Abstract
The aim of the present study was to determine the prognostic significance of a novel marker, the red cell distribution width to lymphocyte percentage (RDW-to-LYM%) ratio, in patients with upper tract urothelial carcinoma (UTUC) after radical nephroureterectomy (RNU). The clinical and follow-up data of 625 patients with UTUC receiving RNU were retrospectively analyzed. The optimal cut-off value of the pre-treatment RDW-to-LYM% ratio was determined as 0.80 using receiver operating characteristic (ROC) analysis according to cancer-specific death. The associations between low (≤0.80) and high (>0.8) RDW-to-LYM% ratio and other clinicopathological parameters were evaluated using the χ2 test and logistic regression analysis. The impact of the RDW-to-LYM% ratio on overall survival (OS), cancer-specific survival (CSS) and progression-free survival (PFS) rates was assessed using the Kaplan-Meier method and Cox regression analysis. A high RDW-to-LYM% ratio (>0.80) was significantly associated with impaired kidney function, previous/concurrent bladder cancer, tumors involving both the pelvis and ureter, advanced pathological T stage, lymph node involvement and lymphovascular invasion (LVI). Kaplan-Meier analysis revealed that a high RDW-to-LYM% ratio was associated with poorer OS, CSS and PFS than a low RDW-to-LYM% ratio (all P<0.001). The multiple logistic regression analysis revealed that high RDW-to-LYM% ratio was associated with non-organ-confined (NOC) disease [odd ratio (OR), 2.107; 95% confidence interval (CI), 1.446-3.069; P<0.001] and positive LVI (OR, 1.978; 95% CI, 1.338-2.916; P<0.001). Furthermore, the multivariate analysis showed that the RDW-to-LYM% ratio was an independent factor for predicting OS [hazard ratio (HR), 2.046; P<0.001], CSS (HR, 2.041; P<0.001) and PFS (HR, 1.502; P=0.009). In conclusion, the pre-treatment RDW-to-LYM% ratio was found to be a significant predictor of both NOC and the presence of LVI in patients with UTUC. Moreover, an elevated pre-treatment RDW-to-LYM% ratio was identified as an independent factor for unfavorable survival outcomes in patients with UTUC undergoing RNU.
Keywords: red cell distribution width to lymphocyte percentage ratio, upper tract urothelial carcinoma, outcomes
Introduction
Upper tract urothelial carcinoma (UTUC) is a rare malignant disease of the urinary system, with a relatively higher prevalence in Taiwan compared with other countries (1-3). Due to the high risk of disease recurrence and progression, radical nephroureterectomy (RNU) is considered the gold-standard treatment for localized UTUC (4). Recent evidence has shown that neoadjuvant and adjuvant systemic therapy can improve outcomes in patients with UTUC who have unfavorable pathological characteristics, including advanced tumor stage, regional lymph node (LN) metastasis and lymphovascular invasion (LVI) positivity (5,6). While pathological tumor stage, LN status and LVI have been considered the most important factors for survival in patients with UTUC, these factors cannot provide preoperative treatment planning. Therefore, identifying preoperative prognostic factors is clinically valuable to make more precise pre-treatment assessments, such as whether to use systemic therapy before or after surgery, and to improve postoperative surveillance.
Aside from the conventional Tumor-Node-Metastasis (TNM) system, evidence suggests that systemic inflammation is also strongly associated with cancer growth/development and progression, and influences survival outcomes. Several clinical studies reported that pre-operative peripheral blood inflammation biomarkers, such as the neutrophil-lymphocyte ratio (NLR) (7,8), the platelet-lymphocyte ratio (PLR) (9-11), the monocyte-lymphocyte ratio (MLR) (11-13) and the systemic immune-inflammation index (SII; neutrophil x platelet/lymphocyte) (14,15) were associated with prognosis in several solid malignancies, including UTUC.
In addition, some studies reported that an elevated red cell distribution width (RDW) (16,17) or a reduced pretreatment lymphocyte percentage (LYM%) (18) was associated with poor survival. While increased RDW was considered to indirectly reflect progressive inflammation state, less circulating lymphocytes implied reduced antitumor immunity. Nevertheless, to the best of our knowledge, the clinical significance of integrating RDW and LYM% has not yet been evaluated in UTUC. Combining RDW and LYM% provides a better indicator than using RDW or LYM% alone, as it effectively reflects the balance between tumor inflammation and antitumor immunity. Therefore, the present study aimed to explore the associations between the pre-treatment RDW-to-LYM% ratio and clinicopathological parameters, and to identify the prognostic value of this ratio in UTUC in clinical practice.
Patients and methods
Study populations and data collection
The present study was approved by the Institutional Review Board of National Cheng Kung University Hospital (Tainan, Taiwan; approval no. NCKUH-B-ER-112-218). The cases of 625 patients (mean age, 69.3±11.0 years) with UTUC who had received an RNU between January 2008 and June 2020 were retrospectively analyzed. RNU was performed based on standard procedures, and the regional LNs were generally dissected if there was a preoperative presentation of enlargement on imaging studies or intraoperative findings of palpable nodes. In this study, exclusion criteria included active infection status, a lack of differential count information from preoperative complete blood counts (CBCs) 30 days prior to surgery, bone or distant metastasis at the time of diagnosis, other cancer diseases, current administration of immunosuppressive agents and a postoperative follow-up duration of <30 days. None of the patients received neo-adjuvant chemotherapy, radiotherapy or any other antitumor therapy. Adjuvant chemotherapy, consisting of at least 3 cycles of gemcitabine (800-1,000 mg/m2) and cisplatin (35-70 mg/m2) (or carboplatin; AUC, 4-6), was administered to patients with pathological T stage (pT≥2) and over or pathological node positivity (pN+) within 3 months of RNU.
