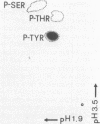
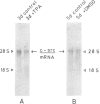
Abstract
The proto-oncogene c-src, the cellular homolog of the Rous sarcoma virus (RSV) transforming gene v-src, is expressed in a tissue-specific and age-dependent manner. Its physiological function, although still unknown, appears to be more closely related to differentiation processes than to proliferation processes. To obtain more information about the physiological role of the c-src gene in cells, we have studied differentiation-dependent alterations using the human HL-60 leukaemia cell line as a model system. Induction of monocytic and granulocytic differentiation of HL-60 cells by 12-O-tetradecanoylphorbol-13-acetate (TPA) and dimethylsulfoxide (DMSO) is associated with an activation of the pp60c-src tyrosine kinase, but not with increased c-src gene expression. Control experiments exclude an interaction of TPA and DMSO themselves with the pp60c-src kinase.
Full text
PDF

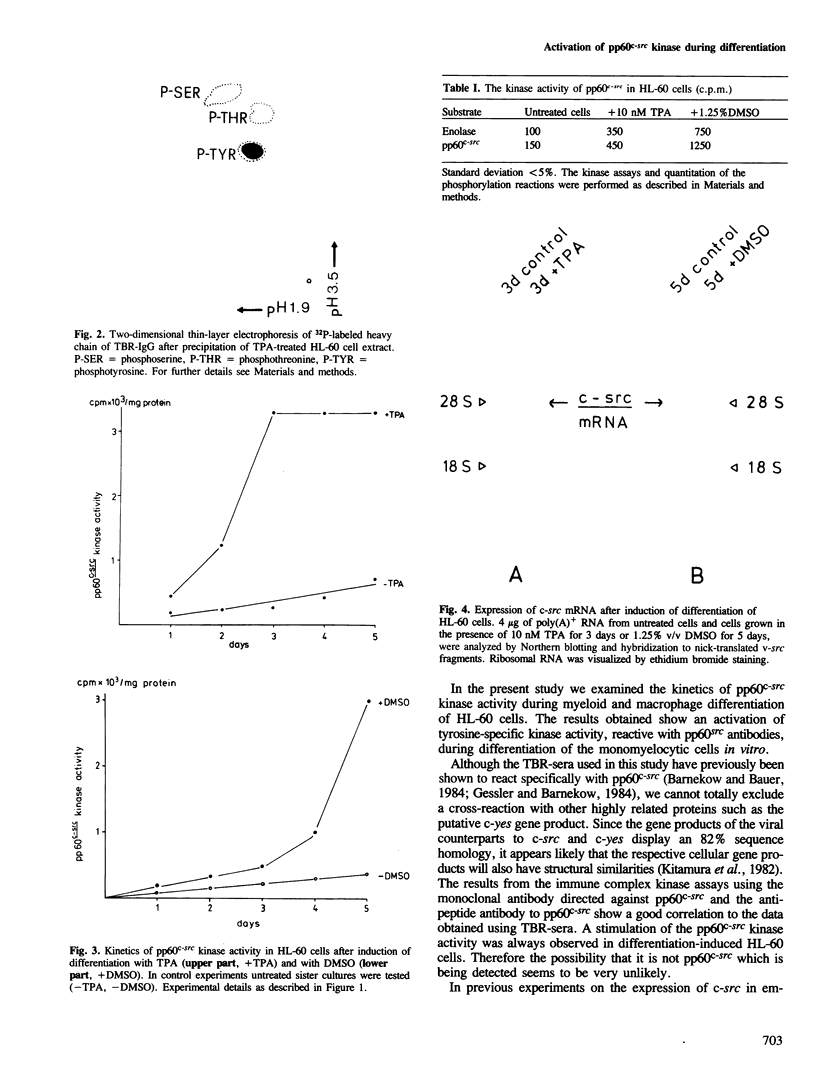
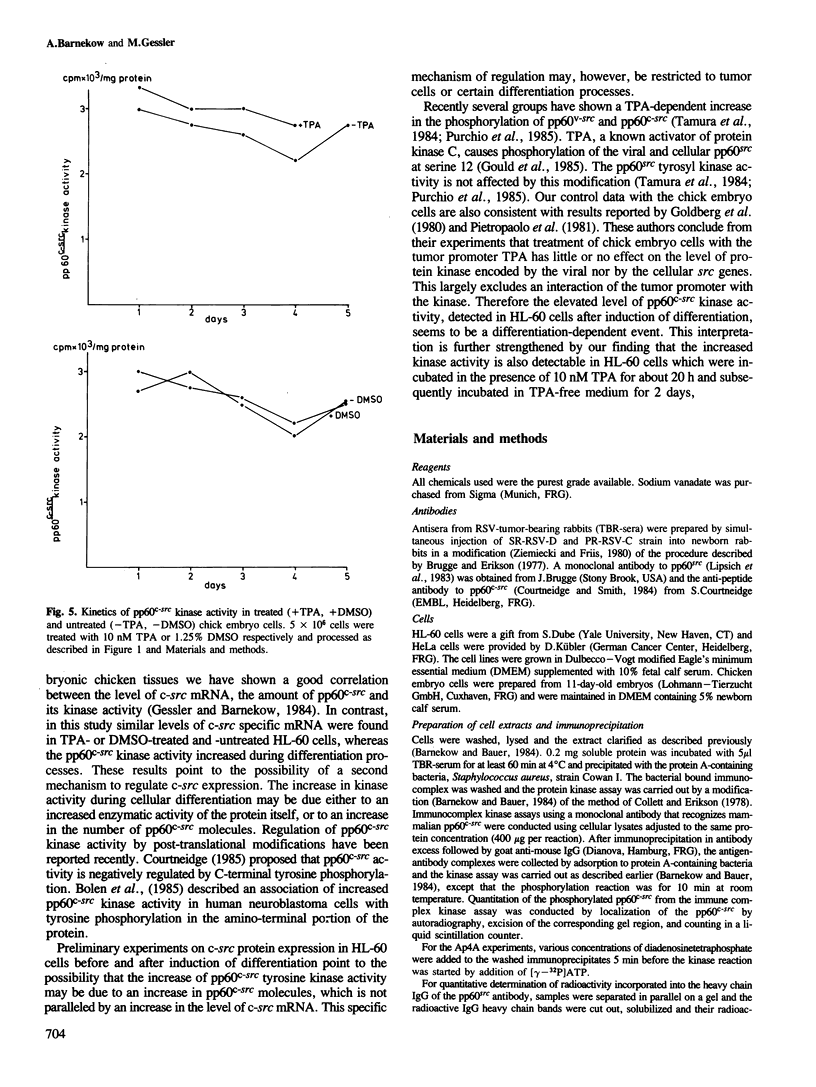

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnekow A., Bauer H. The differential expression of the cellular src-gene product pp60src and its phosphokinase activity in normal chicken cells and tissues. Biochim Biophys Acta. 1984 May 15;782(1):94–102. doi: 10.1016/0167-4781(84)90110-6. [DOI] [PubMed] [Google Scholar]
- Barnekow A. Effect of several nucleotides on the phosphorylating activities of the Rous-sarcoma-virus transforming protein pp60v-src and its cellular homologue, pp60c-src. Biosci Rep. 1983 Feb;3(2):153–162. doi: 10.1007/BF01121946. [DOI] [PubMed] [Google Scholar]
- Barnekow A., Schartl M. Cellular src gene product detected in the freshwater sponge Spongilla lacustris. Mol Cell Biol. 1984 Jun;4(6):1179–1181. doi: 10.1128/mcb.4.6.1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen J. B., Rosen N., Israel M. A. Increased pp60c-src tyrosyl kinase activity in human neuroblastomas is associated with amino-terminal tyrosine phosphorylation of the src gene product. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7275–7279. doi: 10.1073/pnas.82.21.7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Cotton P. C., Queral A. E., Barrett J. N., Nonner D., Keane R. W. Neurones express high levels of a structurally modified, activated form of pp60c-src. Nature. 1985 Aug 8;316(6028):554–557. doi: 10.1038/316554a0. