Abstract
To construct pepper development simulation models under drought, experiments of water capacities of 45–55%, 55–65%, 65–75% or 75–85% and exposure (2, 4, 6 or 8 d) (Exp. 1 & 2), of 50–60%, 60–70% or 70–80% and exposure (3, 5, and 7 d) (Exp. 3) were conducted with “Sanying” pepper. Physiological development time (PDT), product of thermal effectiveness and PAR (photosynthetically active radiation) (TEP) and growing degree days (GDD) were used to simulate growth under various treatments in Exp. 1. Plant development was influenced by the severity and drought duration. Mild water deficits (65–75% for 2–6 d or 55–65% for 2–4 d) accelerated development, while severe water deficits (65–75% for 8 d, 55–65% for 6–8 d or 45–55% for 2–8 d) delayed development. The PDT gave the highest coefficient of determination (R2, 0.89–0.94) and the lowest root mean squared error (RMSE, average of 1.03–1.50 d) and relative error (RE, average of 1.60–1.88%) for simulating three growth periods (Exp. 2). It was therefore used to construct growth models under water capacity of 45–85% over 2–8 d with spline, cubic, makima, linear, and nearest interpolation. Validation in Exp. 3 indicated that the spline model was optimal, having the highest R2 (0.96–0.97) and the lowest RMSE (average of 1.31–1.75 d) and RE (average of 1.18–2.06%). The results of the study can help producers to optimize water management and to develop drought strategies for production.
Keywords: pepper, water capacity, physiological development time, spline interpolation, phenology simulation
1. Introduction
Pepper (Capsicum annuum L.) is an important greenhouse crop in China, and is rich in magnesium, iron, calcium, and zinc (Dai et al., 2022; Padilla et al., 2023a). It is a shallow rooted crop with thin and weak roots, and susceptible to drought (Zhang et al., 2023a). Water deficits can significantly affect pepper growth and development (Kurucz et al., 2023; Padilla et al., 2023b). In recent decades, many studies have been carried out on the effects of water deficits on growth and development in peppers (Sanogo, 2006; Peng et al., 2010; Zhu et al., 2012; Lv et al., 2019; Castronuovo et al., 2023). Yang et al. (2016) suggested that mild water deficits may not affect the growth of above-ground parts, while sever deficits can significantly reduce the shoot length and photosynthesis (Adnew et al., 2023; Molla et al., 2023), and had an impact on fruit yield (Admassie et al., 2022). Pan (2007) concluded that the optimal field water capacity is 80–90% based on a series of experiments. However, some studies suggest that short and long-term stress can cause different effects on crops (Widuri et al., 2020; Xu et al., 2020a; Zhang et al., 2023b). We were interested in studying the effects of different water deficit levels over different durations from a few days to a week or more.
Current studies on models of crop growth have been developed for several decades (Ahmad et al., 2017; Perera et al., 2020; Barriball et al., 2022; Didevarasl et al., 2023). Diao et al. (2009) simulated the development and yield of pepper using a product of thermal effectiveness and PAR (TEP). Torrion et al. (2011) simulated soybean growth in fields of the North-Central United States according to a soybean model (SoySim). Ma and Tian (2016) modelled phenology and leaf area of watermelon based on physiological development time (PDT) and logistic function, respectively. Chen et al. (2019) proposed a sugarcane development simulation model (SDSM) to predict growth of newly planted and perennial sugarcane based on clock model. Niu et al. (2021) used PDT and growing degree day (GDD) to simulate growth of greenhouse cherry tomatoes. Cai et al. (2021) modelled the plant nutrition and physiology in Chinese cabbage using light and temperature data. Leguízamo-Medina et al. (2023) simulated the growth and flowering of carnation based on cumulative GDD. Xu et al. (2020a) compared three models to simulate growth and yield in strawberry and recommended PDT method. Therefore, PDT, TEP and GDD had been widely used to model growth in crops (Chen et al., 2021; Shi and Li, 2021; Chen et al., 2022; Liu et al., 2022; Sun et al., 2022; Wang et al., 2023).
Current studies on the modeling of crop growth under water deficits have focused on a single orthogonal treatment. Interpolation can be used to predict values of growth between data of known points. These methods, including nearest, linear, cubic, spline, and makima, have different applications under various environmental conditions (Gore et al., 2023). For example, Chen et al. (2011) suggested that the surface interpolated by a spline method was smoother. Xiao et al. (2021) concluded that a cubic model is optimal to simulate temperature in a greenhouse.
This study compared PDT, TEP or GDD to model growth of pepper under water capacities of 45–55%, 55–65%, 65–75%, and 75–85% over 2, 4, 6 or 8 days. Then interpolation was used to construct a three-dimension models under water capacity of 45–85% over 2–8 days. Finally, another independent experiment of water capacity (50–60%, 60–70% or 70–80%) and treatment days (3, 5 or 7 d) was conducted to screen out the optimal interpolation model. We expected that the study could provide a scientific method for application of crop simulation models in agriculture.
2. Materials and methods
2.1. Material
The widely-used cultivar, “Sanying” (Capsicum annuum L.) was utilized. It has great disease and pest resistance as well as high production.
2.2. Experiment design
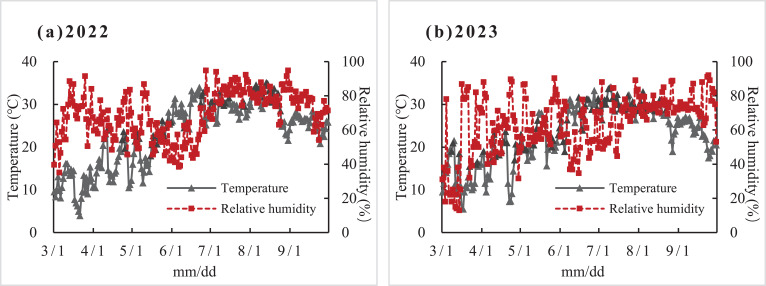
The Experiments 1, 2 and 3 were conducted at the Agrometeorological Experimental Station of Shangqiu Meteorological Service (34.2°N, 115.6°E, 44 m of elevation) from March to September in 2022 and 2023, respectively. The air temperature and relative humidity during the experiments were showed in Figure 1 .
