Abstract
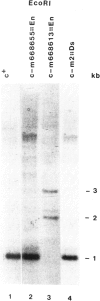
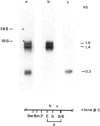
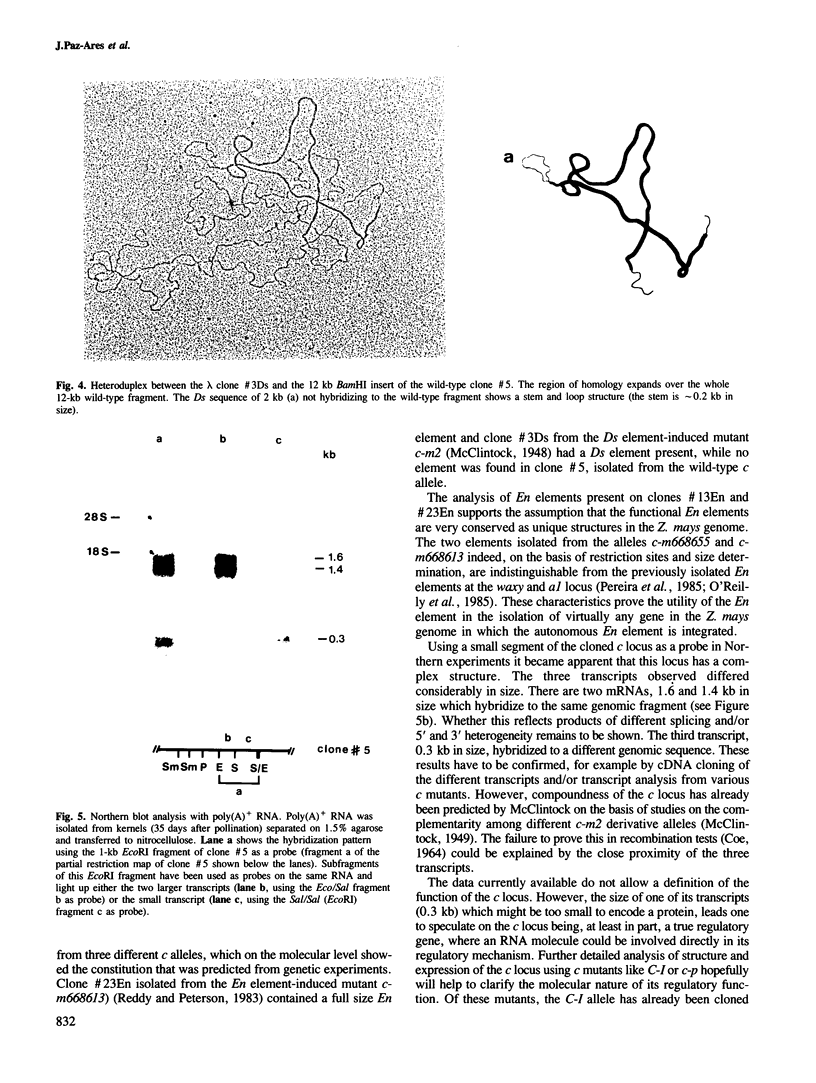
The c locus of Zea mays, involved in the regulation of anthocyanin biosynthesis, has been cloned by transposon tagging. A clone (#18En) containing a full size En1 element was initially isolated from the En element-induced mutable allele c-m668655. Sequences of clone #18En flanking the En1 element were used to clone other c mutants, whose structure was predicted genetically. Clone #23En (isolated from c-m668613) contained a full size En1 element, clone #3Ds (isolated from c-m2) a Ds element and clone #5 (isolated from c+) had no element on the cloned fragment. From these data we conclude that the clones obtained contain at least part of the c locus. Preliminary data on transcript analysis using a 1-kb DNA fragment from wild-type clone #5 showed that at least three transcripts are encoded by that part of the locus, indicating that c is a complex locus.