Preoperative clinical and pathological data, including sex, age, comorbidities (diabetes mellitus and hypertension), symptoms (hydronephrosis and hematuria), preoperative estimated glomerular filtration rate (eGFR), prior or concomitant bladder urothelial carcinoma, tumor location (renal pelvis, ureter or synchronous), tumor size, tumor necrosis, pT stage, LN metastasis, tumor grade, LVI and CBC parameters (neutrophil/monocyte/lymphocyte percentages, white blood cell counts and platelet counts) were also recorded for further analysis. The overall survival (OS) time was defined as the time from RNU to death from any cause or the last follow-up. The cancer-specific survival (CSS) time was defined as the time from RNU until death due to UTUC. The progression-free survival (PFS) time was defined as the time from RNU until disease progression, including local recurrence or distant metastasis or death. Furthermore, bladder or contralateral upper urinary tract relapse was not defined as local recurrence. TNM classification was determined according to American Joint Committee on Cancer staging, 7th edition (18), and pathological grade was based on the 2004 World Health Organization classification (19). Preoperative eGFR was calculated with the Modification of Diet in Renal Disease Study equation as follows: 186 x (serum creatinine)-1.154 x (age)-0.203 x (0.742 if female) (19). Patients were considered to have chronic kidney disease if they had an eGFR value of <60 ml/min/1.73 m2 or if they received regular dialysis. Postoperative follow-up strategies included interval history taking, physical examination, urinalysis, urine cytology, abdominal ultrasonography and abdominal computed tomography every 6-12 months. Cystoscopy was performed every 3 months for the first 2 years, every 6 months for the next 2 years and annually thereafter.
Statistical analysis
Clinical data, including continuous and categorical variables, were analyzed using SPSS software (version 22.0; IBM Corp.). Based on cancer-specific death as the endpoint, the optimal cut-off value of the RDW-to-LYM% ratio was determined using a receiver operating characteristic (ROC) curve analysis and Youden's index. After all included patients were dichotomized into two groups based on the cut-off value of the RDW-to-LYM% ratio, Fisher's exact test and the χ2 test were used to compare the differences in clinicopathological variables between the two groups. Kaplan-Meier analysis was conducted to evaluate the association between RDW-to-LYM% ratio and OS, CSS and PFS, and the significant differences were determined using the log-rank test. Univariate and multivariate Cox regression analyses were performed to assess each variable for significance in terms of OS, CSS and PFS. P<0.05 was considered to indicate a statistically significant difference.
Results
Baseline characteristics of patients with localized UTUC and high or low pretreatment RDW-to-LYM% ratios
A total of 625 patients who received RNU for UTUC were enrolled in this study, with 47.8 months as the median follow-up time (Table I). In terms of preoperative clinical baseline parameters, 330 (53%) patients were >69 years old, 354 (57%) were female, 372 (60%) had chronic kidney disease (eGFR <60 ml/min/1.73 m2), 361 (58%) had underlying hypertension or diabetes mellitus, 543 (87%) had microscopic or gross hematuria and 492 (79%) presented with hydronephrosis (Table I). In terms of pathological information, prior and concomitant bladder tumors were noted in 92 (15%) and 121 (19%) patients, respectively, while tumor location was in the renal pelvis in 284 (45%) patients, in the ureter in 206 (33%) patients and in both locations in 135 (22%) patients. pTis/a/1, T2 and T3/4 stages were found in 229 (37%), 122 (20%) and 274 (44%) patients, respectively, 38 (6%) had positive LN metastasis and 595 (95%) had a high tumor grade. A total of 453 (72%) patients had larger tumor size (>2 cm), 181 (29%) had LVI, 122 (20%) had tumor necrosis and 61 (10%) received adjuvant chemotherapy (Table I).
Table I.
Associations between clinicopathological characteristics and high (n=470) or low (n=155) pre-treatment RDW-to-LYM% ratio in patients with upper tract urothelial carcinoma.
| RDW-to-LYM% ratio | ||||
|---|---|---|---|---|
| Variables | All patients | Low (≤0.80) | High (>0.80) | P-value |
| Mean age ± SD, years | 69.3±11.0 | 69.3±10.7 | 69.1±11.8 | |
| Median follow-up time after surgery, months | 47.8 | 51.5 | 31.1 | |
| Age, n (%) | 0.598 | |||
| ≤69 years | 295(47) | 219(47) | 76(49) | |
| >69 years | 330(53) | 251(53) | 79(51) | |
| Sex, n (%) | 0.969 | |||
| Male | 271(43) | 204(43) | 67(43) | |
| Female | 354(57) | 266(57) | 88(57) | |
| Renal function, n (%) | 0.005 | |||
| Pre-eGFR ≥60 ml/min/1.73 m2 | 253(40) | 205(44) | 48(31) | |
| Pre-eGFR <60 ml/min/1.73 m2 | 372(60) | 265(56) | 107(69) | |
| HTN or DM, n (%) | 0.300 | |||
| No | 264(42) | 193(41) | 71(46) | |
| Yes | 361(58) | 277(59) | 84(54) | |
| Hematuria, n (%) | 0.522 | |||
| No | 82(13) | 64(14) | 18(12) | |
| Yes | 543(87) | 406(86) | 137(88) | |
| Hydronephrosis, n (%) | 0.363 | |||
| No | 133(21) | 96(20) | 37(24) | |
| Yes | 492(79) | 374(80) | 118(76) | |
| Previous BC, n (%) | 0.016 | |||
| No | 533(85) | 410(87) | 123(79) | |
| Yes | 92(15) | 60(13) | 32(21) | |
| Concomitant BC, n (%) | 0.010 | |||
| No | 504(81) | 390(83) | 114(74) | |
| Yes | 121(19) | 80(17) | 41(26) | |
| Tumor location, n (%) | 0.003 | |||
| Pelvis | 284(45) | 210(45) | 74(48) | |
| Ureter | 206(33) | 170(36) | 36(23) | |
| Both | 135(22) | 90(19) | 45(29) | |
| Pathologic T stage, n (%) | <0.001 | |||
| Tis/a/1 | 229(37) | 184(39) | 45(29) | |
| T2 | 122(20) | 101(21) | 21(14) | |
| T3/4 | 274(44) | 185(39) | 89(57) | |
| Lymph node status, n (%) | <0.001 | |||
| N0 | 109(17) | 70(15) | 39(25) | |
| Nx | 478(76) | 380(81) | 98(63) | |
| N+ | 38(6) | 20(4) | 18(12) | |
| Tumor grade, n (%) | 0.290 | |||
| Low | 30(5) | 25(5) | 5(3) | |
| High | 595(95) | 445(95) | 150(97) | |
| Tumor size, n (%) | 0.045 | |||
| ≤2 cm | 172(28) | 139(30) | 33(21) | |
| >2 cm | 453(72) | 331(70) | 122(79) | |
| Lymphovascular invasion, n (%) | <0.001 | |||
| Absent | 444(71) | 351(75) | 93(60) | |
| Present | 181(29) | 119(25) | 62(40) | |
| Tumor necrosis, n (%) | 0.070 | |||
| Absent | 503(80) | 386(82) | 117(75) | |
| Present | 122(20) | 84(18) | 38(25) | |
| Adjuvant chemotherapy, n (%) | 0.968 | |||
| No | 564(90) | 424(90) | 140(90) | |
| Yes | 61(10) | 46(10) | 15(10) | |
RDW-to-LYM% ratio, red cell distribution width to lymphocyte percentage ratio; pre-eGFR, preoperative estimated glomerular filtration rate; BC, bladder cancer; HTN, hypertension; DM, diabetes mellitus.
