Abstract
Animal models constructed using pathogenic factors have significantly advanced drug development for Alzheimer’s disease (AD). These predominantly transgenic models, mainly in mice, replicate pathological phenotypes through gene mutations associated with familial AD cases, thus serving as vital tools for assessing drug efficacy and for performing mechanistic studies. However, the species-specific differences and complex, heterogeneous nature of AD etiology pose considerable challenges for the translatability of these animal models, limiting their utility in drug development. This review offers a comprehensive analysis of widely employed rodent (mice and rats) and non-rodent models ( Danio rerio (zebrafish), Drosophila melanogaster, and Caenorhabditis elegans), detailing their phenotypic features and specific research applications. This review also examines the limitations inherent in these models and introduces various strategies for expanding AD modeling across diverse species, emphasizing recent advancement in non-human primates (NHPs) as valuable models. Furthermore, potential insights from the integration of innovative technologies in AD research are discussed, while providing valuable perspectives on the future development of AD animal models.
Keywords: Alzheimer’s disease, Animal models, Aβ, Tau, Non-human primates
INTRODUCTION
Alzheimer’s disease (AD), the most prevalent neurodegenerative disorder, accounts for over 60% of dementia cases ( 2024 Alzheimer's disease facts and figures, 2024). Clinically, AD manifests initially as episodic and short-term memory impairment, progressing to extensive deficits in both declarative and non-declarative memory functions. Pathological hallmarks include dystrophic neurites, significant synaptic loss, neuronal degeneration, and gliosis within the brain ( Guo et al., 2020; Knopman et al., 2021).
Typical pathological features of AD include extracellular amyloid plaques and intracellular neurofibrillary tangles (NFTs) in the cortical and limbic regions ( Guo et al., 2020). Targeting β-amyloid (Aβ), the core protein of amyloid plaques, several monoclonal antibodies (e.g., aducanumab, lecanemab, donanemab) have recently gained approval for AD therapy, marking a significant milestone in the therapeutic landscape ( Sevigny et al., 2016; Sims et al., 2023; Van Dyck et al., 2023). Nonetheless, current therapeutics and medications are confined to mitigating cognitive decline, lacking the efficacy to halt or reverse the fundamental neurodegenerative mechanisms. Furthermore, limited translatability of preclinical studies using animal models of AD remains a major issue for drug development ( Padmanabhan & Götz, 2023).
Pedigree analyses of familial AD (FAD) and frontotemporal dementia (FTD) have identified mutations in the amyloid precursor protein (encoded by the APP gene) and microtubule-associated protein tau (encoded by the MAPT gene) ( Figure 1), revealing key molecular underpinnings linked to these neurodegenerative disorders ( Bugiani et al., 1999; Clark et al., 1995; Levy et al., 1990). This discovery has led to the development of various animal models carrying these mutations, a widely adopted approach in neurodegenerative disease research. The first transgenic mouse model of AD, established in 1995, involved the microinjection of a platelet-derived growth factor (PDGF)-driven construct encoding full-length human APP with the V717F mutation ( Games et al., 1995). The resulting PDAPP mice exhibited extracellular Aβ deposition, along with dystrophic neurites and loss of synaptic density in the hippocampus ( Dodart et al., 1999; Hartman et al., 2005). However, as designed, these PDAPP mice did not develop tangles or show significant neuronal loss, limiting their utility in AD research. Currently, widely used transgenic rodent models in AD research encompass several key lines, including Tg2576 ( Hsiao et al., 1996), APP23 ( Sturchler-Pierrat et al., 1997), APPswe/PS1dE9 ( Hsia et al., 1999), 3xTg ( Oddo et al., 2003b), 5xFAD ( Oakley et al., 2006), rTg4510 ( Berger et al., 2007), and PS19 mice ( Santacruz et al., 2005), among others.
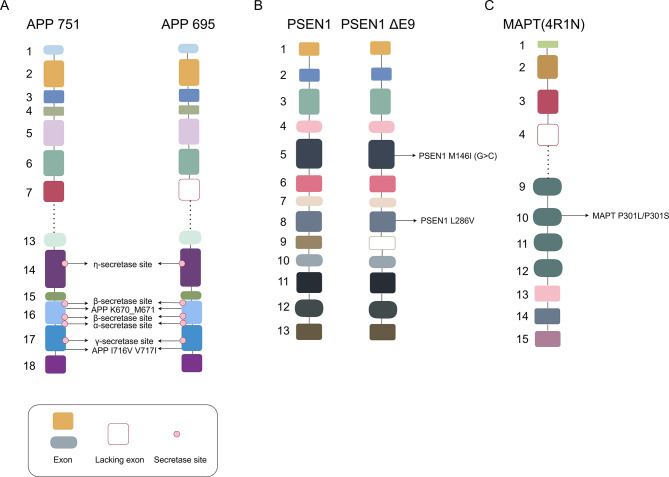
Figure 1.