Keywords: Zea mays, c locus, molecular cloning, transposable elements
Full text
PDF
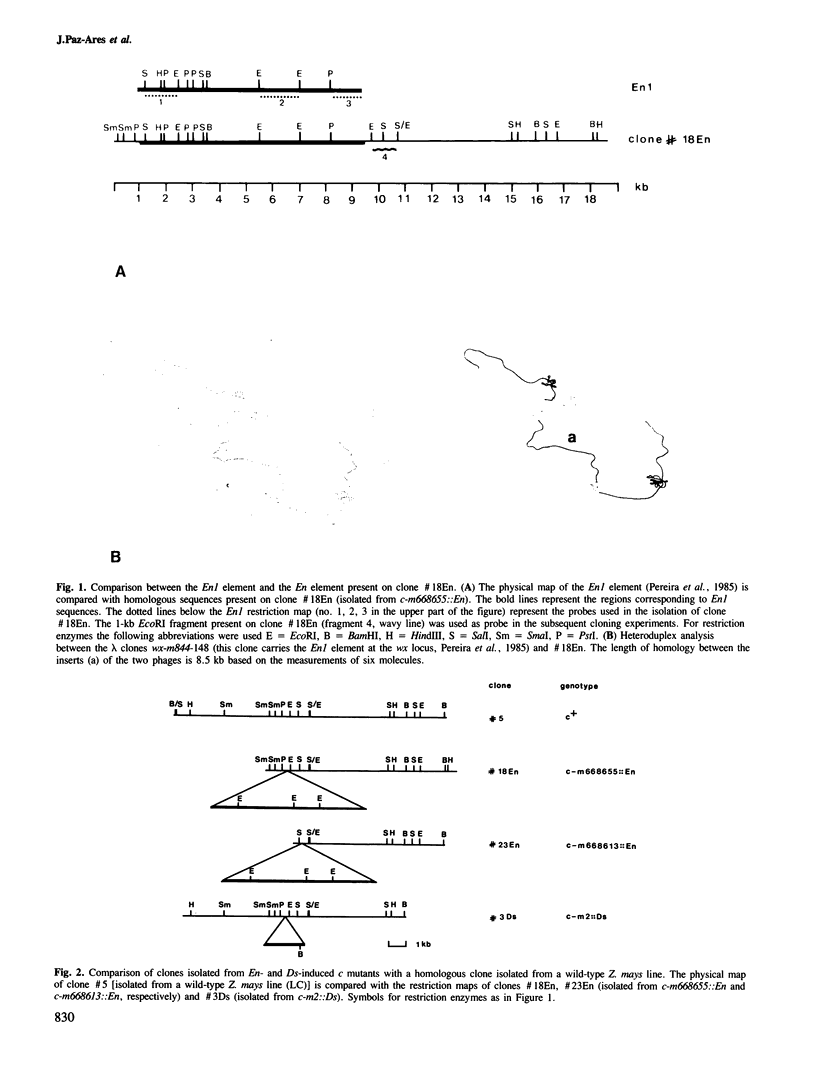
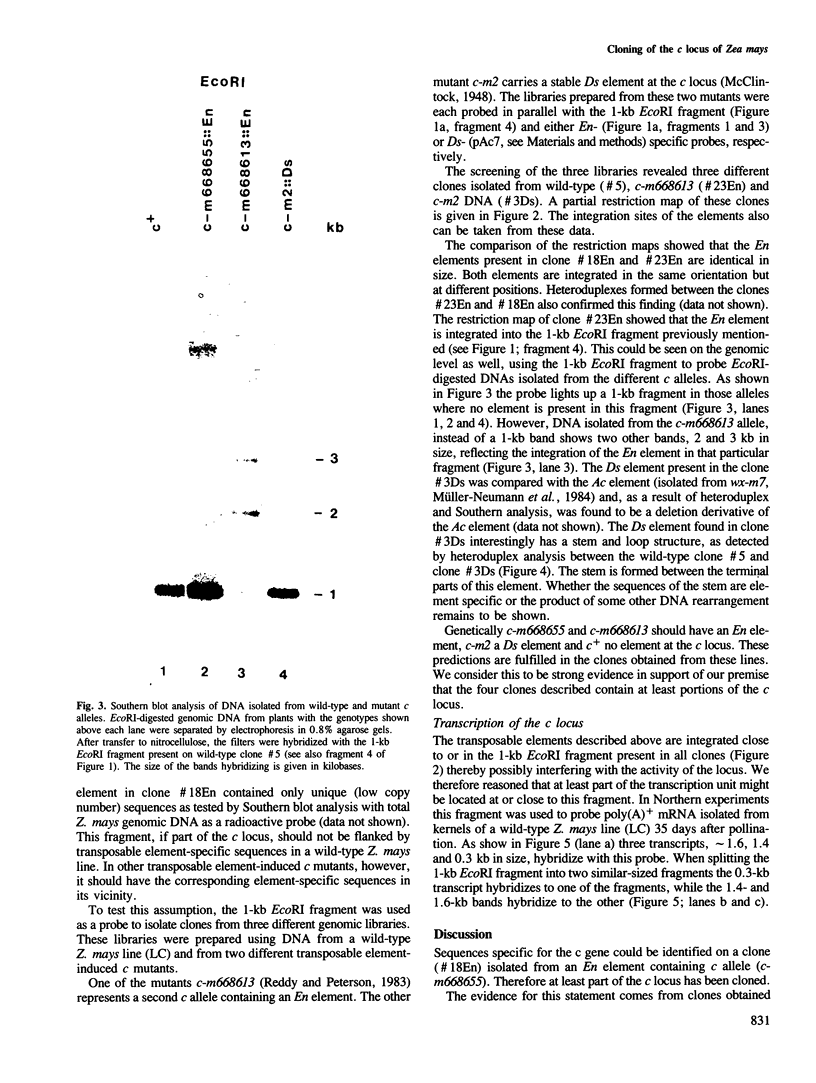

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Bingham P. M., Levis R., Rubin G. M. Cloning of DNA sequences from the white locus of D. melanogaster by a novel and general method. Cell. 1981 Sep;25(3):693–704. doi: 10.1016/0092-8674(81)90176-8. [DOI] [PubMed] [Google Scholar]
- Chen S. M., Coe E. H., Jr Control of anthocyanin synthesis by the C locus in maize. Biochem Genet. 1977 Apr;15(3-4):333–346. doi: 10.1007/BF00484464. [DOI] [PubMed] [Google Scholar]
- Davis R. W., Hyman R. W. A study in evolution: the DNA base sequence homology between coliphages T7 and T3. J Mol Biol. 1971 Dec 14;62(2):287–301. doi: 10.1016/0022-2836(71)90428-1. [DOI] [PubMed] [Google Scholar]
- Dooner H. K., Nelson O. E. Genetic control of UDPglucose:flavonol 3-O-glucosyltransferase in the endosperm of maize. Biochem Genet. 1977 Jun;15(5-6):509–519. doi: 10.1007/BF00520194. [DOI] [PubMed] [Google Scholar]
- Dooner H. K., Nelson O. E. Interaction among C, R and Vp in the Control of the Bz Glucosyltransferase during Endosperm Development in Maize. Genetics. 1979 Feb;91(2):309–315. doi: 10.1093/genetics/91.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooner H. K. Viviparous-1 mutation in maize conditions pleiotropic enzyme deficiencies in the aleurone. Plant Physiol. 1985 Feb;77(2):486–488. doi: 10.1104/pp.77.2.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff N. V., Furtek D. B., Nelson O. E. Cloning of the bronze locus in maize by a simple and generalizable procedure using the transposable controlling element Activator (Ac). Proc Natl Acad Sci U S A. 1984 Jun;81(12):3825–3829. doi: 10.1073/pnas.81.12.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Holmes D. S., Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981 Jun;114(1):193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Larson R. L., Coe E. H., Jr Gene-dependent flavonoid glucosyltransferase in maize. Biochem Genet. 1977 Feb;15(1-2):153–156. doi: 10.1007/BF00484558. [DOI] [PubMed] [Google Scholar]
