Abstract
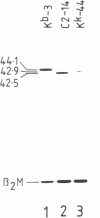
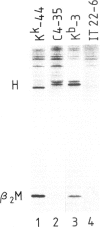
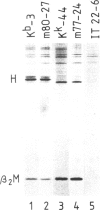
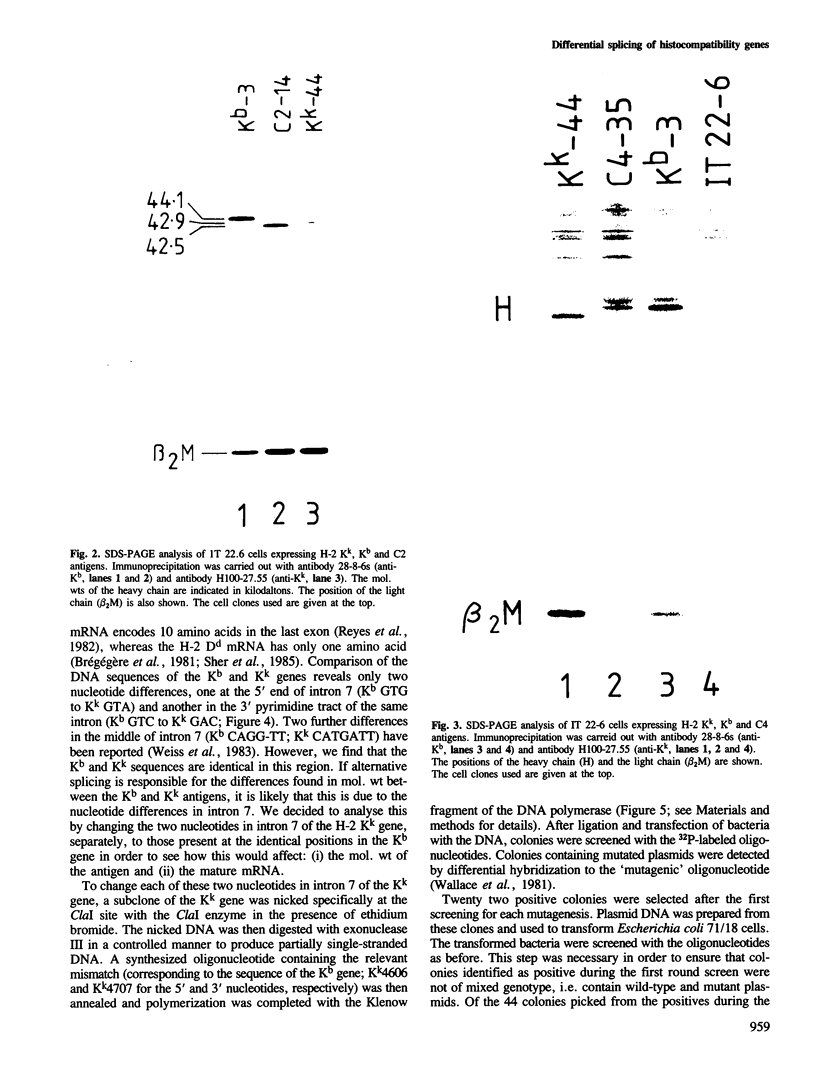
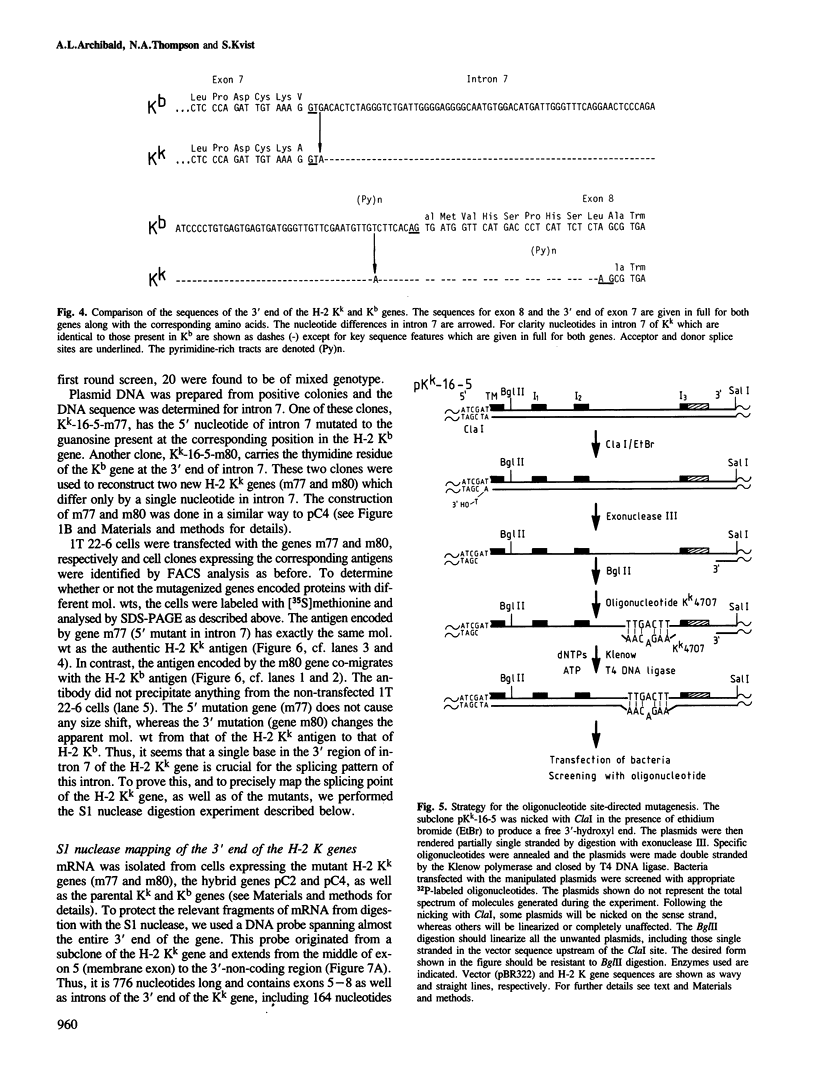
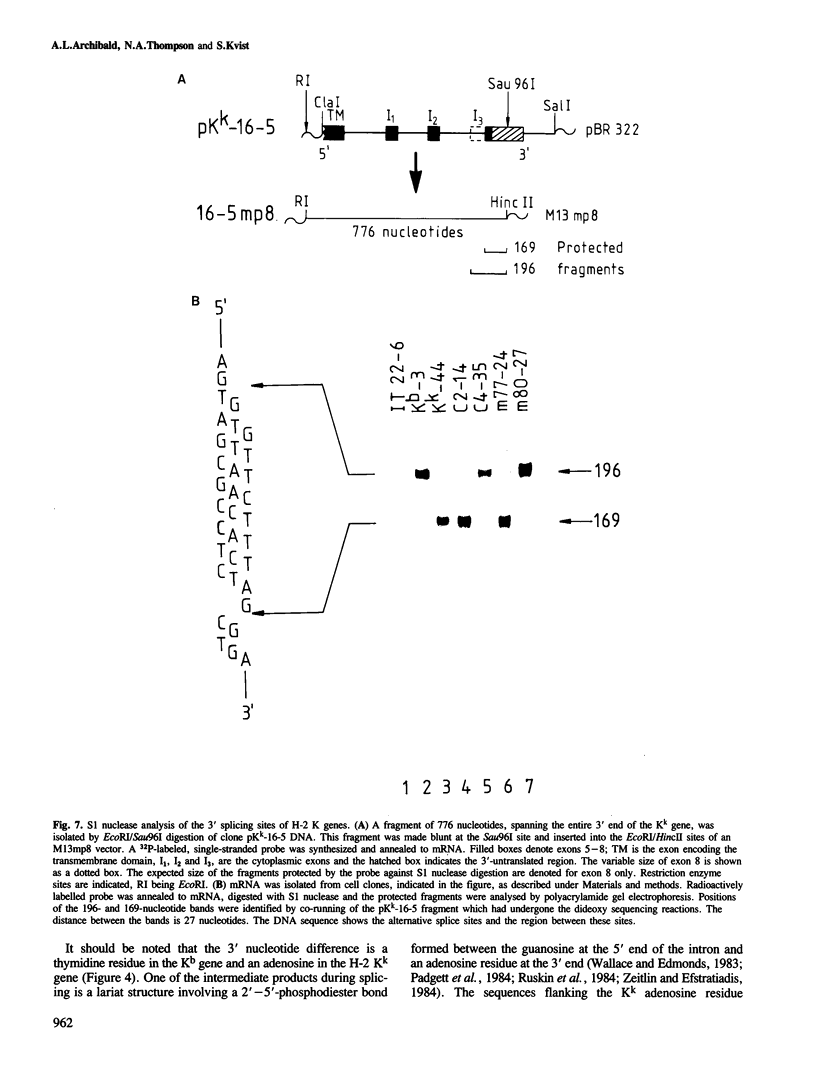
The murine histocompatibility class I genes, H-2 Kb and Kk, display considerable homology at their 3' ends. In fact, from exon 5 to the termination codon, only two nucleotides differ between the two genes, one at the 5' end and the other at the 3' end of intron 7. Despite this similarity, the gene products have distinctly different mol. wts as determined by SDS-PAGE. By constructing two hybrid genes, pC2 and pC4, we demonstrated that it is the cytoplasmic parts of the antigens (encoded by exons 6-8) which are responsible for the major difference in mol. wt. We have used site-directed mutagenesis to change the two nucleotides in intron 7 of the H-2 Kk gene to those present in the H-2 Kb gene. S1 nuclease mapping has been used to identify the actual splice site of the authentic Kb and Kk genes, the hybrid genes and the mutagenized genes. We have shown that it is the 3' nucleotide difference, nine nucleotides upstream of the 3' splice site, which causes the different excision of intron 7 of the Kb gene. The 5' nucleotide difference does not alter the splicing. The choice of branch points and 3' splice signals for intron 7 of five H-2 class I genes, is discussed.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold B., Burgert H. G., Archibald A. L., Kvist S. Complete nucleotide sequence of the murine H-2Kk gene. Comparison of three H-2K locus alleles. Nucleic Acids Res. 1984 Dec 21;12(24):9473–9487. doi: 10.1093/nar/12.24.9473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold B., Burgert H. G., Hamann U., Hämmerling G., Kees U., Kvist S. Cytolytic T cells recognize the two amino-terminal domains of H-2 K antigens in tandem in influenza A infected cells. Cell. 1984 Aug;38(1):79–87. doi: 10.1016/0092-8674(84)90528-2. [DOI] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brégégère F., Abastado J. P., Kvist S., Rask L., Lalanne J. L., Garoff H., Cami B., Wiman K., Larhammar D., Peterson P. A. Structure of C-terminal half of two H-2 antigens from cloned mRNA. Nature. 1981 Jul 2;292(5818):78–81. doi: 10.1038/292078a0. [DOI] [PubMed] [Google Scholar]