Subsequently, with cancer-specific death as the endpoint, the optimal cut-off value of the pretreatment RDW-to-LYM% ratio was determined as 0.80 using ROC analysis (Fig. S1). After stratifying the 625 patients with UTUC into high- and low-level RDW-to-LYM% ratios (>0.80 and ≤0.80), the clinical and pathological characteristics were compared between the two groups. As shown in Table I, patients with pre-existing renal impairment, prior/concomitant bladder cancer, tumors located in both the renal pelvis and ureter, advanced pT stage (≥T3), lymph node metastasis, larger tumor size (>2 cm), and positive LVI tended to have high-level RDW-to-LYM% ratios (all P<0.05). Conversely, the differences in age, sex, underlying diseases (diabetes mellitus or hypertension), preoperative clinical symptoms (hematuria or hydronephrosis), tumor grade, tumor necrosis and administration of adjuvant chemotherapy were not noted to be significant between the two groups.
Association between pre-treatment RDW-to-LYM% ratio and NOC disease and LVI
In the present study, 278 (44%) patients had NOC disease and 181 (29%) were positive for LVI. Among them, 50% (138/278) with NOC disease and 54% (98/181) who were positive for LVI were classified as having a high-level RDW-to-LYM% ratio (data not shown). In the pre-treatment model to predict NOC disease and the presence of LVI, multivariate logistic regression analyses revealed that a high-level RDW-to-LYM% ratio was significantly associated with NOC disease [odds ratio (OR), 2.107; 95% confidence interval (CI), 1.446-3.069; P<0.001] and positive LVI (OR, 1.978; 95% CI, 1.338-2.916; P<0.001) (Tables II and III). However, other preoperative variables, including age, sex, renal function, underlying HTN or DM, and hematuria/hydronephrosis and bladder cancer history, were not associated.
Table II.
Univariate and multivariate logistic regression preoperative model analyses for prediction of NOC disease (≥pT3 and/or positive lymph nodes) based on the pre-treatment RDW-to-LYM% ratio.
| NOC disease | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age at RNU | ||||
| >69 vs. ≤69 years | 0.955 (0.696-1.309) | 0.774 | 1.015 (0.731-1.410) | 0.929 |
| Sex | ||||
| Female vs. male | 0.739 (0.537-1.016) | 0.063 | 0.731 (0.527-1.014) | 0.061 |
| Pre-eGFR | ||||
| <60 vs. ≥60 ml/min/1.73 m2 | 0.911 (0.661-1.256) | 0.570 | 0.882 (0.630-1.236) | 0.466 |
| DM or HTN | ||||
| Present vs. absent | 0.934 (0.679-1.285) | 0.675 | 0.998 (0.716-1.390) | 0.998 |
| Previous BC | ||||
| Yes vs. no | 0.773 (0.492-1.215) | 0.264 | 0727 (0.456-1.160) | 0.181 |
| Hematuria | ||||
| Present vs. absent | 0.867 (0.544-1.380) | 0.547 | 0.816 (0.50-1.314) | 0.428 |
| Hydronephrosis | ||||
| Present vs. absent | 0.862 (0.587-1.266) | 0.450 | 0.864 (0.581-1.283) | 0.469 |
| RDW-to-LYM% ratio | ||||
| High vs. low | 2.005 (1.388-2.897) | <0.001 | 2.107 (1.446-3.069) | <0.001 |
NOC, non-organ confined; RDW-to-LYM% ratio, red cell distribution width to peripheral lymphocyte percentage ratio; RNU, radical nephroureterectomy; pre-eGFR, preoperative estimated glomerular filtration rate; DM, diabetes mellitus; HTN, hypertension; BC, bladder cancer; HR, hazard ratio; CI, confidence interval.
Table III.
Univariate and multivariate logistic regression preoperative model analyses for prediction of presence of LVI based on pre-treatment RDW-to-LYM% ratio.
| LVI | ||||
|---|---|---|---|---|
| Univariate | Multivariate | |||
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age at RNU | ||||
| >69 vs. ≤69 years | 0.923 (0.653-1.305) | 0.650 | 1.036 (0.697-1.539) | 0.861 |
| Sex | ||||
| Female vs. male | 0.814 (0.575-1.153) | 0.246 | 0.799 (0.560-1.140) | 0.216 |
| Pre-eGFR | ||||
| <60 vs. ≥60 ml/min/1.73 m2 | 1.009 (0.709-1.435) | 0.961 | 0.965 (0.667-1.395) | 0.457 |
| DM or HTN | ||||
| Present vs. absent | 0.952 (0.671-1.350) | 0.783 | 1.020 (0.711-1.464) | 0.914 |
| Previous BC | ||||
| Yes vs. no | 1.087 (0.671-1.760) | 0.736 | 1.015 (0.617-1.667 | 0.955 |
| Hematuria | ||||
| Present vs. absent | 0.806 (0.491-1.326) | 0.396 | 0.744 (0.448-1.236) | 0.254 |
| Hydronephrosis | ||||
| Present vs. absent | 0.747 (0.496-1.126) | 0.163 | 0.742 (0.487-1.130) | 0.164 |
| RDW-to-LYM% ratio | ||||
| High vs. low | 1.966 (1.341-2.883) | <0.001 | 1.978 (1.338-2.916) | <0.001 |
LVI, lymphovascular invasion; RNU, radical nephroureterectomy; pre-eGFR, preoperative estimated glomerular filtration rate; DM, diabetes mellitus; HTN, hypertension; BC, bladder cancer; RDW-to-LYM% ratio, red cell distribution width to peripheral lymphocyte percentage ratio; HR, hazard ratio; CI, confidence interval.