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Gallo R. C., Gallagher R. E. Continuous growth and differentiation of human myeloid leukaemic cells in suspension culture. Nature. 1977 Nov 24;270(5635):347–349. doi: 10.1038/270347a0. [DOI] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton P. C., Brugge J. S. Neural tissues express high levels of the cellular src gene product pp60c-src. Mol Cell Biol. 1983 Jun;3(6):1157–1162. doi: 10.1128/mcb.3.6.1157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A. Activation of the pp60c-src kinase by middle T antigen binding or by dephosphorylation. EMBO J. 1985 Jun;4(6):1471–1477. doi: 10.1002/j.1460-2075.1985.tb03805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtneidge S. A., Smith A. E. The complex of polyoma virus middle-T antigen and pp60c-src. EMBO J. 1984 Mar;3(3):585–591. doi: 10.1002/j.1460-2075.1984.tb01852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Wong-Staal F., Gallo R. C. Onc gene amplification in promyelocytic leukaemia cell line HL-60 and primary leukaemic cells of the same patient. Nature. 1982 Sep 2;299(5878):61–63. doi: 10.1038/299061a0. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filmus J., Buick R. N. Relationship of c-myc expression to differentiation and proliferation of HL-60 cells. Cancer Res. 1985 Feb;45(2):822–825. [PubMed] [Google Scholar]
- Gessler M., Barnekow A. Differential expression of the cellular oncogenes c-src and c-yes in embryonal and adult chicken tissues. Biosci Rep. 1984 Sep;4(9):757–770. doi: 10.1007/BF01128817. [DOI] [PubMed] [Google Scholar]
- Goldberg A. R., Delclos K. B., Blumberg P. M. Phorbol ester action is independent of viral and cellular src kinase levels. Science. 1980 Apr 11;208(4440):191–193. doi: 10.1126/science.6244621. [DOI] [PubMed] [Google Scholar]
- Gonda T. J., Metcalf D. Expression of myb, myc and fos proto-oncogenes during the differentiation of a murine myeloid leukaemia. Nature. 1984 Jul 19;310(5974):249–251. doi: 10.1038/310249a0. [DOI] [PubMed] [Google Scholar]
- Gould K. L., Woodgett J. R., Cooper J. A., Buss J. E., Shalloway D., Hunter T. Protein kinase C phosphorylates pp60src at a novel site. Cell. 1985 Oct;42(3):849–857. doi: 10.1016/0092-8674(85)90281-8. [DOI] [PubMed] [Google Scholar]