Figure 1.
Temperature and relative humidity of environment during the experiments in 2022 (A) and 2023 (B).
The plants were sown in polypropylene plastic pots (21.0 cm × 21.4 cm × 19.1 cm) filled with a vermiculite: substrate: perlite mixture of 1:1:1 (v: v: v). There are no holes at the bottom of plastic basins to prevent water loss due to gravity. The water capacity of each pot was kept within a set range by weighing with electronic scale (Lichen YP300001D, accuracy of 0.1 g) at 7:00 and 18:00 every day. There were four water capacities (75–85%, 65–75%, 55–65% or 45–55%) and four treatment days (2, 4, 6 or 8 days) were therefore designed for Exp. 1 & 2 ( Table 1 ). The data from Exp. 1 were used to construct models, while the data from Exp. 2 were used to validate and screen out the optimal simulations. Plants were grown at water capacities of 70–80%, 60–70% or 50–60% over 3, 5 or 7 days for model validation (Exp.3, Table 2 ). The water capacities and treatment durations used were the median values from the treatments in Exp. 1. The water capacity of 75–85% was set as the control (CK) in all experiments. Thirty plants with healthy and similar growth were selected for each experiment when four true leaves appeared. The water capacities of all the plants were kept at 75–85% before and after the treatments during the experiments. Awnings were used to ensure that the plants would not be wet by rain.
Table 1.
Treatments used in Experiments 1 & 2.
| Water capacity (%) | Days | |||
|---|---|---|---|---|
| 2 | 4 | 6 | 8 | |
| 75–85% | CK | |||
| 65–75% | W1D1 | W1D2 | W1D3 | W1D4 |
| 55–65% | W2D1 | W2D2 | W2D3 | W2D4 |
| 45–55% | W3D1 | W3D2 | W3D3 | W3D4 |
Table 2.
Treatments used in Experiment 3.
| Water capacity (%) | Treatment days (d) | ||
|---|---|---|---|
| 3 | 5 | 7 | |
| 70%–80% | V1 | V2 | V3 |
| 60%–70% | V4 | V5 | V6 |
| 50%–60% | V7 | V8 | V9 |
2.3. Methods
2.3.1. Phenology
We recorded the start and end dates of each pepper growth period, including planting, flowering (the first flower with more than 30% of plants blooming), fruit set (the first fruit with more than 30% of plants setting), and harvest stage (all of the pepper fruits turning red) were observed and recorded.
2.3.2. Meteorological data
Air temperature, relative humidity and photosynthetic active radiation were automatically recorded with a FT-QC7-RP sensor every 10 minutes. Averages values per hour or a day were used in the models.
2.3.3. Physiological development time
Physiological development time (PDT) is the cumulative time of growth under the optimum temperature and light (Xu et al., 2020a; Zhang et al., 2022). For a specific cultivar, the cumulative PDT at each growth period is theoretically constant (Cheng et al., 2019; Giolo et al., 2021; Zhang et al., 2022). Cumulative PDT is computed by relative thermal effectiveness (RTE) and relative photoperiod effectiveness (RPE).
| (1) |
where i and n represent the ith day and the total number of days of the plant growth period, respectively.
RTE represents relative growth of a plant at actual temperature for one day relative to those at the optimal temperature.
| (2) |
where T represents the actual temperature of environment, Tm and Tb represent upper and lower limit temperatures, and Tou and Tob represent upper and lower limit of optima. Three critical temperatures at various stages of peppers are presented in Table 3 (Yue et al., 2018).
Table 3.
The three critical temperatures for the growth of peppers.
| Growth period | Lower temperature (°C) |
Optimum temperature (°C) | Upper temperature (°C) |
|---|---|---|---|
| Seedling | 10 | 25 | 35 |
| Flowering | 15 | 20 | 35 |
| Fruit setting | 15 | 25 | 35 |
RPE represents the growth of plants under an actual photoperiod for one day relative to that under the optimal photoperiod.
| (3) |
where DLc denotes the critical day-length of peppers (16 hours), and DLo denotes the optimal day-length (10 hours). DL represents the actual day-length:
| (4) |
| (5) |
| (6) |
| (7) |
| (8) |
where λ denotes the latitude of the study region (34°45’ N), δ denotes the obliquity of the ecliptic, and DOY is day of the year.
2.3.4. Product of thermal effectiveness and PAR
The product of thermal effectiveness and photosynthetically active radiation (PAR), which is defined as TEP, can be used to model growth (Wu et al., 2021). Cumulative TEP is obtained from the accumulation of daily relative TEP (DTEP):
| (9) |
where DTEPi (MJ·m-2), RTEi (MJ·m-2) and PARi (MJ·m−2·d−1) represent TEP, daily mean thermal effectiveness and PAR of the ith day, and n denotes the total number of days of the period.
2.3.5. Growing degree day
GDD is used to express the relationship between effective accumulated temperature and development (Wu et al., 2021). Only temperature is required in the calculation. GDD is the summation of effective temperature, i.e., the cumulative difference between daily mean temperature and lower limit temperature:
| (10) |
| (11) |
where Tavg represents daily mean temperature (°C), Tn represents daily minimum temperature (°C), Tx represents daily maximum temperature (°C), Tupper and Tbase represent the upper and lower limit temperature (°C), respectively, and n represents the total number of days of the period.
2.3.6. Interpolation methods
Based on MATLAB R2018a, the five interpolation methods, including linear, nearest, cubic, makima and spline, were used to construct growth models under various field water deficits of 45%–85% over 2–8 days.
2.3.7. Model construction and validation
Based on the results of Exp. 1, the PDT, TEP and GDD models were used to simulate pepper growth. The meteorological data and phenology from Exp. 2 were used to validate and screen out the optimal method according to the root mean squared error (RMSE), relative error (RE), and coefficient of determination (R2) (Tang et al., 2007; Shi et al., 2022). The optimal simulation method was selected to construct models of growth based on values of RMSE, RE and R2.
| (12) |
| (13) |
where OBSi and SIMi represent observed and simulated values, and n is the number of samples.
| (14) |
where residual SS denotes residual sum of squares and corrected SS denotes corrected sum of squares.
