Abstract
Polyclonal and monoclonal antibodies were raised against a synthetic peptide containing the 15 carboxy-terminal amino acids (497-511) of vesicular stomatitis virus glycoprotein (VSV-G). The polyclonal antibodies (alpha P4) reacted with epitopes distributed along the 15-residue peptide, whereas the monoclonal antibody (P5D4) reacted with one epitope containing the five carboxy-terminal amino acids. Both types of antibodies recognized the cytoplasmic domain of VSV-G synthesized by tissue culture cells infected with the temperature-sensitive 045-VSV mutant (ts045-VSV). They recognized immature forms of VSV-G in the rough endoplasmic reticulum (RER) and Golgi complex, as well as mature VSV-G at the cell surface and in budding virus. The effect of these antibodies on intracellular transport and maturation of VSV-G was studied by microinjection. Both divalent antibodies (alpha P4 and P5D4) blocked transport of VSV-G to the cell surface. Monovalent Fab' fragments of alpha P4 (alpha P4-Fabs) also interfered with the appearance of VSV-G at the cell surface; Fab fragments of P5D4 (P5D4-Fabs), however, had no inhibitory effect. These results suggest that accessibility of a cytoplasmic domain, located within the sequence of amino acids 497-506 of the carboxy-terminal tail, is essential for transport of VSV-G to the cell surface.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansorge W. Improved system for capillary microinjection into living cells. Exp Cell Res. 1982 Jul;140(1):31–37. doi: 10.1016/0014-4827(82)90152-5. [DOI] [PubMed] [Google Scholar]
- Arnheiter H., Dubois-Dalcq M., Lazzarini R. A. Direct visualization of protein transport and processing in the living cell by microinjection of specific antibodies. Cell. 1984 Nov;39(1):99–109. doi: 10.1016/0092-8674(84)90195-8. [DOI] [PubMed] [Google Scholar]
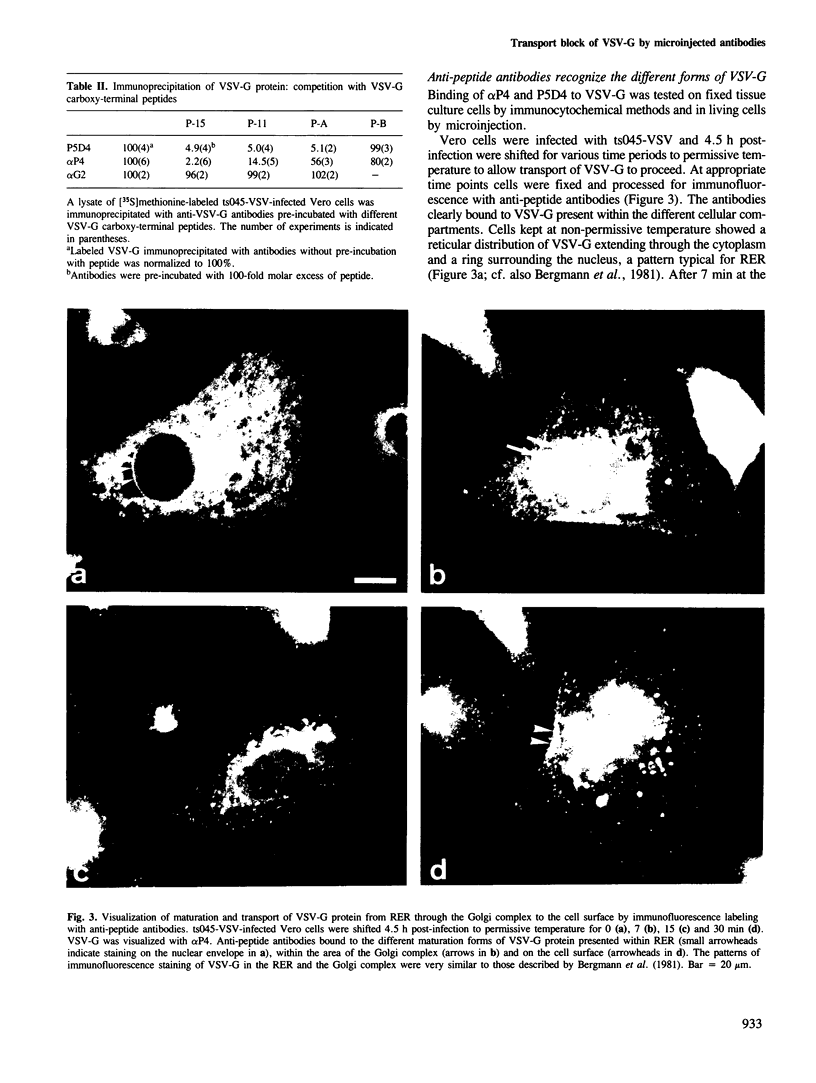
- Bergmann J. E., Tokuyasu K. T., Singer S. J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1746–1750. doi: 10.1073/pnas.78.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Conjugates of immunoglobulin G with different fluorochromes. I. Characterization by anionic-exchange chromatography. Scand J Immunol. 1973;2(3):273–290. doi: 10.1111/j.1365-3083.1973.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Burke B., Griffiths G., Reggio H., Louvard D., Warren G. A monoclonal antibody against a 135-K Golgi membrane protein. EMBO J. 1982;1(12):1621–1628. doi: 10.1002/j.1460-2075.1982.tb01364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. P. Fluorographic detection of radioactivity in polyacrylamide gels with the water-soluble fluor, sodium salicylate. Anal Biochem. 1979 Sep 15;98(1):132–135. doi: 10.1016/0003-2697(79)90716-4. [DOI] [PubMed] [Google Scholar]
