Abstract
Concentrations of mRNA coding for the opioid peptide precursor proenkephalin A (mRNAENK) were measured in primary cultures of bovine adrenal chromaffin cells maintained in serum-free medium. Using a sensitive solution hybridization assay, an increase in mRNAENK levels from 45 to 300% above control with K+ (10-20 mM), Ba2+ (1 mM) and veratridine (5 microM) was found. The highest increase (300% above control) was obtained with the Na+ channel agonist veratridine. This effect was nearly abolished in the presence of the Na+ channel antagonist tetrodotoxin (TTX) (1 microM). Moreover, TTX partially inhibited the increase in mRNAENK levels caused by K+ (20 mM) depolarization (from 185 to 130% of control), but had no effect on the stimulation by Ba2+ (1 mM). The Ca2+ channel antagonists D600 (50 microM) verapamil (50 microM) and Co2+ (1 mM) inhibited the responses to either K+, Ba2+ or veratridine, whereas the Ca2+ channel agonist Bay K 8644 (0.1 microM) potentiated the effect of 20 mM K+ from 185 to 230% of control. The K+-induced increase in the mRNAENK levels was associated with an increase of immunoreactive proenkephalin A-derived peptides in both tissue and medium, indicating an enhanced production of opioid peptides. These results suggest that membrane depolarization may play an important role in the regulation of proenkephalin A gene expression in bovine adrenal chromaffin cells. It may represent a mode by which substances acting directly on Na+ or Ca2+ channels may modulate the regulation of proenkephalin A mRNA biosynthesis and opioid peptide production.
Full text
PDF
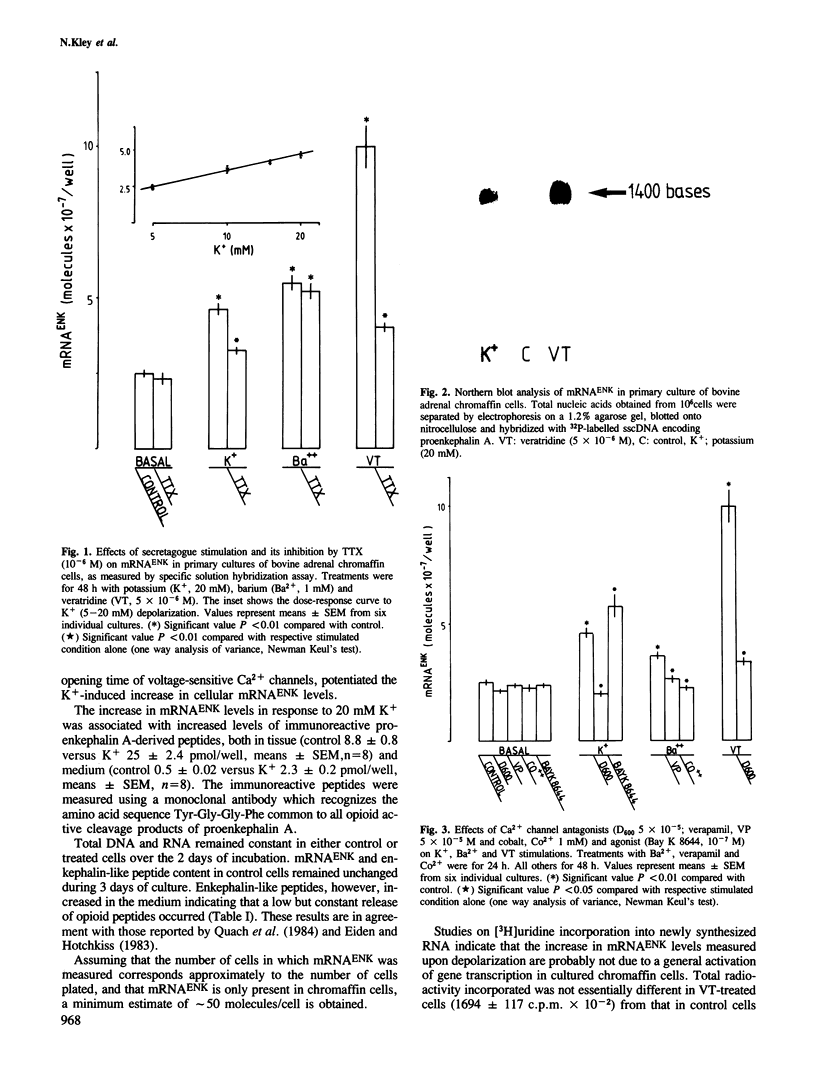


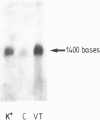
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biales B., Dichter M., Tischler A. Electrical excitability of cultured adrenal chromaffin cells. J Physiol. 1976 Nov;262(3):743–753. doi: 10.1113/jphysiol.1976.sp011618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Constanti A., Adams P. R. Slow cholinergic and peptidergic transmission in sympathetic ganglia. Fed Proc. 1981 Sep;40(11):2625–2630. [PubMed] [Google Scholar]
- Chen C. L., Dionne F. T., Roberts J. L. Regulation of the pro-opiomelanocortin mRNA levels in rat pituitary by dopaminergic compounds. Proc Natl Acad Sci U S A. 1983 Apr;80(8):2211–2215. doi: 10.1073/pnas.80.8.2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Kanno T., Sampson S. R. Influence of the ionic environment on the membrane potential of adrenal chromaffin cells and on the depolarizing effect of acetylcholine. J Physiol. 1967 Jul;191(1):107–121. doi: 10.1113/jphysiol.1967.sp008239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S., Tomiko S. A. Secretagogue effect of barium on output of melanocyte-stimulating hormone from pars intermedia of the mouse pituitary. J Physiol. 1983 May;338:243–257. doi: 10.1113/jphysiol.1983.sp014671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durnam D. M., Palmiter R. D. A practical approach for quantitating specific mRNAs by solution hybridization. Anal Biochem. 1983 Jun;131(2):385–393. doi: 10.1016/0003-2697(83)90188-4. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Giraud P., Dave J. R., Hotchkiss A. J., Affolter H. U. Nicotinic receptor stimulation activates enkephalin release and biosynthesis in adrenal chromaffin cells. Nature. 1984 Dec 13;312(5995):661–663. doi: 10.1038/312661a0. [DOI] [PubMed] [Google Scholar]