- Burgert H. G., Kvist S. An adenovirus type 2 glycoprotein blocks cell surface expression of human histocompatibility class I antigens. Cell. 1985 Jul;41(3):987–997. doi: 10.1016/s0092-8674(85)80079-9. [DOI] [PubMed] [Google Scholar]
- Connolly B. A., Potter B. V., Eckstein F., Pingoud A., Grotjahn L. Synthesis and characterization of an octanucleotide containing the EcoRI recognition sequence with a phosphorothioate group at the cleavage site. Biochemistry. 1984 Jul 17;23(15):3443–3453. doi: 10.1021/bi00310a010. [DOI] [PubMed] [Google Scholar]
- Dente L., Cesareni G., Cortese R. pEMBL: a new family of single stranded plasmids. Nucleic Acids Res. 1983 Mar 25;11(6):1645–1655. doi: 10.1093/nar/11.6.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domdey H., Apostol B., Lin R. J., Newman A., Brody E., Abelson J. Lariat structures are in vivo intermediates in yeast pre-mRNA splicing. Cell. 1984 Dec;39(3 Pt 2):611–621. doi: 10.1016/0092-8674(84)90468-9. [DOI] [PubMed] [Google Scholar]
- Felber B. K., Orkin S. H., Hamer D. H. Abnormal RNA splicing causes one form of alpha thalassemia. Cell. 1982 Jul;29(3):895–902. doi: 10.1016/0092-8674(82)90451-2. [DOI] [PubMed] [Google Scholar]
- Frendewey D., Keller W. Stepwise assembly of a pre-mRNA splicing complex requires U-snRNPs and specific intron sequences. Cell. 1985 Aug;42(1):355–367. doi: 10.1016/s0092-8674(85)80131-8. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Garoff H., Lehrach H. A subcloning strategy for DNA sequence analysis. Nucleic Acids Res. 1980 Dec 11;8(23):5541–5549. doi: 10.1093/nar/8.23.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowski P. J., Padgett R. A., Sharp P. A. Messenger RNA splicing in vitro: an excised intervening sequence and a potential intermediate. Cell. 1984 Jun;37(2):415–427. doi: 10.1016/0092-8674(84)90372-6. [DOI] [PubMed] [Google Scholar]
- Green M. R., Maniatis T., Melton D. A. Human beta-globin pre-mRNA synthesized in vitro is accurately spliced in Xenopus oocyte nuclei. Cell. 1983 Mar;32(3):681–694. doi: 10.1016/0092-8674(83)90054-5. [DOI] [PubMed] [Google Scholar]