Association between pre-treatment RDW-to-LYM% ratio and poor survival outcomes
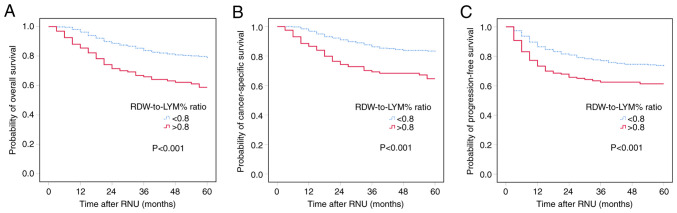
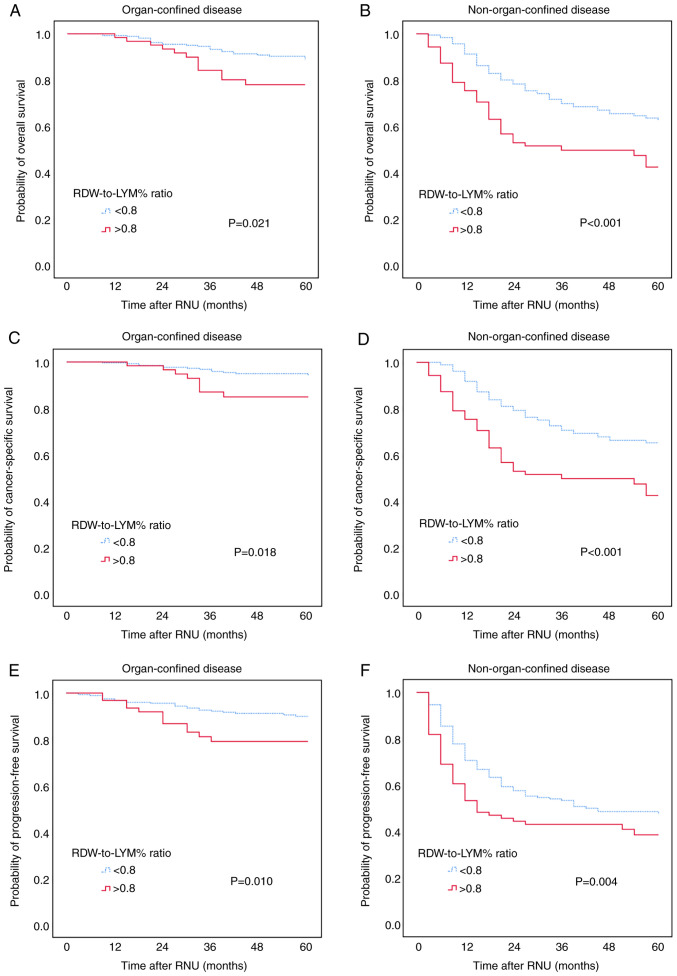
Kaplan-Meier analysis was performed to evaluate the associations between pre-treatment RDW-to-LYM% ratio and OS, CSS and PFS. The results showed that the high-level RDW-to-LYM% ratio was significantly associated with worse OS, CSS and PFS, with all P<0.001 for all comparisons (Fig. 1). The effect of the pre-treatment RDW-to-LYM% ratio on OS, CSS and PFS in different pathological tumor stages was also assessed. The patients were sub-grouped into organ-confined and NOC UTUC. Kaplan-Meier analysis showed that a high-level RDW-to-LYM% ratio was associated with significantly shorter OS, CSS and PFS times (all P<0.001) compared with a low RDW-to-LYM% ratio in both organ-confined and NOC disease (Fig. 2).
Figure 1.
Kaplan-Meier analysis for (A) overall survival, (B) cancer-specific survival and (C) progression-free survival in patients with upper tract urothelial carcinoma based on a high and low pre-treatment RDW-to-LYM% ratio. RDW-to-LYM% ratio, red cell distribution width to lymphocyte percentage ratio; RNU, radical nephroureterectomy.
Figure 2.
Kaplan-Meier analysis for (A and B) overall survival, (C and D) cancer-specific survival and (E and F) progression-free survival in patients with (A, C and E) non-organ-confined and (B, D and F) organ-confined upper tract urothelial carcinoma stratified based on the pre-treatment RDW-to-LYM% ratio. RDW-to-LYM% ratio, red cell distribution width to lymphocyte percentage ratio; RNU, radical nephroureterectomy.
Furthermore, a multivariate Cox regression analysis was conducted to determine the prognostic factors that independently influenced survival in the patients. From the results of the multivariate analyses (Table IV), old age, male, tumor localizing in the ureter or both ureter and pelvis, advanced pT stage (pT3/4), pN+, positive LVI and high RDW-to-LYM% ratio significantly conferred the negative impacts on OS and CSS. Regarding PFS, the high RDW-to-LYM% ratio was still an independent predicting factor, and other significant factors included older age, sex, tumors localizing in the ureter or both ureter and pelvis, advanced pT3/4 stage, LN-positive status and the presence of LVI.
Table IV.
Multivariate analyses for OS, CSS and PFS.
