastien A, List JF. Effect of dapagliflozin in patients with type 2 diabetes who have inadequate glycaemic control with metformin: a randomised, double-blind, placebo-controlled trial. Lancet (London, England). 2010;375(9733):2223–33. [DOI] [PubMed] [Google Scholar]
- 42.Weng J, Zeng L, Zhang Y, Qu S, Wang X, Li P, Fu L, et al. Henagliflozin as add-on therapy to metformin in patients with type 2 diabetes inadequately controlled with metformin: A multicentre, randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Obes Metab. 2021;23(8):1754–64. [DOI] [PubMed] [Google Scholar]
- 43.Lu J, Fu L, Li Y, Geng J, Qin L, Li P, Zheng H, et al. Henagliflozin monotherapy in patients with type 2 diabetes inadequately controlled on diet and exercise: A randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Obes Metab. 2021;23(5):1111–20. [DOI] [PubMed] [Google Scholar]
- 44.Rosenstock J, Frias J, Páll D, Charbonnel B, Pascu R, Saur D, Darekar A, Huyck S, Shi H, Lauring B, et al. Effect of ertugliflozin on glucose control, body weight, blood pressure and bone density in type 2 diabetes mellitus inadequately controlled on metformin monotherapy (VERTIS MET). Diabetes Obes Metab. 2018;20(3):520–29. [DOI] [PubMed]
- 45.Lingvay I, Sumithran P, Cohen RV, le Roux CW. Obesity management as a primary treatment goal for type 2 diabetes: time to reframe the conversation. Lancet (London, England). 2022;399(10322):394–405. [DOI] [PubMed] [Google Scholar]
- 46.Peikert A, Vaduganathan M, Claggett BL, Kulac IJ, Foà A, Desai AS, Jhund PS, et al. Dapagliflozin in patients with heart failure and previous myocardial infarction: a participant-level pooled analysis of DAPA-HF and DELIVER. Eur J Heart Fail. 2024;26(4):912–24. [DOI] [PubMed] [Google Scholar]
- 47.Brown E, Heerspink HJL, Cuthbertson DJ, Wilding JPH. SGLT2 inhibitors and GLP-1 receptor agonists: established and emerging indications. Lancet (London, England). 2021;398(10296):262–76. [DOI] [PubMed] [Google Scholar]
- 48.Hussein H, Zaccardi F, Khunti K, Davies MJ, Patsko E, Dhalwani NN, Kloecker DE, et al. Efficacy and tolerability of sodium-glucose co-transporter-2 inhibitors and glucagon-like peptide-1 receptor agonists: a systematic review and network meta-analysis. Diabetes Obes Metab. 2020;22(7):1035–46. [DOI] [PubMed] [Google Scholar]
- 49.Zhou Z, Zheng M, Zuo Z, Wu T. Comparison of cardiovascular outcomes of new antihyperglycemic agents in type 2 diabetes mellitus: a meta-analysis. ESC Heart Fail. 2024;11(3):1647–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shi Q, Nong K, Vandvik PO, Guyatt GH, Schnell O, Rydén L, Marx N, et al. Benefits and harms of drug treatment for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ (Clinical research ed). 2023;381:e074068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this published article.