- Grosso L. E., Pitot H. C. Transcriptional regulation of c-myc during chemically induced differentiation of HL-60 cultures. Cancer Res. 1985 Feb;45(2):847–850. [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Kitamura A., Toyoshima K., Hirayama Y., Yoshida M. Avian sarcoma virus Y73 genome sequence and structural similarity of its transforming gene product to that of Rous sarcoma virus. Nature. 1982 May 20;297(5863):205–208. doi: 10.1038/297205a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lipsich L. A., Lewis A. J., Brugge J. S. Isolation of monoclonal antibodies that recognize the transforming proteins of avian sarcoma viruses. J Virol. 1983 Nov;48(2):352–360. doi: 10.1128/jvi.48.2.352-360.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell R. L., Zokas L., Schreiber R. D., Verma I. M. Rapid induction of the expression of proto-oncogene fos during human monocytic differentiation. Cell. 1985 Jan;40(1):209–217. doi: 10.1016/0092-8674(85)90324-1. [DOI] [PubMed] [Google Scholar]
- Murray M. J., Cunningham J. M., Parada L. F., Dautry F., Lebowitz P., Weinberg R. A. The HL-60 transforming sequence: a ras oncogene coexisting with altered myc genes in hematopoietic tumors. Cell. 1983 Jul;33(3):749–757. doi: 10.1016/0092-8674(83)90017-x. [DOI] [PubMed] [Google Scholar]
- Müller R., Curran T., Müller D., Guilbert L. Induction of c-fos during myelomonocytic differentiation and macrophage proliferation. Nature. 1985 Apr 11;314(6011):546–548. doi: 10.1038/314546a0. [DOI] [PubMed] [Google Scholar]
- Müller R., Müller D., Guilbert L. Differential expression of c-fos in hematopoietic cells: correlation with differentiation of monomyelocytic cells in vitro. EMBO J. 1984 Aug;3(8):1887–1890. doi: 10.1002/j.1460-2075.1984.tb02063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietropaolo C., Laskin J. D., Weinstein I. B. Effect of tumor promoters on sarc gene expression in normal and transformed chick embryo fibroblasts. Cancer Res. 1981 Apr;41(4):1565–1571. [PubMed] [Google Scholar]
- Purchio A. F., Shoyab M., Gentry L. E. Site-specific increased phosphorylation of pp60v-src after treatment of RSV-transformed cells with a tumor promoter. Science. 1985 Sep 27;229(4720):1393–1395. doi: 10.1126/science.2994221. [DOI] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rüther U., Wagner E. F., Müller R. Analysis of the differentiation-promoting potential of inducible c-fos genes introduced into embryonal carcinoma cells. EMBO J. 1985 Jul;4(7):1775–1781. doi: 10.1002/j.1460-2075.1985.tb03850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sariban E., Mitchell T., Kufe D. Expression of the c-fms proto-oncogene during human monocytic differentiation. Nature. 1985 Jul 4;316(6023):64–66. doi: 10.1038/316064a0. [DOI] [PubMed] [Google Scholar]