- Doyle C., Roth M. G., Sambrook J., Gething M. J. Mutations in the cytoplasmic domain of the influenza virus hemagglutinin affect different stages of intracellular transport. J Cell Biol. 1985 Mar;100(3):704–714. doi: 10.1083/jcb.100.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallione C. J., Rose J. K. A single amino acid substitution in a hydrophobic domain causes temperature-sensitive cell-surface transport of a mutant viral glycoprotein. J Virol. 1985 May;54(2):374–382. doi: 10.1128/jvi.54.2.374-382.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garoff H., Kondor-Koch C., Pettersson R., Burke B. Expression of Semliki Forest virus proteins from cloned complementary DNA. II. The membrane-spanning glycoprotein E2 is transported to the cell surface without its normal cytoplasmic domain. J Cell Biol. 1983 Sep;97(3):652–658. doi: 10.1083/jcb.97.3.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B., Singer S. J. The participation of alpha-actinin in the capping of cell membrane components. Cell. 1979 Jan;16(1):213–222. doi: 10.1016/0092-8674(79)90202-2. [DOI] [PubMed] [Google Scholar]
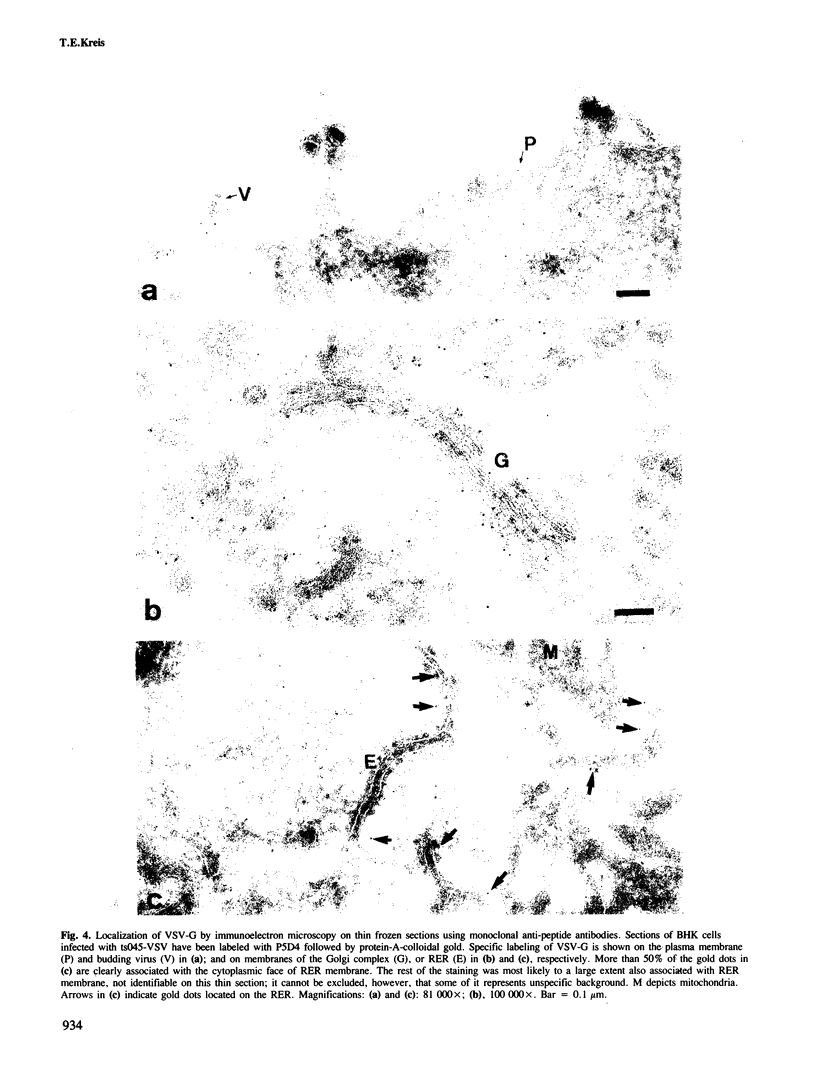
- Griffiths G., McDowall A., Back R., Dubochet J. On the preparation of cryosections for immunocytochemistry. J Ultrastruct Res. 1984 Oct;89(1):65–78. doi: 10.1016/s0022-5320(84)80024-6. [DOI] [PubMed] [Google Scholar]
- Katz F. N., Rothman J. E., Lingappa V. R., Blobel G., Lodish H. F. Membrane assembly in vitro: synthesis, glycosylation, and asymmetric insertion of a transmembrane protein. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3278–3282. doi: 10.1073/pnas.74.8.3278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Maturation of viral proteins in cells infected with temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1149–1158. doi: 10.1128/jvi.21.3.1149-1158.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis T. E., Birchmeier W. Microinjection of fluorescently labeled proteins into living cells with emphasis on cytoskeletal proteins. Int Rev Cytol. 1982;75:209–214. doi: 10.1016/s0074-7696(08)61005-0. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schlessinger J. Mobility of microinjected rhodamine actin within living chicken gizzard cells determined by fluorescence photobleaching recovery. Cell. 1982 Jul;29(3):835–845. doi: 10.1016/0092-8674(82)90445-7. [DOI] [PubMed] [Google Scholar]
- Kreis T. E., Geiger B., Schmid E., Jorcano J. L., Franke W. W. De novo synthesis and specific assembly of keratin filaments in nonepithelial cells after microinjection of mRNA for epidermal keratin. Cell. 1983 Apr;32(4):1125–1137. doi: 10.1016/0092-8674(83)90296-9. [DOI] [PubMed] [Google Scholar]