- Guo L. H., Wu R. Exonuclease III: use for DNA sequence analysis and in specific deletions of nucleotides. Methods Enzymol. 1983;100:60–96. doi: 10.1016/0076-6879(83)00046-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hernandez N., Keller W. Splicing of in vitro synthesized messenger RNA precursors in HeLa cell extracts. Cell. 1983 Nov;35(1):89–99. doi: 10.1016/0092-8674(83)90211-8. [DOI] [PubMed] [Google Scholar]
- Keller E. B., Noon W. A. Intron splicing: a conserved internal signal in introns of animal pre-mRNAs. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7417–7420. doi: 10.1073/pnas.81.23.7417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller W. The RNA lariat: a new ring to the splicing of mRNA precursors. Cell. 1984 Dec;39(3 Pt 2):423–425. doi: 10.1016/0092-8674(84)90449-5. [DOI] [PubMed] [Google Scholar]
- Konarska M. M., Grabowski P. J., Padgett R. A., Sharp P. A. Characterization of the branch site in lariat RNAs produced by splicing of mRNA precursors. Nature. 1985 Feb 14;313(6003):552–557. doi: 10.1038/313552a0. [DOI] [PubMed] [Google Scholar]
- Krainer A. R., Maniatis T., Ruskin B., Green M. R. Normal and mutant human beta-globin pre-mRNAs are faithfully and efficiently spliced in vitro. Cell. 1984 Apr;36(4):993–1005. doi: 10.1016/0092-8674(84)90049-7. [DOI] [PubMed] [Google Scholar]
- Kress M., Glaros D., Khoury G., Jay G. Alternative RNA splicing in expression of the H-2K gene. Nature. 1983 Dec 8;306(5943):602–604. doi: 10.1038/306602a0. [DOI] [PubMed] [Google Scholar]
- Kvist S., Roberts L., Dobberstein B. Mouse histocompatibility genes: structure and organisation of a Kd gene. EMBO J. 1983;2(2):245–254. doi: 10.1002/j.1460-2075.1983.tb01413.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvist S., Wiman K., Claesson L., Peterson P. A., Dobberstein B. Membrane insertion and oligomeric assembly of HLA-DR histocompatibility antigens. Cell. 1982 May;29(1):61–69. doi: 10.1016/0092-8674(82)90090-3. [DOI] [PubMed] [Google Scholar]
- Lalanne J. L., Delarbre C., Gachelin G., Kourilsky P. A cDNA clone containing the entire coding sequence of a mouse H-2Kd histocompatibility antigen. Nucleic Acids Res. 1983 Mar 11;11(5):1567–1577. doi: 10.1093/nar/11.5.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford C. J., Klinz F. J., Donath C., Gallwitz D. Point mutations identify the conserved, intron-contained TACTAAC box as an essential splicing signal sequence in yeast. Cell. 1984 Mar;36(3):645–653. doi: 10.1016/0092-8674(84)90344-1. [DOI] [PubMed] [Google Scholar]