- Eiden L. E., Hotchkiss A. J. Cyclic adenosine monophosphate regulates vasoactive intestinal polypeptide and enkephalin biosynthesis in cultured bovine chromaffin cells. Neuropeptides. 1983 Dec;4(1):1–9. doi: 10.1016/0143-4179(83)90002-1. [DOI] [PubMed] [Google Scholar]
- Fenwick E. M., Marty A., Neher E. Sodium and calcium channels in bovine chromaffin cells. J Physiol. 1982 Oct;331:599–635. doi: 10.1113/jphysiol.1982.sp014394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleminger G., Lahm H. W., Udenfriend S. Changes in rat adrenal catecholamines and proenkephalin metabolism after denervation. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3587–3590. doi: 10.1073/pnas.81.11.3587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman J. E., Lelkes P. I., Lavie E., Rosenheck K., Schneeweiss F., Schneider A. S. Membrane potential and catecholamine secretion by bovine adrenal chromaffin cells: use of tetraphenylphosphonium distribution and carbocyanine dye fluorescence. J Neurochem. 1985 May;44(5):1391–1402. doi: 10.1111/j.1471-4159.1985.tb08775.x. [DOI] [PubMed] [Google Scholar]
- Gramsch C., Meo T., Riethmüller G., Herz A. Binding characteristics of a monoclonal beta-endorphin antibody recognizing the N-terminus of opioid peptides. J Neurochem. 1983 May;40(5):1220–1226. doi: 10.1111/j.1471-4159.1983.tb13560.x. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Byerly L. Calcium channel. Annu Rev Neurosci. 1981;4:69–125. doi: 10.1146/annurev.ne.04.030181.000441. [DOI] [PubMed] [Google Scholar]
- Ito S., Nakazato Y., Ohga A. The effect of veratridine on the release of catecholamines from the perfused adrenal gland. Br J Pharmacol. 1979 Feb;65(2):319–330. doi: 10.1111/j.1476-5381.1979.tb07833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka Y., Gutman Y., Guidotti A., Panula P., Wroblewski J., Cosenza-Murphy D., Wu J. Y., Costa E. Intrinsic GABAergic system of adrenal chromaffin cells. Proc Natl Acad Sci U S A. 1984 May;81(10):3218–3222. doi: 10.1073/pnas.81.10.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler J. A., Adler J. E., Bohn M. C., Black I. B. Substance P in principal sympathetic neurons: regulation by impulse activity. Science. 1981 Oct 16;214(4518):335–336. doi: 10.1126/science.6169153. [DOI] [PubMed] [Google Scholar]
- Kessler J. A., Black I. B. Regulation of substance P in adult rat sympathetic ganglia. Brain Res. 1982 Feb 18;234(1):182–187. doi: 10.1016/0006-8993(82)90485-1. [DOI] [PubMed] [Google Scholar]
