Abstract
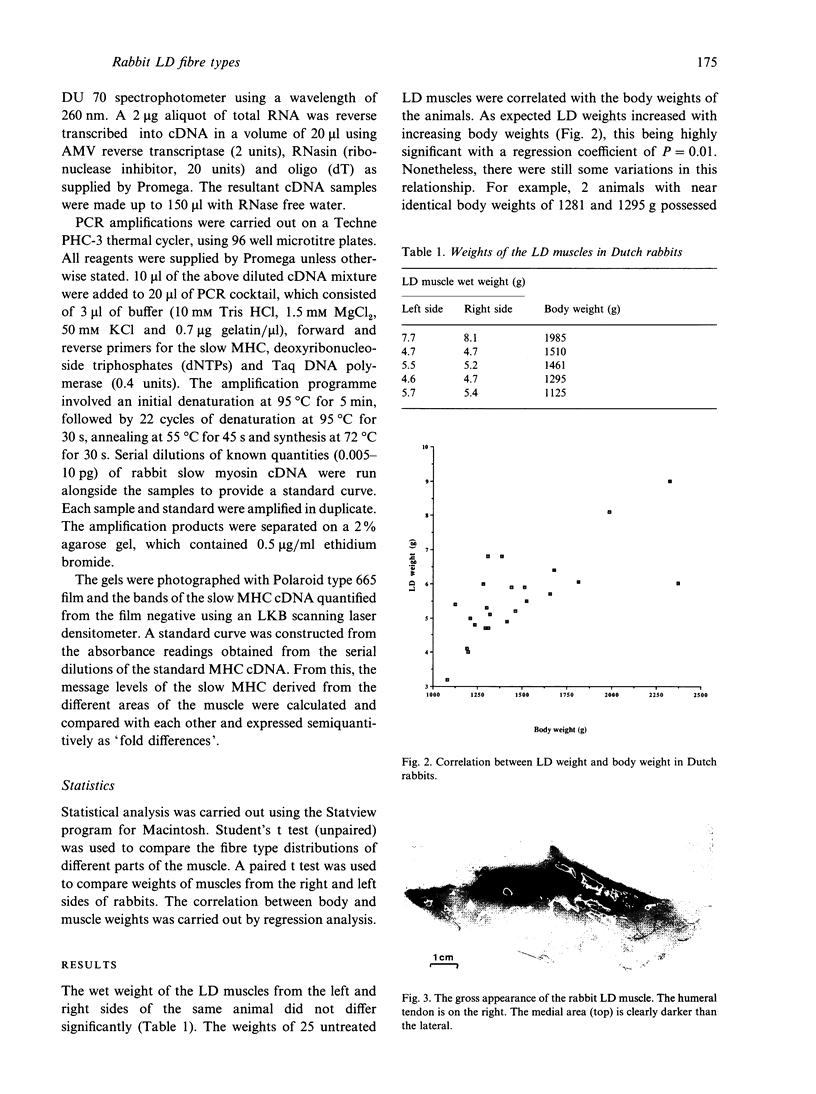
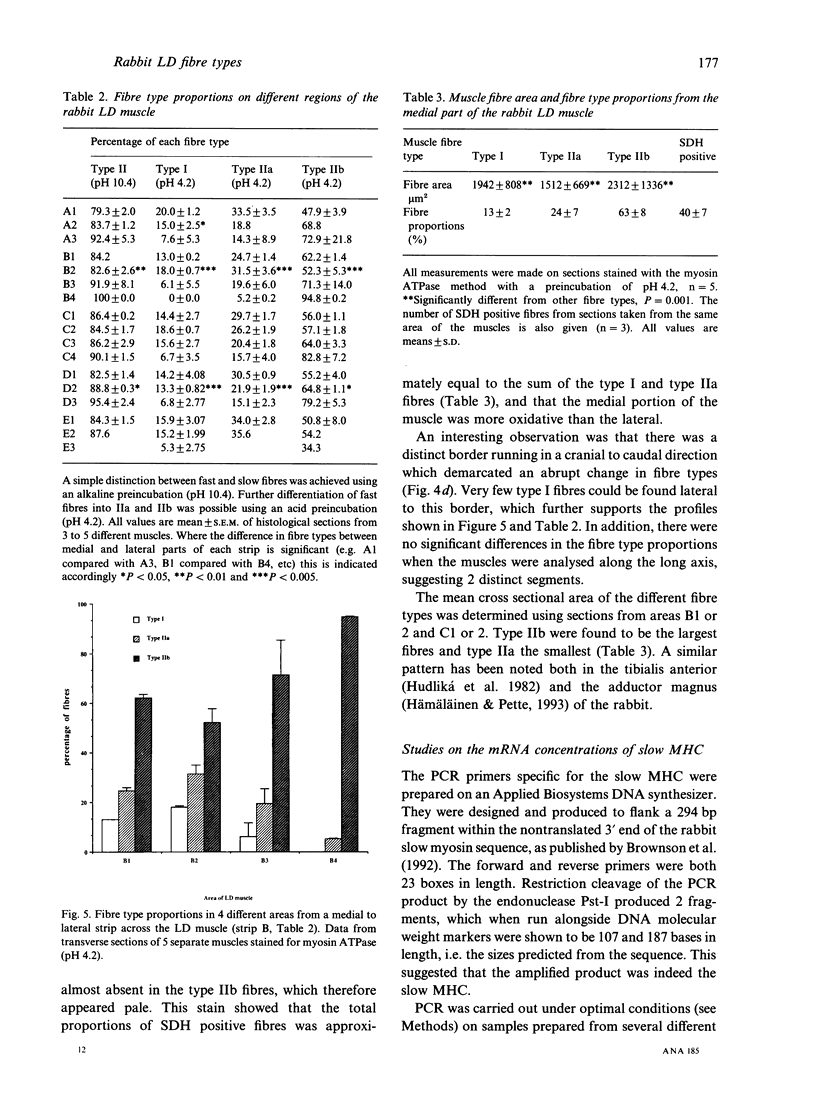
The fibre type distribution has been mapped in the latissimus dorsi muscle of the Dutch rabbit. Using the myosin ATPase stain, a distinct border was found to run in a cranial to caudal direction, which effectively divided the muscle into 2 segments of different fibre type proportions. Although both segments contained mostly fast twitch fibres, the medial areas were found to contain approximately 10-20% slow (i.e. type I) fibres while the lateral portions contained very few, if any, slow fibres. Significantly fewer type IIa fibres were also found in the lateral areas of the muscle. These histochemical findings were confirmed by the use of the reverse transcriptase polymerase chain reaction, which demonstrated that more messenger RNA of the slow myosin heavy chain was found in the medial regions compared with the lateral segment. These results demonstrate the importance of choosing well defined sampling sites when evaluating regimes designed to transform this heterogeneous muscle for use in subsequent myoplasty procedures.