- Lemke H., Hämmerling G. J., Hämmerling U. Fine specificity analysis with monoclonal antibodies of antigens controlled by the major histocompatibility complex and by the Qa/TL region in mice. Immunol Rev. 1979;47:175–206. doi: 10.1111/j.1600-065x.1979.tb00293.x. [DOI] [PubMed] [Google Scholar]
- Lewin B. Alternatives for splicing: recognizing the ends of introns. Cell. 1980 Nov;22(2 Pt 2):324–326. doi: 10.1016/0092-8674(80)90340-2. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Moore K. W., Sher B. T., Sun Y. H., Eakle K. A., Hood L. DNA sequence of a gene encoding a BALB/c mouse Ld transplantation antigen. Science. 1982 Feb 5;215(4533):679–682. doi: 10.1126/science.7058332. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murre C., Reiss C. S., Bernabeu C., Chen L. B., Burakoff S. J., Seidman J. G. Construction, expression and recognition of an H-2 molecule lacking its carboxyl terminus. Nature. 1984 Feb 2;307(5950):432–436. doi: 10.1038/307432a0. [DOI] [PubMed] [Google Scholar]
- Ozato K., Sachs D. H. Monoclonal antibodies to mouse MHC antigens. III. Hybridoma antibodies reacting to antigens of the H-2b haplotype reveal genetic control of isotype expression. J Immunol. 1981 Jan;126(1):317–321. [PubMed] [Google Scholar]
- Padgett R. A., Hardy S. F., Sharp P. A. Splicing of adenovirus RNA in a cell-free transcription system. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5230–5234. doi: 10.1073/pnas.80.17.5230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Konarska M. M., Grabowski P. J., Hardy S. F., Sharp P. A. Lariat RNA's as intermediates and products in the splicing of messenger RNA precursors. Science. 1984 Aug 31;225(4665):898–903. doi: 10.1126/science.6206566. [DOI] [PubMed] [Google Scholar]
- Pikielny C. W., Teem J. L., Rosbash M. Evidence for the biochemical role of an internal sequence in yeast nuclear mRNA introns: implications for U1 RNA and metazoan mRNA splicing. Cell. 1983 Sep;34(2):395–403. doi: 10.1016/0092-8674(83)90373-2. [DOI] [PubMed] [Google Scholar]