3. Results
3.1. Effect of water deficits on growth
Under the optimal condition (CK), it took 96, 116 and 171 days from planting to flowering, fruit set and harvest, respectively ( Table 4 ). The plants under W1D1, W1D2, W1D3, W2D1 and W2D2 were advanced compared with the controls. Days to flowering, fruit set, and harvest under W1D3 were 3, 4 and 5 days earlier. However, the plants under water capacity of 55–65% for 6 or 8 days, or water capacity of 45–55% for 2, 4, 6, 8 days were all delayed. Days to flowering, fruit set, and harvest under W3D4 were 12, 14 and 18 days slower than the controls.
Table 4.
Effect of water deficits on the phenology of pepper in Exp. 1 (N=30).
| Treatment | Days to flowering (d) |
Days to fruit set (d) | Days to harvest (d) |
|---|---|---|---|
| CK | 96 | 116 | 171 |
| W1D1 | 94 | 114 | 169 |
| W1D2 | 94 | 113 | 168 |
| W1D3 | 93 | 112 | 166 |
| W1D4 | 97 | 118 | 172 |
| W2D1 | 94 | 113 | 168 |
| W2D2 | 93 | 112 | 168 |
| W2D3 | 98 | 119 | 173 |
| W2D4 | 99 | 120 | 174 |
| W3D1 | 101 | 123 | 175 |
| W3D2 | 103 | 125 | 181 |
| W3D3 | 105 | 127 | 185 |
| W3D4 | 108 | 130 | 189 |
3.2. Simulation of growth with PDT, TEP and GDD
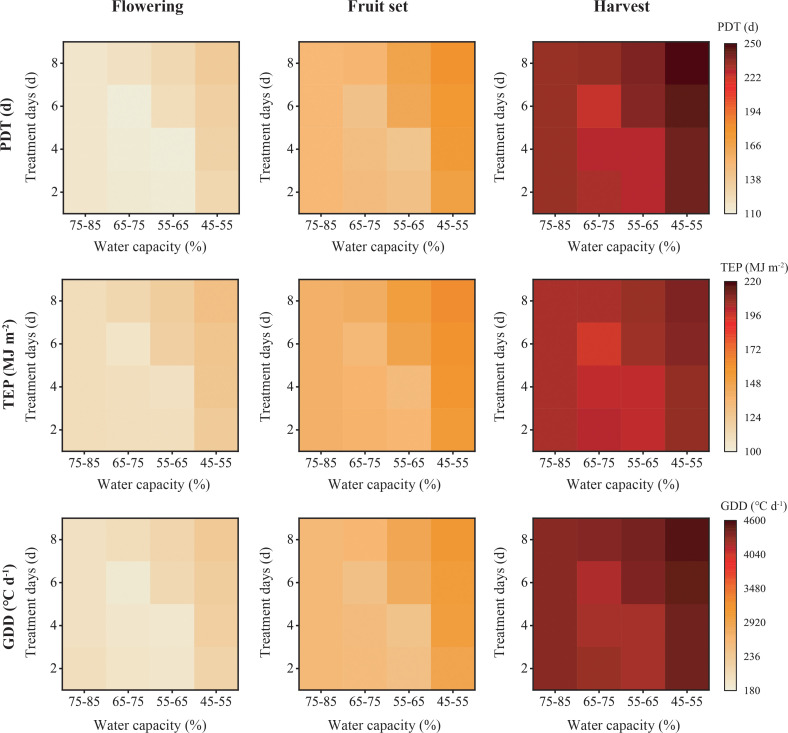
Phenology of the plants is shown in Figure 2 , with the darker the color, the slower growth. Cumulative PDT/TEP/GDD under water capacity of 75–85% for 2, 4, 6 or 8 days were similar. Growth was accelerated for W1D1, W1D2, W1D3, W2D1 and W2D2 (lighter). Plants given a water capacity of 65–75% for 6 days had the quickest growth. However, plants given a water capacity of 55–65% for 6 or 8 days, or a water capacity of 45–55% for 2, 4, 6 or 8 days were slower. Plants given a water capacity of 45–55% for 8 days required higher values of cumulative PDT, TEP, and GDD than the other plants.
Figure 2.
Cumulative physiological development time (PDT), product of thermal effectiveness and PAR (photosynthetically active radiation) (TEP) and growing degree days (GDD) required for pepper plants to flowering, fruit set and harvest under different water deficits (N=30).
3.3. Simulation and validation of growth models using PDT, TEP or GDD
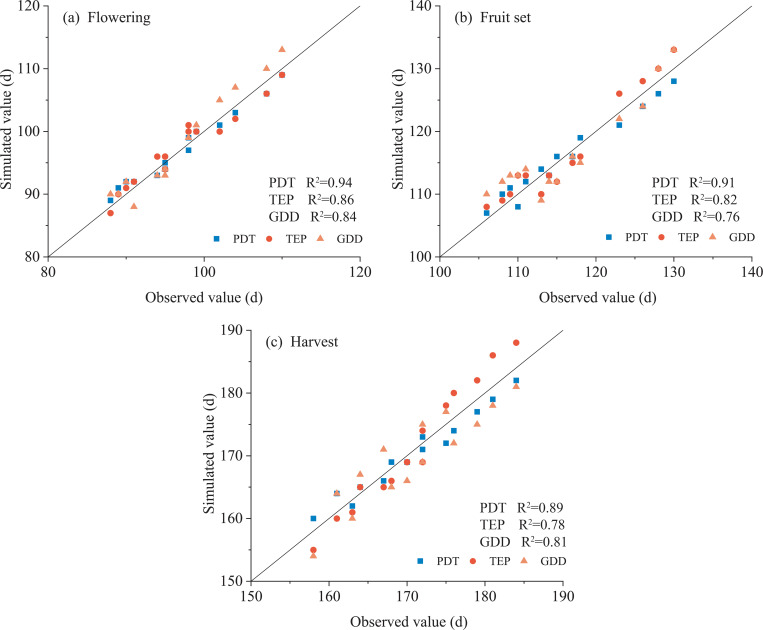
All of three methods accurately simulated growth with the scatter points of the data nearly distributed along the 1:1 line ( Figure 3 ). Overall, the scatter for PDT was closer to the 1:1 line than that for other two methods. Simulation with PDT showed the highest R2 of 0.94, 0.91, and 0.89 for flowering, fruit set, and harvest compared with TEP and GDD. The TEP model had a higher R2 than GDD in simulating flowering (0.86) and fruit set (0.82), with a smaller R2 in simulating harvest (0.78).