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
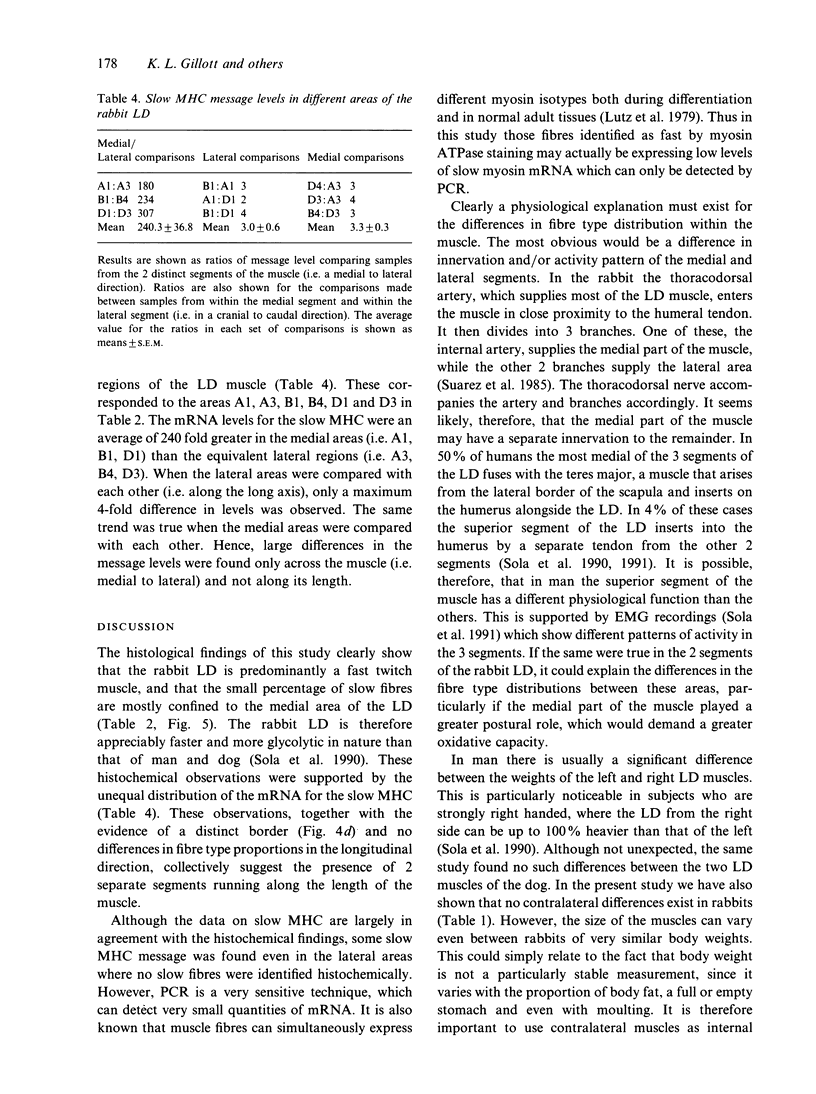
- Brownson C., Little P., Jarvis J. C., Salmons S. Reciprocal changes in myosin isoform mRNAs of rabbit skeletal muscle in response to the initiation and cessation of chronic electrical stimulation. Muscle Nerve. 1992 Jun;15(6):694–700. doi: 10.1002/mus.880150611. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Hudlická O., Dodd L., Renkin E. M., Gray S. D. Early changes in fiber profile and capillary density in long-term stimulated muscles. Am J Physiol. 1982 Oct;243(4):H528–H535. doi: 10.1152/ajpheart.1982.243.4.H528. [DOI] [PubMed] [Google Scholar]
- Hämäläinen N., Pette D. The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem. 1993 May;41(5):733–743. doi: 10.1177/41.5.8468455. [DOI] [PubMed] [Google Scholar]