- Schartl M., Barnekow A. Differential expression of the cellular src gene during vertebrate development. Dev Biol. 1984 Oct;105(2):415–422. doi: 10.1016/0012-1606(84)90298-7. [DOI] [PubMed] [Google Scholar]
- Schartl M., Barnekow A. The expression in eukaryotes of a tyrosine kinase which is reactive with pp60v-src antibodies. Differentiation. 1982;23(2):109–114. doi: 10.1111/j.1432-0436.1982.tb01273.x. [DOI] [PubMed] [Google Scholar]
- Shilo B. Z., Weinberg R. A. DNA sequences homologous to vertebrate oncogenes are conserved in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6789–6792. doi: 10.1073/pnas.78.11.6789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorge L. K., Levy B. T., Maness P. F. pp60c-src is developmentally regulated in the neural retina. Cell. 1984 Feb;36(2):249–257. doi: 10.1016/0092-8674(84)90218-6. [DOI] [PubMed] [Google Scholar]
- Takeya T., Hanafusa H. Structure and sequence of the cellular gene homologous to the RSV src gene and the mechanism for generating the transforming virus. Cell. 1983 Mar;32(3):881–890. doi: 10.1016/0092-8674(83)90073-9. [DOI] [PubMed] [Google Scholar]
- Tamura T., Friis R. R., Bauer H. pp60c-src is a substrate for phosphorylation when cells are stimulated to enter cycle. FEBS Lett. 1984 Nov 5;177(1):151–156. doi: 10.1016/0014-5793(84)81001-7. [DOI] [PubMed] [Google Scholar]
- Watanabe T., Sariban E., Mitchell T., Kufe D. Human c-myc and N-ras expression during induction of HL-60 cellular differentiation. Biochem Biophys Res Commun. 1985 Feb 15;126(3):999–1005. doi: 10.1016/0006-291x(85)90284-0. [DOI] [PubMed] [Google Scholar]
- Westin E. H., Wong-Staal F., Gelmann E. P., Dalla-Favera R., Papas T. S., Lautenberger J. A., Eva A., Reddy E. P., Tronick S. R., Aaronson S. A. Expression of cellular homologues of retroviral onc genes in human hematopoietic cells. Proc Natl Acad Sci U S A. 1982 Apr;79(8):2490–2494. doi: 10.1073/pnas.79.8.2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemiecki A., Friis R. R. Simultaneous injection of newborn rabbits with the Schmidt-Ruppin and prague strains of Rous sarcoma virus induces antibodies which recognize the pp60src of both strains. J Gen Virol. 1980 Sep;50(1):211–216. doi: 10.1099/0022-1317-50-1-211. [DOI] [PubMed] [Google Scholar]