| OS | CSS | PFS | ||||
|---|---|---|---|---|---|---|
| Parameter | HR (95% CI) | P-value | HR (95% CI) | P-value | HR (95% CI) | P-value |
| Age at RNU years (continuous variable) | 1.025 (1.009-1.043) | 0.003 | 1.023 (1.005-1.040) | 0.011 | 1.015 (1.001-1.029) | 0.030 |
| Sex | ||||||
| Female vs. male | 0.627 (0.453-0.868) | 0.005 | 0.575 (0.408-0.810) | 0.002 | 0.729 (0.542-0.979) | 0.036 |
| Renal function | ||||||
| ≤60 vs. >60 ml/min/1.73 m2 | 1.109 (0.762-1.615) | 0.589 | 1.021 (0.691-1.508) | 0.918 | 0.934 (0.673-1.269) | 0.683 |
| HTN or DM | ||||||
| Yes vs. no | 1.374 (0.972-1.941) | 0.072 | 1.330 (0.927-1.909) | 0.121 | 1.302 (0.967-1.752) | 0.082 |
| Previous BC | ||||||
| Yes vs. no | 0.958 (0.602-1.525) | 0.905 | 0.889 (0.534-1.480) | 0.651 | 1.196 (0.811-1.765) | 0.367 |
| Concomitant BC | ||||||
| Yes vs. no | 1.024 (0.695-1.509) | 0.856 | 1.047 (0.699-1.570) | 0.823 | 1.131 (0.810-1.578) | 0.471 |
| Hydronephrosis | ||||||
| Present vs. absent | 0.824 (0.535-1.269) | 0.380 | 0.826 (0.524-1.302) | 0.410 | 0.784 (0.545-1.128) | 0.190 |
| Hematuria | ||||||
| Present vs. absent | 1.157 (0.703-1.902) | 0.567 | 1.126 (0.673-1.886) | 0.652 | 1.013 (0.661-1.552) | 0.954 |
| Tumor location | ||||||
| Ureter vs. renal pelvis | 1.694 (1.068-2.687) | 0.025 | 1.730 (1.065-2.809) | 0.027 | 1.588 (1.079-2.337) | 0.019 |
| Both vs. renal pelvis | 2.197 (1.462-3.302) | <0.001 | 2.170 (1.412-3.335) | <0.001 | 1.843 (1.290-2.632) | 0.001 |
| Pathological T stage | ||||||
| T2 vs. Tis/a/1 | 1.398 (0.777-2.516) | 0.263 | 3.194 (1.501-6.795) | 0.003 | 1.430 (0.994-2.409) | 0.179 |
| T3/4 vs. Tis/a/1 | 2.730 (1.667-4.469) | <0.001 | 6.209 (3.135-12.299) | <0.001 | 3.242 (2.111-4.979) | <0.001 |
| Lymph node stage | ||||||
| Nx vs. N0 | 0.907 (0.593-1.387) | 0.651 | 0.804 (0.520-1.244) | 0.327 | 1.079 (0.742-1.569) | 0.689 |
| N+ vs. N0 | 2.948 (1.656-5.246) | <0.001 | 2.803 (1.569-5.009) | 0.001 | 2.994 (1.801-4.997) | <0.001 |
| Tumor grade | ||||||
| High vs. low | 1.737 (0.521-5.793) | 0.369 | 4.355 (0.567-33.426) | 0.157 | 1.369 (0.423-4.429) | 0.600 |
| Tumor size | ||||||
| >2 vs. ≤2 cm | 1.218 (0.762-1.948) | 0.410 | 1.180 (0.711-1.959) | 0.453 | 1.429 (0.960-2.126) | 0.078 |
| Lymphovascular invasion | ||||||
| Present vs. absent | 1.798 (1.235-2.619) | 0.002 | 1.785 (1.208-2.638) | 0.004 | 1.933 (1.403-2.662) | <0.001 |
| Tumor necrosis | ||||||
| Present vs. absent | 1.321 (0.898-1.944) | 0.157 | 1.260 (0.841-1.89) | 0.263 | 1.089 (0.7776-1.527) | 0.622 |
| Adjuvant chemotherapy | ||||||
| Yes vs. no | 0.590 (0.330-1.056) | 0.076 | 0.545 (0.302-1.014) | 0.064 | 1.012 (0.957-1.577) | 0.957 |
| RDW-to-LYM% ratio | ||||||
| High (>0.80) vs. low (<0.80) | 2.046 (1.441-2.906) | <0.001 | 2.041 (1.415-2.945) | <0.001 | 1.502 (1.105-2.042) | 0.009 |
OS, overall survival; CSS, cancer-specific survival; PFS, progression-free survival; RNU, radical nephroureterectomy; eGFR, preoperative estimated glomerular filtration rate; HTN, hypertension; DM, diabetes mellitus; BC, bladder cancer; RDW-to-LYM% ratio, red cell distribution width to peripheral lymphocyte percentage ratio.
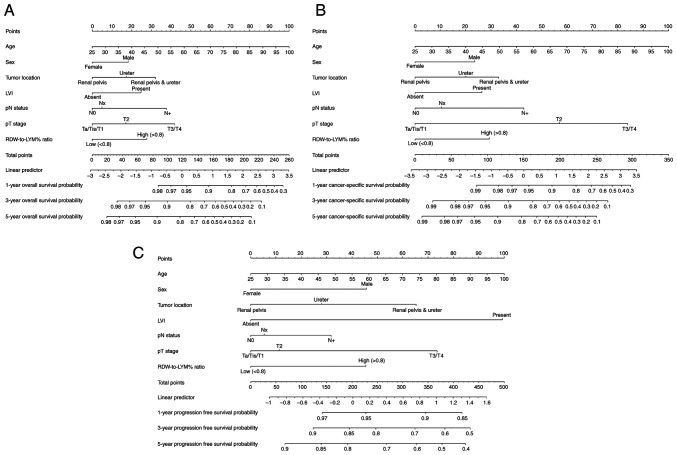
Development of a nomogram that includes pre-treatment RDW-to-LYM% ratio to predict survival outcomes
Considering all independent variables for survival outcomes in multivariate analyses, a prognostic nomogram was developed for OS, CSS and PFS in patients with UTUC after RNU (Fig. 3). This nomogram incorporated pre-treatment RDW-to-LYM% ratio with age, sex, tumor location, pT stage, LN status and LVI. According to this nomogram, the 1-, 3- and 5-year survival rates can be easily obtained.
Figure 3.
Nomograms for predicting OS, CSS and PFS. Nomograms based on the independent prognostic factors for the prediction of 1-, 3- and 5-year (A) OS, (B) CSS and (C) PFS rates. LVI, lymphovascular invasion; RDW-to-LYM% ratio, red cell distribution width to lymphocyte percentage ratio; OS, overall survival; CSS, cause-specific survival; PFS, progression-free survival; pN, pathological node; pT, pathological tumor.