- Kidokoro Y., Ritchie A. K. Chromaffin cell action potentials and their possible role in adrenaline secretion from rat adrenal medulla. J Physiol. 1980 Oct;307:199–216. doi: 10.1113/jphysiol.1980.sp013431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Howells R. D., Fleminger G., Udenfriend S. Denervation of rat adrenal glands markedly increases preproenkephalin mRNA. Proc Natl Acad Sci U S A. 1984 Nov;81(22):7221–7223. doi: 10.1073/pnas.81.22.7221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilpatrick D. L., Slepetis R., Kirshner N. Ion channels and membrane potential in stimulus-secretion coupling in adrenal medulla cells. J Neurochem. 1981 Mar;36(3):1245–1255. doi: 10.1111/j.1471-4159.1981.tb01724.x. [DOI] [PubMed] [Google Scholar]
- LaGamma E. F., Adler J. E., Black I. B. Impulse activity differentially regulates [Leu]enkephalin and catecholamine characters in the adrenal medulla. Science. 1984 Jun 8;224(4653):1102–1104. doi: 10.1126/science.6144183. [DOI] [PubMed] [Google Scholar]
- LaGamma E. F., White J. D., Adler J. E., Krause J. E., McKelvy J. F., Black I. B. Depolarization regulates adrenal preproenkephalin mRNA. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8252–8255. doi: 10.1073/pnas.82.23.8252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis R. V., Stern A. S. Biosynthesis of the enkephalins and enkephalin-containing polypeptides. Annu Rev Pharmacol Toxicol. 1983;23:353–372. doi: 10.1146/annurev.pa.23.040183.002033. [DOI] [PubMed] [Google Scholar]
- Livett B. G. Adrenal medullary chromaffin cells in vitro. Physiol Rev. 1984 Oct;64(4):1103–1161. doi: 10.1152/physrev.1984.64.4.1103. [DOI] [PubMed] [Google Scholar]
- Livett B. G., Dean D. M., Whelan L. G., Udenfriend S., Rossier J. Co-release of enkephalin and catecholamines from cultured adrenal chromaffin cells. Nature. 1981 Jan 22;289(5795):317–319. doi: 10.1038/289317a0. [DOI] [PubMed] [Google Scholar]
- Pittius C. W., Kley N., Loeffler J. P., Höllt V. Quantitation of proenkephalin A messenger RNA in bovine brain, pituitary and adrenal medulla: correlation between mRNA and peptide levels. EMBO J. 1985 May;4(5):1257–1260. doi: 10.1002/j.1460-2075.1985.tb03769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quach T. T., Tang F., Kageyama H., Mocchetti I., Guidotti A., Meek J. L., Costa E., Schwartz J. P. Enkephalin biosynthesis in adrenal medulla. Modulation of proenkephalin mRNA content of cultured chromaffin cells by 8-bromo-adenosine 3',5'-monophosphate. Mol Pharmacol. 1984 Sep;26(2):255–260. [PubMed] [Google Scholar]
- Romey G., Lazdunski M. Lipid-soluble toxins thought to be specific for Na+ channels block Ca2+ channels in neuronal cells. Nature. 1982 May 6;297(5861):79–78. doi: 10.1038/297079a0. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viveros O. H., Diliberto E. J., Jr, Hazum E., Chang K. J. Opiate-like materials in the adrenal medulla: evidence for storage and secretion with catecholamines. Mol Pharmacol. 1979 Nov;16(3):1101–1108. [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]