- Reed R., Maniatis T. Intron sequences involved in lariat formation during pre-mRNA splicing. Cell. 1985 May;41(1):95–105. doi: 10.1016/0092-8674(85)90064-9. [DOI] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Itakura K., Wallace R. B. Isolation of a cDNA clone for the murine transplantation antigen H-2Kb. Proc Natl Acad Sci U S A. 1982 May;79(10):3270–3274. doi: 10.1073/pnas.79.10.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez J. R., Pikielny C. W., Rosbash M. In vivo characterization of yeast mRNA processing intermediates. Cell. 1984 Dec;39(3 Pt 2):603–610. doi: 10.1016/0092-8674(84)90467-7. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Green M. R. Role of the 3' splice site consensus sequence in mammalian pre-mRNA splicing. Nature. 1985 Oct 24;317(6039):732–734. doi: 10.1038/317732a0. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Greene J. M., Green M. R. Cryptic branch point activation allows accurate in vitro splicing of human beta-globin intron mutants. Cell. 1985 Jul;41(3):833–844. doi: 10.1016/s0092-8674(85)80064-7. [DOI] [PubMed] [Google Scholar]
- Ruskin B., Krainer A. R., Maniatis T., Green M. R. Excision of an intact intron as a novel lariat structure during pre-mRNA splicing in vitro. Cell. 1984 Aug;38(1):317–331. doi: 10.1016/0092-8674(84)90553-1. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. On the origin of RNA splicing and introns. Cell. 1985 Sep;42(2):397–400. doi: 10.1016/0092-8674(85)90092-3. [DOI] [PubMed] [Google Scholar]
- Sher B. T., Nairn R., Coligan J. E., Hood L. E. DNA sequence of the mouse H-2Dd transplantation antigen gene. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1175–1179. doi: 10.1073/pnas.82.4.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Steinmetz M., Hood L. Genes of the major histocompatibility complex in mouse and man. Science. 1983 Nov 18;222(4625):727–733. doi: 10.1126/science.6356354. [DOI] [PubMed] [Google Scholar]
- Treisman R., Orkin S. H., Maniatis T. Specific transcription and RNA splicing defects in five cloned beta-thalassaemia genes. Nature. 1983 Apr 14;302(5909):591–596. doi: 10.1038/302591a0. [DOI] [PubMed] [Google Scholar]
- Treisman R., Proudfoot N. J., Shander M., Maniatis T. A single-base change at a splice site in a beta 0-thalassemic gene causes abnormal RNA splicing. Cell. 1982 Jul;29(3):903–911. doi: 10.1016/0092-8674(82)90452-4. [DOI] [PubMed] [Google Scholar]