Discussion
Various predictive factors derived from blood-based inflammatory immune cells have been used to assess the prognosis of patients with UTUC undergoing RNU. For example, RDW, lymphocyte counts, NLR, PLR, MLR, lymphocyte-to-monocyte ratio and SII, among others, have been demonstrated to predict oncological outcomes in patients with non-metastatic UTUC (7,9,11,13,18). Previous studies reported that the RDW-to-lymphocyte count ratio was associated with prognosis in cutaneous malignant melanoma (20) and renal cell carcinoma (21). Simultaneously assessing RDW and LYM% for patients with cancer could provide information about systemic inflammation and immunity in the presence of tumors. However, to the best of our knowledge, the combination of RDW and LYM% has not been evaluated for predicting oncological outcomes in patients with UTUC after RNU.
In the present study, an increased RDW-to-LYM% ratio (>0.80) was markedly associated with more aggressive pathological phenotypes, including synchronous involvement of the ureter and pelvis, advanced tumor stage, regional LN metastasis and positive LVI, in patients with UTUC. Patients with renal impairment or previous/concomitant BC also tended to have a high RDW-to-LYM% ratio. In the preoperative model, a relatively higher RDW-to-LYM% ratio had the potency to predict NOC disease and the presence of LVI. Furthermore, the high-level RDW-to-LYM% ratio was associated with decreased OS, CSS and PFS times, particularly in NOC disease. Furthermore, in the present cohort, <20% of patients with localized advanced (≥pT2 or pN+) UTUC received adjuvant chemotherapy, as there was no significant evidence on the survival benefit of adjuvant platinum-based chemotherapy before the POUT trial (5). In the present study, adjuvant chemotherapy possibly provided a trend in survival benefits, but did not reach significance. Notably, the RDW-to-LYM% ratio was identified as an independent prognostic factor for predicting worse outcomes, which could potentially aid in treatment decision-making, such as administering systemic therapy before or after surgery. To the best of our knowledge, the present study is the first to describe the prognostic significance of the pre-treatment RDW-to-LYM% ratio in UTUC.
Tumor progression and metastases are closely associated with systemic inflammation (22,23). There is a mutually promoting interaction between inflammation and tumor progression. Inappropriate inflammation precedes and promotes tumor development/growth and progression, and tumor progression induces and drives inflammatory reactions. Numerous clinical studies in various cancer types, including breast, esophageal, gastrointestinal, pancreatic and upper urinary tract cancer, found that changes in peripheral blood immune cell numbers or proportions indirectly denoted tumor-related inflammation status (7-15). According to previous studies, peripheral neutrophils, monocytes or platelets were considered to facilitate tumor malignant behaviors, whereas lymphocytes were responsible for weakening tumor cell viability. Decreased lymphocyte counts (abnormally low absolute lymphocyte counts) were associated with poor outcomes in some solid cancer types, such as oropharyngeal, cervical, lung and breast cancer (24-27). Furthermore, several studies adopted other blood parameters unrelated to immune cells, such as RDW or C-reactive protein (CRP), as inflammation biomarkers for predicting outcomes in patients with cancer (16,17,28-31). Increasing preoperative RDW was reported to negatively affect prognosis in bladder and prostate cancer (16,17,28). RDW was used to diagnose anemia, and indirectly reflected oxidative stress and inflammation status (32). RDW was also closely linked to various inflammatory cytokines, such as interleukin-6, enterococcal surface protein and CRP (33,34). Elevated RDW may imply an increase in systemic inflammation, thus potentially enhancing tumor aggressiveness and resulting in tumor progression and metastasis.
In the present study, increased RDW combined with decreased LYM% was significantly associated with poorer OS, CSS and PFS times in patients with UTUC after RNU, particularly in those with NOC disease. ROC analysis revealed that on their own, RDW or LYM% did not better predict survival outcomes compared with the RDW-to-LYM% ratio (Fig. S1). The pretreatment RDW-to-LYM% ratio as a combination marker is derived from RDW and LYM% values. Given that RDW and LYM% are pro-tumor and antitumor inflammation markers, respectively, the RDW-to-LYM% ratio could reflect the imbalance between pro- and anti-tumor immunity, rather than RDW or LYM% alone. An increase in the RDW-to-LYM% ratio value is mathematically attributed to relatively increased RDW and/or decreased LYM%. As aforementioned, increased RDW levels may indicate an increase in the systemic inflammation state, and a reduced lymphocyte distribution may imply a weak immune response to the tumor. We suggest that an elevated RDW-to-LYM% ratio in patients UTUC may reflect a clinical state with more inflammatory burden and less antitumor immunity. As such, patients with a higher RDW-to-LYM% ratio are at risk of worse survival times and disease progression. The present study emphasized that the pre-treatment RDW-to-LYM% ratio remained statistically significant for predicting OS, CSS and PFS after adjusting several important and relevant risk factors, including tumor location, pT stage, LN status and LVI. Furthermore, the pretreatment RDW-to-LYM% ratio had the potency to predict NOC and the presence of LVI. Since the clinical staging of UTUC has been dependent on imaging and ureterorenoscopy biopsy to date, the pretreatment RDW-to-LYM% ratio will provide additional assistance to improve the accuracy of the clinical staging of UTUC.
Taken together, the results of the present study are, to the best of our knowledge, the first to report the prognostic significance of combining RDW and LYM% for determining OS, CSS and PFS in patients with UTUC after RNU. The optimal cut-off value of 0.80 for the pre-treatment RDW-to-LYM% ratio significantly distinguished high-risk patients who had unfavorable outcomes after surgery from the cohort. As the rising RDW-to-LYM% ratio was attributed to increased RDW or/and decreased LYM%, it contributed to benefit the survival and proliferation of the tumor, and even metastasis, eventually leading to adverse patient survival outcomes. Assessment of the pre-treatment RDW-to-LYM% ratio before surgery was low in cost and easily accessible in clinical practice. Furthermore, a pre-treatment RDW-to-LYM% ratio was employed to establish a nomogram for predicting prognosis in UTUC. This nomogram can be clinically applied to understand the specific survival probabilities and risk factors for each patient; it can serve as a decision-support tool, helping clinicians identify patients with a high risk of post-operative recurrence, and it further assists in deciding the timing and intensity of adjuvant treatment.
There are some limitations to the present study. First, this was a single-center and retrospective study, which might give rise to selection bias. Secondly, all patients in this study were Taiwanese, and Taiwan is known for its high incidence of UTUC. The single ethnic group and limited area might affect the result. Last but not least, this study lacked convincing molecular biomarkers that involved the systemic inflammation and tumor microenvironment, such as CRP, CD4 or CD8 for T cells, or CD68 and CD163 for macrophages. All of these limitations may indicate that future multicenter and prospective studies are required.