- Martin C., Carpenter R., Sommer H., Saedler H., Coen E. S. Molecular analysis of instability in flower pigmentation of Antirrhinum majus, following isolation of the pallida locus by transposon tagging. EMBO J. 1985 Jul;4(7):1625–1630. doi: 10.1002/j.1460-2075.1985.tb03829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick S. Pigment synthesis in maize aleurone from precursors fed to anthocyanin mutants. Biochem Genet. 1978 Aug;16(7-8):777–785. doi: 10.1007/BF00484735. [DOI] [PubMed] [Google Scholar]
- O'Reilly C., Shepherd N. S., Pereira A., Schwarz-Sommer Z., Bertram I., Robertson D. S., Peterson P. A., Saedler H. Molecular cloning of the a1 locus of Zea mays using the transposable elements En and Mu1. EMBO J. 1985 Apr;4(4):877–882. doi: 10.1002/j.1460-2075.1985.tb03713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A., Schwarz-Sommer Z., Gierl A., Bertram I., Peterson P. A., Saedler H. Genetic and molecular analysis of the Enhancer (En) transposable element system of Zea mays. EMBO J. 1985 Jan;4(1):17–23. doi: 10.1002/j.1460-2075.1985.tb02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REDDY G. M., COE E. H., Jr Inter-tissue complemention: a simple technique for direct analysis of gene-action sequence. Science. 1962 Oct 12;138(3537):149–150. doi: 10.1126/science.138.3537.149. [DOI] [PubMed] [Google Scholar]
- Schwarz-Sommer Z., Gierl A., Klösgen R. B., Wienand U., Peterson P. A., Saedler H. The Spm (En) transposable element controls the excision of a 2-kb DNA insert at the wx allele of Zea mays. EMBO J. 1984 May;3(5):1021–1028. doi: 10.1002/j.1460-2075.1984.tb01922.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]