- Wallace J. C., Edmonds M. Polyadenylylated nuclear RNA contains branches. Proc Natl Acad Sci U S A. 1983 Feb;80(4):950–954. doi: 10.1073/pnas.80.4.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. B., Johnson P. F., Tanaka S., Schöld M., Itakura K., Abelson J. Directed deletion of a yeast transfer RNA intervening sequence. Science. 1980 Sep 19;209(4463):1396–1400. doi: 10.1126/science.6997991. [DOI] [PubMed] [Google Scholar]
- Wallace R. B., Schold M., Johnson M. J., Dembek P., Itakura K. Oligonucleotide directed mutagenesis of the human beta-globin gene: a general method for producing specific point mutations in cloned DNA. Nucleic Acids Res. 1981 Aug 11;9(15):3647–3656. doi: 10.1093/nar/9.15.3647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E., Golden L., Zakut R., Mellor A., Fahrner K., Kvist S., Flavell R. A. The DNA sequence of the H-2kb gene: evidence for gene conversion as a mechanism for the generation of polymorphism in histocompatibilty antigens. EMBO J. 1983;2(3):453–462. doi: 10.1002/j.1460-2075.1983.tb01444.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C. Molecular biology. Excision of introns in lariat form. Nature. 1984 Sep 13;311(5982):103–104. doi: 10.1038/311103a0. [DOI] [PubMed] [Google Scholar]
- Wieringa B., Hofer E., Weissmann C. A minimal intron length but no specific internal sequence is required for splicing the large rabbit beta-globin intron. Cell. 1984 Jul;37(3):915–925. doi: 10.1016/0092-8674(84)90426-4. [DOI] [PubMed] [Google Scholar]
- Wieringa B., Meyer F., Reiser J., Weissmann C. Unusual splice sites revealed by mutagenic inactivation of an authentic splice site of the rabbit beta-globin gene. Nature. 1983 Jan 6;301(5895):38–43. doi: 10.1038/301038a0. [DOI] [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin J. H., Kvist S., Dobberstein B. Identification of an H-2Kd gene using a specific cDNA probe. EMBO J. 1982;1(4):467–471. doi: 10.1002/j.1460-2075.1982.tb01192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin S., Efstratiadis A. In vivo splicing products of the rabbit beta-globin pre-mRNA. Cell. 1984 Dec;39(3 Pt 2):589–602. doi: 10.1016/0092-8674(84)90466-5. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]
- Zuniga M. C., Malissen B., McMillan M., Brayton P. R., Clark S. S., Forman J., Hood L. Expression and function of transplantation antigens with altered or deleted cytoplasmic domains. Cell. 1983 Sep;34(2):535–544. doi: 10.1016/0092-8674(83)90386-0. [DOI] [PubMed] [Google Scholar]