In conclusion, the present study found that an elevated pre-treatment RDW-to-LYM% ratio was associated with more advanced stage UTUC and a poorer prognosis after RNU. Moreover, the pre-treatment RDW-to-LYM% ratio was demonstrated to be an independent prognostic factor, potentially aiding in treatment planning for patients with UTUC.
Supplementary Material
Acknowledgements
The authors are grateful to Dr Sheng-Hsiang Lin and Ms. Wan-Ni Chen from the Biostatistics Consulting Center, Clinical Medicine Research Center, National Cheng Kung University Hospital (Tainan, Taiwan) for providing statistical consulting services.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The data generated in the present study may be requested from the corresponding author.
Authors' contributions
YCH and HCJ conceived the study and analyzed data. THC, CYH, WHY, CHO and HCJ collected data. CAW, CYH, WHY and CHO interpreted the data. YCH, THC and CAW wrote the manuscript draft. CHO and HCJ supervised the study and reviewed and edited the manuscript. YCH, THC, CAW, CHO and HCJ confirm the authenticity of all the raw data. All authors read and approved the final version of the manuscript.
Ethics approval and consent to participate
The study received approval from the Institutional Review Board of National Cheng-Kung University Hospital (Tainan, Taiwan; approval no. NCKUH-B-ER-112-218). The board waived the necessity for informed consent from the participants and granted access to follow-up clinical records. The study adhered to the guidelines outlined in the Declaration of Helsinki.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Use of artificial intelligence tools
During the preparation of this work, AI tools were used to improve and revise the manuscript's grammar as necessary. The authors take full responsibility for the ultimate content of the present manuscript.
References
- 1.Rouprêt M, Babjuk M, Burger M, Capoun O, Cohen D, Compérat EM, Cowan NC, Dominguez-Escrig JL, Gontero P, Hugh Mostafid A, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2020 Update. Eur Urol. 2021;79:62–79. doi: 10.1016/j.eururo.2020.05.042. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A . Cancer statistics, 2019. CA Cancer J Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 3.Chang YH, Hsu WL, Lee YK, Chiang CJ, Yang YW, You SL, Chen YC, Lai TS. Trends and sex-specific incidence of upper urinary tract cancer in Taiwan: A birth cohort study. Cancer Med. 2023;12:15350–15357. doi: 10.1002/cam4.6084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Margulis V, Shariat SF, Matin SF, Kamat AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD, Wood CG. Outcomes of radical nephroureterectomy: A series from the upper tract urothelial carcinoma collaboration. Cancer. 2009;115:1224–1233. doi: 10.1002/cncr.24135. Upper Tract Urothelial Carcinoma CollaborationThe Upper Tract Urothelial Carcinoma Collaboration. [DOI] [PubMed] [Google Scholar]
- 5.Birtle A, Johnson M, Chester J, Jones R, Dolling D, Bryan RT, Harris C, Winterbottom A, Blacker A, Catto JWF, et al. Adjuvant chemotherapy in upper tract urothelial carcinoma (the POUT trial): A phase 3, open-label, randomised controlled trial. Lancet. 2020;395:1268–1277. doi: 10.1016/S0140-6736(20)30415-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leow JJ, Chong YL, Chang SL, Valderrama BP, Powles T, Bellmunt J. Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: A 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur Urol. 2021;79:635–654. doi: 10.1016/j.eururo.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 7.Vartolomei MD, Kimura S, Ferro M, Vartolomei L, Foerster B, Abufaraj M, Shariat SF. Is neutrophil-to-lymphocytes ratio a clinical relevant preoperative biomarker in upper tract urothelial carcinoma? A meta-analysis of 4385 patients. World J Urol. 2018;36:1019–1029. doi: 10.1007/s00345-018-2235-5. [DOI] [PubMed] [Google Scholar]
- 8.Duan J, Pan L, Yang M. Preoperative elevated neutrophil-to-lymphocyte ratio (NLR) and derived NLR are associated with poor prognosis in patients with breast cancer: A meta-analysis. Medicine (Baltimore) 2018;97(e13340) doi: 10.1097/MD.0000000000013340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dalpiaz O, Krieger D, Ehrlich GC, Pohlmann K, Stojakovic T, Pummer K, Zigeuner R, Pichler M, Hutterer GC. Validation of the preoperative platelet-to-lymphocyte ratio as a prognostic factor in a European cohort of patients with upper tract urothelial carcinoma. Urol Int. 2017;98:320–327. doi: 10.1159/000452109. [DOI] [PubMed] [Google Scholar]
- 10.Feng JF, Huang Y, Chen QX. Preoperative platelet lymphocyte ratio (PLR) is superior to neutrophil lymphocyte ratio (NLR) as a predictive factor in patients with esophageal squamous cell carcinoma. World J Surg Oncol. 2014;12(58) doi: 10.1186/1477-7819-12-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jan HC, Hu CY, Yang WH, Ou CH. Combination of platelet-lymphocyte ratio and monocyte-lymphocyte ratio as a new promising prognostic factor in upper tract urothelial carcinoma with large tumor sizes >3 cm. Clin Genitourin Cancer. 2020;18:e484–e500. doi: 10.1016/j.clgc.2019.12.008. [DOI] [PubMed] [Google Scholar]
- 12.Li T, Xu H, Yang L, Tan P, Wei Q. Predictive value of preoperative lymphocyte-to-monocyte ratio for patients with upper tract urothelial carcinoma. Clin Chim Acta. 2019;492:50–56. doi: 10.1016/j.cca.2019.02.007. [DOI] [PubMed] [Google Scholar]
- 13.Cananzi FCM, Minerva EM, Samà L, Ruspi L, Sicoli F, Conti L, Fumagalli Romario U, Quagliuolo VL. Preoperative monocyte-to-lymphocyte ratio predicts recurrence in gastrointestinal stromal tumors. J Surg Oncol. 2019;119:12–20. doi: 10.1002/jso.25290. [DOI] [PubMed] [Google Scholar]