Figure 3.
Comparison of observed and simulated days to flowering (A), fruit set (B) and harvest (C) in pepper with physiological development time (PDT), product of thermal effectiveness and PAR (photosynthetically active radiation) (TEP) and growing degree days (GDD) models. Dashed line represents the 1:1 line.
Simulation errors increased as growth increased ( Table 5 ). For example, the errors from planting to flowering with PDT, TEP or GDD were 0–2, 1–3 or 1–3 days. The errors from planting to harvest using PDT, TEP or GDD increased to 1–3, 1–5 or 2–4 days. Simulations using PDT were overall better with smaller errors. Simulations using PDT with water capacity of 75–85% were more accurate than those for the other treatments with the errors only 1 or less day for three growth periods. The GDD models had a larger error in simulating growth (mostly 3–4 days).
Table 5.
Simulated errors (observed - simulated values) for the number of days from planting to flowering, fruit set, and harvest of peppers using models based on physiological development time (PDT), product of thermal effectiveness and PAR (photosynthetically active radiation) (TEP) and growing degree days (GDD).
| Period | Water capacity | PDT | TEP | GDD | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Days | Days | Days | |||||||||||
| 2 | 4 | 6 | 8 | 2 | 4 | 6 | 8 | 2 | 4 | 6 | 8 | ||
| Planting to flowering | 75–85% 65–75% 55–65% 45–55% |
95 (0) 93 (1) 92 (-2) 101 (1) |
94 (1) 92 (-1) 89 (-1) 103 (1) |
95 (0) 91 (-2) 99 (-1) 106 (2) |
94 (1) 97 (1) 100 (-1) 109 (1) |
94 (-1) 96 (2) 91 (1) 100 (-2) |
96 (1) 92 (1) 87 (-1) 102 (-2) |
94 (-1) 90 (1) 101 (3) 106 (-2) |
96 (1) 100 (2) 100 (1) 109 (-1) |
93 (-2) 93 (-1) 92 (2) 105 (3) |
94 (-1) 88 (-3) 90 (2) 107 (3) |
93 (-2) 90 (1) 99 (1) 110 (2) |
94 (-1) 99 (1) 101 (2) 113 (3) |
| Planting to fruit set | 75–85% 65–75% 55–65% 45–55% |
114 (-1) 112 (-1) 108 (2) 121 (2) |
113 (1) 111 (-2) 107 (-1) 124 (2) |
114 (-1) 110 (-2) 116 (-1) 126 (2) |
113 (1) 116 (1) 119 (-1) 128 (2) |
110 (-3) 113 (2) 113 (3) 126 (3) |
113 (-1) 110 (1) 108 (2) 128 (2) |
110 (-3) 109 (1) 112 (-3) 130 (2) |
113 (-1) 115 (-2) 116 (-2) 133 (3) |
109 (-4) 114 (3) 113 (3) 122 (-1) |
112 (-2) 113 (4) 110 (4) 124 (-2) |
109 (-4) 112 (4) 112 (-3) 130 (2) |
112 (-2) 116 (-1) 115 (-3) 133 (3) |
| Planting to harvest | 75–85% 65–75% 55–65% 45–55% |
169 (1) 166 (1) 162 (1) 174 (2) |
169 (-1) 165 (-1) 160 (-2) 177 (2) |
169 (1) 164 (-3) 171 (1) 179 (2) |
169 (-1) 173 (-1) 172 (3) 182 (2) |
169 (-1) 165 (-2) 161 (-2) 180 (4) |
166 (-2) 165 (1) 155 (-3) 182 (3) |
169 (-1) 160 (-1) 174 (2) 186 (5) |
166 (-2) 169 (-3) 178 (3) 188 (4) |
166 (-4) 171 (4) 160 (-3) 172 (-4) |
165 (-3) 167 (3) 154 (-4) 175 (-4) |
166 (-4) 164 (3) 175 (3) 178 (-3) |
165 (-3) 169 (-3) 177 (2) 181 (-3) |
N=30.
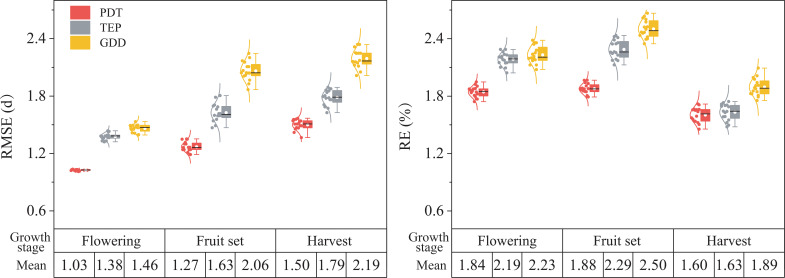
Overall, the RMSE values of simulations with PDT, TEP, and GDD increased with growth, while the RE of the simulations increased and then decreased ( Figure 4 ). The simulation of peppers from planting to flowering with PDT had the lowest RMSE (average of 1.03 d) compared with other methods. However, the mean RE of simulation for planting–harvest was 0.24 and 0.28 lower than that for planting–flowering and planting–fruit set. The GDD model had the highest RMSE and RE in simulating growth.
Figure 4.
Boxplots of RMSE (d) and RE (%) for the simulation of growth in pepper from planting to flowering, fruit set, and harvest, with physiological development time (PDT), product of thermal effectiveness and PAR (photosynthetically active radiation) (TEP) and growing degree days (GDD) models under different water deficits.