- Lutz H., Weber H., Billeter R., Jenny E. Fast and slow myosin within single skeletal muscle fibres of adult rabbits. Nature. 1979 Sep 13;281(5727):142–144. doi: 10.1038/281142a0. [DOI] [PubMed] [Google Scholar]
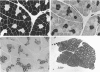
- NACHLAS M. M., TSOU K. C., DE SOUZA E., CHENG C. S., SELIGMAN A. M. Cytochemical demonstration of succinic dehydrogenase by the use of a new p-nitrophenyl substituted ditetrazole. J Histochem Cytochem. 1957 Jul;5(4):420–436. doi: 10.1177/5.4.420. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Sola O. M., Haines L. C., Kakulas B. A., Ivey T., Dillard D. H., Thomas R., Shoji Y., Fujimura Y., Dahm L. Comparative anatomy and histochemistry of human and canine latissimus dorsi muscle. J Heart Transplant. 1990 Mar-Apr;9(2):151–159. [PubMed] [Google Scholar]
- Suarez Nieto C., Martinez Vidal A., Suarez Garcia M. J., Barthe Garcia P., Escudero Comis J. Latissimus dorsi myocutaneous flap: vascular anatomy and experimental viability. J Otolaryngol. 1985 Dec;14(6):394–398. [PubMed] [Google Scholar]