- Köhler G., Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975 Aug 7;256(5517):495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lafay F. Envelope proteins of vesicular stomatitis virus: effect of temperature-sensitive mutations in complementation groups III and V. J Virol. 1974 Nov;14(5):1220–1228. doi: 10.1128/jvi.14.5.1220-1228.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehrman M. A., Goldstein J. L., Brown M. S., Russell D. W., Schneider W. J. Internalization-defective LDL receptors produced by genes with nonsense and frameshift mutations that truncate the cytoplasmic domain. Cell. 1985 Jul;41(3):735–743. doi: 10.1016/s0092-8674(85)80054-4. [DOI] [PubMed] [Google Scholar]
- Little S. P., Huang A. S. Shedding of the glycoprotein from vesicular stomatitis virus-infected cells. J Virol. 1978 Aug;27(2):330–339. doi: 10.1128/jvi.27.2.330-339.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mage M. G. Preparation of Fab fragments from IgGs of different animal species. Methods Enzymol. 1980;70(A):142–150. doi: 10.1016/s0076-6879(80)70045-9. [DOI] [PubMed] [Google Scholar]
- McAllister P. E., Wagner R. R. Differential inhibition of host protein synthesis in L cells infected with RNA - temperature-sensitive mutants of vesicular stomatitis virus. J Virol. 1976 May;18(2):550–558. doi: 10.1128/jvi.18.2.550-558.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison T. G. Site of synthesis of membrane and nonmembrane proteins of vesicular stomatitis virus. J Biol Chem. 1975 Sep 10;250(17):6955–6962. [PubMed] [Google Scholar]
- Murre C., Reiss C. S., Bernabeu C., Chen L. B., Burakoff S. J., Seidman J. G. Construction, expression and recognition of an H-2 molecule lacking its carboxyl terminus. Nature. 1984 Feb 2;307(5950):432–436. doi: 10.1038/307432a0. [DOI] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Bergmann J. E. Altered cytoplasmic domains affect intracellular transport of the vesicular stomatitis virus glycoprotein. Cell. 1983 Sep;34(2):513–524. doi: 10.1016/0092-8674(83)90384-7. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Relation of fatty acid attachment to the translation and maturation of vesicular stomatitis and Sindbis virus membrane glycoproteins. J Biol Chem. 1980 Apr 25;255(8):3334–3339. [PubMed] [Google Scholar]
- Zilberstein A., Snider M. D., Porter M., Lodish H. F. Mutants of vesicular stomatitis virus blocked at different stages in maturation of the viral glycoprotein. Cell. 1980 Sep;21(2):417–427. doi: 10.1016/0092-8674(80)90478-x. [DOI] [PubMed] [Google Scholar]
- Zuniga M. C., Malissen B., McMillan M., Brayton P. R., Clark S. S., Forman J., Hood L. Expression and function of transplantation antigens with altered or deleted cytoplasmic domains. Cell. 1983 Sep;34(2):535–544. doi: 10.1016/0092-8674(83)90386-0. [DOI] [PubMed] [Google Scholar]