3.4. Construction of growth models with five interpolation methods
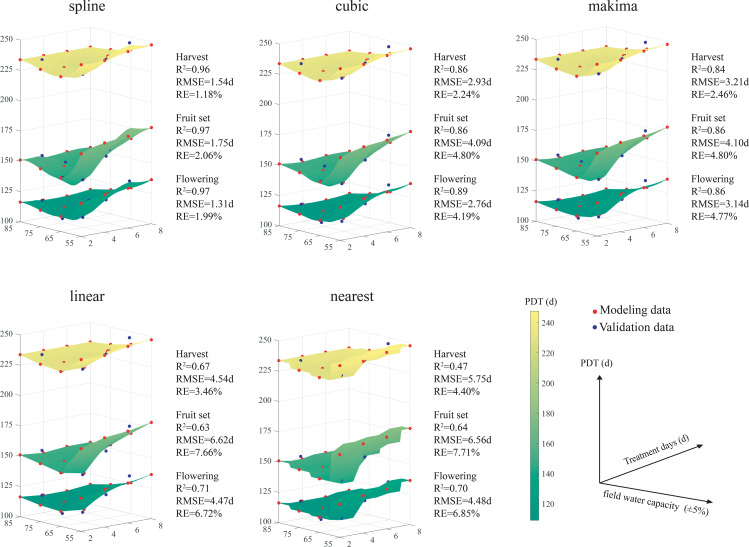
The PDT model was more accurate to predict growth and was selected for the simulation study with water capacities of 45–85% over 2–8 days (red scatter points in Figure 5 ). This experiment then compared and validated the distinct models (blue scatter points in Figure 5 ), based on values of RMSE, RE and R2.
Figure 5.
Three-dimension surface map of flowering models in pepper under water deficits with spline, cubic, makima, linear and nearest interpolation algorithms based on accumulated physiological development time (PDT).
All the models exhibited similar trends under water deficits. Cumulative PDT required for each growth period showed a decreased trend and then an increased trend as water capacity decreased from 85% to 45%. The required PDT of each growth period remained constant under water capacity of 75–85% over 2–8 days, and increased under water capacity of 45–55% over 2–8 days.
The interpolation surfaces for the spline, cubic and makima models were smoother than that of the linear or nearest models. Overall, the validations were better using spline than using the other models. The spline models had the highest R2 (0.96–0.97) and the lowest RMSE (1.31–1.75 d) and RE (1.18–2.06%). The errors with spline were in the order of flowering< fruit set< harvest. The RMSE and RE of each pepper growth period using nearest model were the highest among the five models, except for a slight lower RMSE (0.06 d) than the linear model in planting–fruit set stage. The interpolation images of nearest models for growth were segmentate.
4. Discussion
Pepper is a typical short-day plant (Lu et al., 2020; Zhang et al., 2020; Wang et al., 2021). The PDT model characterized the relationship between temperature and development speed by converting the response of crop to temperature into relative thermal effectiveness on the basis of three critical points of temperature, but also considered the relative photoperiod effectiveness of crops (Zhu et al., 2023). Thus, this model was more accurate and robust than those based on TEP and GDD.
Combined stresses of different water capacities and duration days might affect the growth differently. Interpolation models can approximately simulate growth under various water capacity and duration days. Spline models gave the highest values of R2 and the lowest RMSE and RE across all of the growth. This response is consistent with the results of Gore et al. (2023). Development with water capacities of 75–85% for 2, 4, 6, and 8 days was similar possibly, because 75–85% is the optimal water level for peppers. Phenology was accelerated as water capacity decreased from 85% to 45%, and then was delayed. Cumulative PDT for each growth period under water capacity of 65–75% for 2–6 days and 55–65% for 2–4 days was lower than that under the other treatments, indicating a mild water deficit may promote crop growth and development (Xu et al., 2020b; Zhang et al., 2023b). However, long-term mild and moderate water deficits (65–75% for 8 days or 55–65% for 6–8 days) and severe water deficits (45–55% for 2–8 days) can significantly decrease growth, protein metabolism and yield (Jiang et al., 2008; Li et al., 2020; Yang et al., 2022).
The spline model was proved to successfully and precisely simulate growth under a water capacity from 45% to 85% for 2–8 days based on accumulated PDT. Nevertheless, some limitations exist in our study.
The size of pots used to plant peppers in this study was based on earlier research (Zhang et al., 2023b; Zhu et al., 2023). However, previous studies have demonstrated that root restrictions can reduce growth (He et al., 2022; Li et al., 2022; Liu et al., 2023). The applicability of the models in our study may not apply to other cultivars and growing system.
We only used water capacities of 45–85% over 2–8 days, which is common in commercial pepper cultivation. Further, studies are required with other watering strategies (Mills et al., 2018; Clifton et al., 2020; Otieno et al., 2022).
5. Conclusion
Three independent experiments were conducted to construct and validate models in pepper growth under water deficits. Mild water deficits (65–75% for 2–6 days or 55–65% for 2–4 days) accelerate development while severe water deficits (65–75% for 8 days, 55–65% for 6–8 d or 45–55% for 2–8 days) delay development. Models based on PDT simulated growth from planting to flowering, fruit set, and harvest compared those based on TEP and GDD. Growth can be simulated using spline interpolation under a water deficit based on a water capacity from 45% to 85% over 2 to 8 days. The study provides a scientific basis for assessment of pepper growth and development under various water deficits to develop irrigation strategies for best commercial production.
Acknowledgments
Special thanks to Changying Xue and Yanling Zhang for their contributions during the manuscript revision process.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. This study was supported by the foundations of Shangqiu Meteorological Bureau (SY202403), and Henan Key Laboratory of Agrometeorological Support and Applied Technique, CMA (KQ202358).