- 14.Jan HC, Yang WH, Ou CH. Combination of the preoperative systemic immune-inflammation index and monocyte-lymphocyte ratio as a novel prognostic factor in patients with upper-tract urothelial carcinoma. Ann Surg Oncol. 2019;26:669–684. doi: 10.1245/s10434-018-6942-3. [DOI] [PubMed] [Google Scholar]
- 15.Aziz MH, Sideras K, Aziz NA, Mauff K, Haen R, Roos D, Saida L, Suker M, van der Harst E, Mieog JS, et al. The systemic-immune-inflammation index independently predicts survival and recurrence in resectable pancreatic cancer and its prognostic value depends on bilirubin levels: A retrospective multicenter cohort study. Ann Surg. 2019;270:139–146. doi: 10.1097/SLA.0000000000002660. [DOI] [PubMed] [Google Scholar]
- 16.Ma W, Mao S, Bao M, Wu Y, Guo Y, Liu J, Wang R, Li C, Zhang J, Zhang W, Yao X. Prognostic significance of red cell distribution width in bladder cancer. Transl Androl Urol. 2020;9:295–302. doi: 10.21037/tau.2020.03.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang TB, Zhu LY, Zhou GC, Ding XF. Pre-treatment red blood cell distribution width as a predictor of clinically significant prostate cancer. Int Urol Nephrol. 2021;53:1765–1771. doi: 10.1007/s11255-021-02900-z. [DOI] [PubMed] [Google Scholar]
- 18.Huang H, Li L, Luo W, Yang Y, Ni Y, Song T, Zhu Y, Yang Y, Zhang L. Lymphocyte percentage as a valuable predictor of prognosis in lung cancer. J Cell Mol Med. 2022;26:1918–1931. doi: 10.1111/jcmm.17214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Modification of diet in renal disease study group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 20.Edge SB, Compton CC. The American joint committee on cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 21.Eble JN, Sauter G, Epstein JI, Sesterhenn IA (eds) Pathology and genetics of tumours of the urinary system and male genital organs. WHO Classification of Tumours. IARC Press, Lyon, 2004. [Google Scholar]
- 22.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolan RD, Lim J, McSorley ST, Horgan PG, McMillan DC. The role of the systemic inflammatory response in predicting outcomes in patients with operable cancer: Systematic review and meta-analysis. Sci Rep. 2017;7(16717) doi: 10.1038/s41598-017-16955-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Price JM, Mistry HB, Betts G, Cheadle EJ, Dixon L, Garcez K, Illidge T, Iyizoba-Ebozue Z, Lee LW, McPartlin A, et al. Pretreatment lymphocyte count predicts benefit from concurrent chemotherapy with radiotherapy in oropharyngeal cancer. J Clin Oncol. 2022;40:2203–2212. doi: 10.1200/JCO.21.01991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Thiangphak E, Pruegsanusak K, Buhachat R. Pretreatment lymphocyte count as independent prognostic factor in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy. Int J Gynaecol Obstet. 2022;159:672–678. doi: 10.1002/ijgo.14229. [DOI] [PubMed] [Google Scholar]
- 26.Kobayashi N, Usui S, Kikuchi S, Goto Y, Sakai M, Onizuka M, Sato Y. Preoperative lymphocyte count is an independent prognostic factor in node-negative non-small cell lung cancer. Lung Cancer. 2012;75:223–227. doi: 10.1016/j.lungcan.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 27.Jimbo H, Horimoto Y, Ishizuka Y, Nogami N, Shikanai A, Saito M, Watanabe J. Absolute lymphocyte count decreases with disease progression and is a potential prognostic marker for metastatic breast cancer. Breast Cancer Res Treat. 2022;196:291–298. doi: 10.1007/s10549-022-06748-4. [DOI] [PubMed] [Google Scholar]
- 28.Fukuokaya W, Kimura T, Miki J, Kimura S, Watanabe H, Bo F, Okada D, Aikawa K, Ochi A, Suzuki K, et al. Red cell distribution width predicts time to recurrence in patients with primary non-muscle-invasive bladder cancer and improves the accuracy of the EORTC scoring system. Urol Oncol. 2020;38:638.e15–638.e23. doi: 10.1016/j.urolonc.2020.01.016. [DOI] [PubMed] [Google Scholar]
- 29.Thurner EM, Krenn-Pilko S, Langsenlehner U, Stojakovic T, Pichler M, Gerger A, Kapp KS, Langsenlehner T. The elevated C-reactive protein level is associated with poor prognosis in prostate cancer patients treated with radiotherapy. Eur J Cancer. 2015;51:610–619. doi: 10.1016/j.ejca.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Szkandera J, Stotz M, Absenger G, Stojakovic T, Samonigg H, Kornprat P, Schaberl-Moser R, Alzoughbi W, Lackner C, Ress AL, et al. Validation of C-reactive protein levels as a prognostic indicator for survival in a large cohort of pancreatic cancer patients. Br J Cancer. 2014;110:183–188. doi: 10.1038/bjc.2013.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Iivanainen S, Ahvonen J, Knuuttila A, Tiainen S, Koivunen JP. Elevated CRP levels indicate poor progression-free and overall survival on cancer patients treated with PD-1 inhibitors. ESMO Open. 2019;4(e000531) doi: 10.1136/esmoopen-2019-000531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. Crit Rev Clin Lab Sci. 2015;52:86–105. doi: 10.3109/10408363.2014.992064. [DOI] [PubMed] [Google Scholar]
- 33.de Gonzalo-Calvo D, de Luxán-Delgado B, Rodríguez-González S, García-Macia M, Suárez FM, Solano JJ, Rodríguez-Colunga MJ, Coto-Montes A. Interleukin 6, soluble tumor necrosis factor receptor I and red blood cell distribution width as biological markers of functional dependence in an elderly population: A translational approach. Cytokine. 2012;58:193–198. doi: 10.1016/j.cyto.2012.01.005. [DOI] [PubMed] [Google Scholar]
- 34.Lippi G, Targher G, Montagnana M, Salvagno GL, Zoppini G, Guidi GC. Relation between red blood cell distribution width and inflammatory biomarkers in a large cohort of unselected outpatients. Arch Pathol Lab Med. 2009;133:628–632. doi: 10.5858/133.4.628. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated in the present study may be requested from the corresponding author.