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
JZ: Funding acquisition, Writing – original draft. YZ: Methodology, Writing – review & editing. GY: Investigation, Writing – review & editing. SL: Validation, Writing – review & editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Admassie M., Woldehawariat Y., Alemu T., Gonzalez E., Jimenez J. F. (2022). The role of plant growth-promoting bacteria in alleviating drought stress on pepper plants. Agric. Water Manage. 272, 107831. doi: 10.1016/j.agwat.2022.107831 [DOI] [Google Scholar]
- Adnew W., Andualem A., Molla A., Tarekegn Z., Aragaw M., Ayana M. (2023). Growth, physiological, and biochemical responses of Ethiopian red pepper (Capsicum annum L.) cultivars to drought stress. Sci. World J. 2023, 1–21. doi: 10.1155/2023/4374318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad S., Abbas Q., Abbas G., Fatima Z., Atique ur R., Naz S., et al. (2017). Quantification of climate warming and crop management impacts on cotton phenology. Plants 6, 7. doi: 10.3390/plants6010007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriball S., Han A., Schlautman B. (2022). Effect of growing degree days, day of the year, and cropping systems on reproductive development of Kernza in Kansas. Agrosyst. Geosci. Environ. 5, 20286. doi: 10.1002/agg2.20286 [DOI] [Google Scholar]
- Cai S. F., Wu B. Y., Lei J. G. (2021). Dynamic simulation effect of physiological characteristics and nutritional quality of chinese cabbage based on light and temperature function. Chin. J. Agrometeorol. 42, 34–43. doi: 10.3969/j.issn.1000-6362.2021.01.004 [DOI] [Google Scholar]
- Castronuovo D., Satriani A., Rivelli A. R., Comegna A., Belviso C., Coppola A., et al. (2023). Effects of zeolite and deficit irrigation on sweet pepper growth. Horticulturae 9, 1230. doi: 10.3390/horticulturae9111230 [DOI] [Google Scholar]
- Chen J., Sun J. H., Fu J. (2011). Using the sub-area dark pixel and spline interpolation approach to estimate the aerosol optical thickness on taihu lake. Remote Sens. Inf. 2011, 33–37. doi: 10.3969/j.issn.1000-3177.2011.03.006 [DOI] [Google Scholar]
- Chen X., Feng L. P., Peng M. X., Chen Y. L. (2019). Establishment of sugarcane development simulation model based on clock model method. Chin. J. Agrometeorol. 40, 186–194. doi: 10.3969/j.issn.1000-6362.2019.03.006 [DOI] [Google Scholar]
- Chen Y. K., Huang Y. Y., Wang T., Kang Y. X. (2021). Growth model of lettuce cultivated by NFT based on product of thermal effectiveness and photosynthesis active radiation. Jiangsu. Agric. Sci. 49, 201–204 + 215. doi: 10.15889/j.issn.1002-1302.2021.19.036 [DOI] [Google Scholar]
- Chen Y., Xu M. Z., Wang Y. H., Bai Y. L., Lu Y. L., Wang L. (2022). Quantitative study on effective accumulated temperature and dry matter and nitrogen accumulation of summer maize under different nitrogen supply levels. Sci. Agricultura. Sin. 55, 2973–2987. doi: 10.3864/j.issn.0578-1752.2022.15.009 [DOI] [Google Scholar]
- Cheng C., Feng L. P., Xue Q. Y., Li C., Gong Z. H., Dong C. Y., et al. (2019). Simulation model for cucumber growth and development in sunlight greenhouse. Chin. J. Appl. Ecol. 30, 3491–3500. doi: 10.13287/i.1001-9332.201910.020 [DOI] [PubMed] [Google Scholar]
- Clifton O., Lombardozzi D., Fiore A., Paulot F., Horowitz L. (2020). Stomatal conductance influences interannual variability and long-term changes in regional cumulative plant uptake of ozone. Environ. Res. Lett. 15, 114059. doi: 10.1088/1748-9326/abc3f1 [DOI] [Google Scholar]
- Dai Z., Zhao X., Yan H., Qin L., Niu X., Zhao L., et al. (2022). Optimizing Water and Nitrogen Management for Green Pepper (Capsicum annuum L.) under Drip Irrigation in Sub-Tropical Monsoon Climate Regions. Agronomy 13, 34. doi: 10.3390/agronomy13010034 [DOI] [Google Scholar]
- Diao M., Dai J. F., Luo W. H., Yuan C. M., Pu C. X., Xian K. M., et al. (2009). Model for simulation of growth and yield of greenhouse sweet pepper. Trans. CSAE. 25, 241–246. doi: 10.3969/j.issn.1002-6819.2009.10.044 [DOI] [Google Scholar]
- Didevarasl A., Costa Saura J. M., Spano D., Deiana P., Snyder R. L., Mulas M., et al. (2023). Modeling phenological phases across olive cultivars in the mediterranean. Plants 12, 3181. doi: 10.3390/plants12183181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giolo M., Sallenave R., Pornaro C., Velasco-Cruz C., Macolino S., Leinauer B. (2021). Base temperatures affect accuracy of growing degree day model to predict emergence of Bermudagrasses. Agron. J. 113, 2960–2966. doi: 10.1002/agj2.v113.3 [DOI] [Google Scholar]
- Gore R., Gawali B., Pachpatte D. (2023). Weather parameter analysis using interpolation methods. Artif. Intell. Appl. 13, 101542. doi: 10.1016/j.asej.2021.07.001 [DOI] [Google Scholar]
- He J., Li X., Tian Y., He X., Qin K., Zhu L., et al. (2022). Effect of lycium barbarum L. Root restriction cultivation method on plant growth and soil bacterial community abundance. Agronomy 13, 14. doi: 10.3390/agronomy13010014 [DOI] [Google Scholar]
- Jiang B. B., Fang W. M., Chen F. D., Gu J. J. (2008). Effects of N, P and K ratio on the growth and development of cut chrysanthemum ‘Jinba’. J. Zhejiang. Forestry. Coll. 25, 692–697. [Google Scholar]
- Kurucz E., Antal G., Kincses I., Sipos M., Fári M., Holb I. (2023). Effect of light treatment and maturity stage on biomass production and bioactive compounds of two pepper cultivars under a deep water culture hydroponic system. Sustainability 15, 13205. doi: 10.3390/su151713205 [DOI] [Google Scholar]
- Leguizamo Medina M., Pinzón-Sandoval E., Balaguera-López H. (2023). Phenology analysis growing and degree days of flower bud growth in three Dianthus caryophyllus L. varieties under greenhouse conditions. Rev. Colombiana. Cienc. Hortícolas. 16, 15296. doi: 10.17584/rcch.2022v16i3.15296 [DOI] [Google Scholar]
- Li J., Li D., Liu B., Wang R., Yan Y., Li G., et al. (2022). Effects of root restriction on phytohormone levels in different growth stages and grapevine organs. Sci. Rep. 12, 1–15. doi: 10.1038/s41598-021-04617-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. S., Xiao S. J., Wang X. R., Liang X. M., Gao S., Pu H. M., et al. (2020). Effects of nitrogen fertilizer rate on yield and economic benefit of winter potato with drip irrigation under mulch. Soils 52, 25–32. doi: 10.13758/j.cnki.tr.2020.01.004 [DOI] [Google Scholar]
- Liu D., Chen J., Hao Y., Yang X., Chen R., Zhang Y. (2023). Effects of extreme root restriction on the nutritional and flavor quality, and sucrose metabolism of tomato (Solanum lycopersicum L.). Horticulturae 9, 813. doi: 10.3390/horticulturae9070813 [DOI] [Google Scholar]
- Liu F. H., Guo S. B., Wang D., Huang B., Cao Y. F. (2022). Construction and verification of an external morphology, substance accumulation, and distribution model of tomatoes in greenhouses. Trans. Chin. Soc. Agric. Eng. 38, 188–196. doi: 10.11975/j.issn.1002-6819.2022.21.022 [DOI] [Google Scholar]
- Lu S. Y., Yang Z. Q., Zhang Y. D., Zheng H., Yang L. (2020). Effect of photoperiod on fluorescence characteristics of photosynthetic system of fresh-cut chrysanthemum leaves under high temperature. Chin. J. Agrometeorol. 41, 632–643. doi: 10.3969/j.issn.1000-6362.2020.10.003 [DOI] [Google Scholar]
- Lv Y., Wei Q., Luan Y., Xu J., Hameed F., Dalson T., et al. (2019). Coupling effect of soil water deficit and air aridity on crop water stress of pepper. Int. J. Agric. Biol. 21, 506–512. doi: 10.17957/IJAB/15.0922 [DOI] [Google Scholar]
- Ma B., Tian J. C. (2016). Growth and development dynamic model of watermelon in gravel-mulched field based on physiological development time. Trans. Chin. Soc. Agric. Eng. 32, 122–128. doi: 10.11975/j. issn.1002-6819.2016.20.016 [Google Scholar]
- Mills G., Sharps K., Simpson D., Pleijel H., Broberg M., Uddling J., et al. (2018). Ozone pollution will compromise efforts to increase global wheat production. Global Change Biol. 24, 3560–3574. doi: 10.1111/gcb.2018.24.issue-8 [DOI] [PubMed] [Google Scholar]
- Molla A., Andualem A., Ayana M., Zeru M. (2023). Effects of drought stress on growth, physiological and biochemical parameters of two Ethiopian red pepper (Capsicum annum L.) cultivars. J. Appl. Horticult. 25, 32–38. doi: 10.37855/jah.2023.v25i01.05 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu N., Jing B., Liu Q., Wang X., Diao M. (2021). Research on the growth and development simulation model of greenhouse cherry tomatoes. J. Shihezi. Univ. (Natural. Science). 39, 318–325. doi: 10.13880/j.cnki.65-1174/n.2021.22.025 [DOI] [Google Scholar]
- Otieno M., Peters M., Duque L., Steffan-Dewenter I. (2022). Interactive effects of ozone and carbon dioxide on plant-pollinator interactions and yields in a legume crop. Environ. Adv. 9, 100285. doi: 10.1016/j.envadv.2022.100285 [DOI] [Google Scholar]
- Padilla Y. G., Gisbert-Mullor R., Bueso E., Zhang L., Forment J., Lucini L., et al. (2023. a). New insights into short-term water stress tolerance through transcriptomic and metabolomic analyses on pepper roots. Plant Sci. 333, 111731. doi: 10.1016/j.plantsci.2023.111731 [DOI] [PubMed] [Google Scholar]
- Padilla Y. G., Gisbert-Mullor R., López-Galarza S., Albacete A., Melgarejo P., Calatayud A. (2023. b). Short-term water stress responses of grafted pepper plants are associated with changes in the hormonal balance. Front. Plant Sci. 14, 1170021. doi: 10.3389/fpls.2023.1170021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. J. (2007). Study on the simulation model of growth and dry matter accumulation of pepper under different irrigation quartity (Changchun: Jilin Agricultural University; ), 1–49. [Google Scholar]
- Peng Q., Liang Y. L., Chen C., Jia W. Y., Tian Z. G., Hao W. L., et al. (2010). Response of physiological characteristics of pepper leaf to different light intensities and soil moisture contents. Trans. Chin. Soc. Agric. Eng. 26, 115–121. doi: 10.3969/j.issn.1002-6819.2010.z1.023 [DOI] [Google Scholar]
- Perera R. S., Cullen B. R., Eckard R. J. (2020). Using leaf temperature to improve simulation of heat and drought stresses in a biophysical model. Plants 9, 8. doi: 10.3390/plants9010008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanogo S. (2006). Predispositional effect of soil water saturation on infection of Chile pepper by phytophthora capsici. HortSci. 41, 172–175. doi: 10.21273/HORTSCI.41.1.172 [DOI] [Google Scholar]
- Shi N., Gao Z. Q., Chen C. Y., Wen S. Y., Shu C., Tang H., et al. (2022). Simulation and analysis of above-ground dry matter and leaf area index of rice based on Logistic model. J. Northeast Agric. Univ. 53, 10–18. doi: 10.19720/j.cnki.issn.1005-9369.2022.03.002 [DOI] [Google Scholar]
- Shi X. H., Li C. (2021). Effects of irrigation amount on dry matter production and its translocation in greenhouse green pepper. J. Irrigation. Drainage. 40, 10–17. doi: 10.13522/j.cnki.ggps.2021056 [DOI] [Google Scholar]
- Sun Y. G., Wang Y., Zhang Y., Wu K. C., Wang Y. H., Wang D. H., et al. (2022). Investigation and utilization of temperature and light characteristics of tobacco: III. Establishment of simulation model of tobacco leaf growth based on temperature and light effects. Chin. Tobacco. Sci. 43, 6–14. doi: 10.13496/j.issn.1007-5119.2022.04.002 [DOI] [Google Scholar]
- Tang L., Zhu Y., Cao W. X. (2007). A simulation model for dynamic green area index in winter rapeseed (Brassica napus). J. Plant Ecol. 31, 897–902. doi: 10.17521/cjpe.2007.0113 [DOI] [Google Scholar]
- Torrion J., Setiyono T., Cassman K., Specht J. (2011). Soybean phenology simulation in the north-central United States. Agron. J. 103, 1661–1667. doi: 10.2134/agronj2011.0141 [DOI] [Google Scholar]
- Wang Z. P., Liang Z. G., Fan F. C., Liu S. Y., Jia S. N., Qin Y. (2023). Dry matter accumulation model of eggplant in solar greenhouse under different nitrogen level. Northern. Horticult. 2023, 48–54. doi: 10.11937/bfyy.20221926 [DOI] [Google Scholar]
- Wang J. J., Tian X., Qin H. B., Wang H. G., Cao X. N., Chen L., et al. (2021). Regulation effects of photoperiod on growth and leaf endogenous hormones in broomcorn millet. Sci. Agricultura. Sin. 54, 286–295. doi: 10.3864/j.issn.0578-1752.2021.02.005 [DOI] [Google Scholar]
- Widuri L., Lakitan B., Sakagami J., Yabuta S., Kartika K., Siaga E. (2020). Short-term drought exposure decelerated growth and photosynthetic activities in chili pepper (Capsicum annuum L.). Ann. Agric. Sci. 65, 149–158. doi: 10.1016/j.aoas.2020.09.002 [DOI] [Google Scholar]
- Wu F., Zou X. Z., Li J., Wang W. W. (2021). Study on prediction model of rape flowering date based on radiant thermal product. Jiangsu. Agric. Sci. 49, 153–157. doi: 10.15889/j.issn.1002-1302.2021.11.027 [DOI] [Google Scholar]
- Xiao X. P., Cheng M., Yuan H. B., Wang Q. F. (2021). Visual simulation analysis of greenhouse temperature field based on interpolation method. J. Chin. Agric. Mechanization. 42, 75–84. doi: 10.13733/j.jcam.issn.2095-5553.2021.01.011 [DOI] [Google Scholar]
- Xu C., Wang M. T., Yang Z. Q., Han W., Zheng S. H. (2020. a). Effect of high temperature in seedling stage on phenological stage of strawberry and its simulation. Chin. J. Agrometeorol. 41, 644–654. doi: 10.3969/j.issn.1000-6362.2020.10.004 [DOI] [Google Scholar]
- Xu C., Wang M. T., Yang Z. Q., Zheng Q. T. (2020. b). Low temperature and low irradiation induced irreversible damage of strawberry seedlings. Photosynthetica 58, 156–164. doi: 10.32615/ps.2020.001 [DOI] [Google Scholar]
- Yang Z. Q., Qiu Y. X., Liu Z. X., Chen Y. Q., Tan W. (2016). The effects of soil moisture stress on the growth of root and above-ground parts of greenhouse tomato crops. Acta Ecol. Sin. 36, 748–757. doi: 10.5846 /stxb201403310606 [Google Scholar]
- Yang P., Yang X., Yan M., Cheng Z. M., Cai Y., Huang S., et al. (2022). Effects of planting density, nitrogen application rate and the number of retained leaves on yield and quality of yunyan 116. Hunan. Agric. Sci. 07), 6–10. doi: 10.16498/j.cnki.hnnykx.2022.007.002 [DOI] [Google Scholar]
- Yue Y. B., Zhao Z. Y., Peng Z. L., Li R. J., Li L. J., Feng E. Y., et al. (2018). Simulation on leaf area and dry matter production of Chilli pepper. Southwest. China J. Agric. Sci. 31, 2653–2658. doi: 10.16213/j.cnki.scjas.2018.12.032 [DOI] [Google Scholar]
- Zhang L. X., Liu W., Tsegaw M., Xu X., Qi Y. P., Sapey E., et al. (2020). Principles and practices of the photo-thermal adaptability improvement in soybean. J. Integr. Agric. 19, 295–310. doi: 10.1016/S2095-3119(19)62850-9 [DOI] [Google Scholar]
- Zhang F., Luo J., Yuan C., Li C., Yang Z. (2022). A model for the effect of low temperature and poor light on the growth of cucumbers in a greenhouse. Agronomy 12, 2992. doi: 10.3390/agronomy12122992 [DOI] [Google Scholar]
- Zhang H., Wang Y., Yu S., Zhou C., Li F., Chen X., et al. (2023. a). Plant photosynthesis and dry matter accumulation response of sweet pepper to water–nitrogen coupling in cold and arid environment. Water 15, 2134. doi: 10.3390/w15112134 [DOI] [Google Scholar]
- Zhang Y. D., Yang Z. Q., Wang P. J., Xu C. (2023. b). Long-term high temperature stress decreases the photosynthetic capacity and induces irreversible damage in chrysanthemum seedlings. Hortic. Sci. 50, 159–173. doi: 10.17221/28/2022-HORTSCI [DOI] [Google Scholar]
- Zhu J., Peng Q., Liang Y., Wu X., Hao W. (2012). Leaf gas exchange, chlorophyll fluorescence, and fruit yield in hot pepper (Capsicum anmuum L.) grown under different shade and soil moisture during the fruit growth stage. J. Integr. Agric. 11, 927–937. doi: 10.1016/S2095-3119(12)60083-5 [DOI] [Google Scholar]
- Zhu J., Xue C., Zhang Y. (2023). Simulation of pepper (Capsicum annuum L.) growth stages under different nitrogen fertiliser levels based on three models. J. Hortic. Sci. Biotechnol. 99, 187–197. doi: 10.1080/14620316.2023.2242403 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.