Main pathogenic mutation sites and related secretase sites in FAD associated with APP and PSEN1 and FTD associated with MAPT
A: APP isoforms containing 751 or 695 amino acids with main pathogenic mutations, as well as primary secretase cleavage sites: In healthy individuals, α-secretase cleaves APP, producing soluble APP; in disease states, β-secretase and γ-secretase abnormally cleave APP in sequence, initially forming the Aβ peptide fragment. B: Full-length PSEN1 fragments and fragments with exon 9 deletion, as well as main pathogenic mutations. C: Tau 4R1N isoform and its main pathogenic mutations. APP: Amyloid precursor protein; MAPT: Microtubule-associated protein tau; PSEN1: Presenilin-1; FAD: Familial Alzheimer’s disease; FTD: Frontotemporal dementia.
In addition to amyloid plaques and NFT pathology, other processes, such as neuroinflammation (activated immune cells and inflammatory mediators), metabolic dysregulation (glucose metabolism and mitochondrial dysfunction), biometal dysregulation (iron and copper accumulation in the brain), and vascular damage (cerebral amyloid angiopathy and microvascular abnormalities), are also highly associated with AD pathogenesis ( Graff-Radford et al., 2021), and are often recapitulated in AD animal models. Genes identified through genome-wide association studies, such as APOE4 and TREM2, have been incorporated into transgenic models to explore the pathogenic mechanisms of AD ( Kotredes et al., 2021), with models such as APOE/TREM2 and Thy1-ApoE4/C/EBPβ double transgenic mice serving as pivotal tools in investigating AD-related pathways ( Kotredes et al., 2021; Qian et al., 2024). Furthermore, disruptions in neurotransmitter systems, especially acetylcholine and glutamate, further complicate AD pathophysiology ( Hampel et al., 2018; Hsieh et al., 2006; Scott et al., 2011), leading to the development of animal models based on these specific deficits.
While transgenic rodent models have greatly expanded our understanding of AD, species differences and the premature onset of disease symptoms in these models often result in disparities with clinical reality. Naturally occurring AD models, such as those in non-human primates (NHPs), incorporate a wider range of genetic and environmental factors, closely mimicking the complexity of human AD populations ( Chen & Zhang, 2022; Yao et al., 2024). These models provide a more comprehensive view of AD heterogeneity, offering potential insights into previously unidentified genetic or environmental contributors to disease development.
This review provides a detailed analysis of the diverse applications of various animal models and examines the three major challenges in AD modeling, namely replicating aging processes, addressing interspecies differences, and capturing sporadic etiology. Additionally, novel animal models designed to overcome these challenges are introduced, offering insights that may enhance future AD model development.
ANIMAL MODELS REPLICATING FAD
Single-transgenic mouse models ( APP or MAPT mutations)
The Tg2576: Tg2576 mouse model, a widely used transgenic model of AD, expresses the APP-Swedish mutation ( K670N, M671L) under the control of the HamPrP promotor ( Hsiao et al., 1996) ( Figure 1), resulting in a 5-fold increase in Aβ40 peptides and a 14-fold increase in the Aβ42/Aβ40 ratio.
As early as 2 months of age, Tg2576 mice exhibit intraneuronal soluble Aβ in the cerebral cortex at detectable pg/mg levels ( Manczak et al., 2006). By 3 months, although Aβ plaques have not yet formed, these mice display hippocampal synaptic dysfunction, increased TUNEL-positive apoptotic cells in dopaminergic neurons, and a marked rise in glial fibrillary acidic protein (GFAP)-positive cells in the ventral tegmental area, findings associated with psychiatric symptoms and suggesting that soluble Aβ assemblies may impair memory and other brain functions ( Nobili et al., 2017). At 6 months, Tg2576 mice show significantly elevated levels of soluble human Aβ42 and Aβ40, with insoluble Aβ deposition (plaques) emerging at 7 months ( Kawarabayashi et al., 2001). By 12 months of age, Aβ plaques become more diffuse, forming dense cores within the neocortex and hippocampus ( Duffy et al., 2015; Sasaki et al., 2002). Microglial and astrocytic up-regulation of interferon-γ (IFN-γ) and interleukin-12 (IL-12) is observed between 10 and 13 months, indicating microglial activation associated with plaque formation ( Abbas et al., 2002; Frautschy et al., 1998). Behavioral changes in Tg2576 mice typically manifest between 8 to 10 months of age ( Stewart et al., 2011). While conventional behavioral assays are often employed to assess generalized memory deficits, it is important to acknowledge that specific tests may capture distinct aspects of memory impairment. For example, the forced-choice alternation T-maze test ( Deacon & Rawlins, 2006) relies on the natural drive of rodents to explore new environments, providing a measure of spatial working memory sensitive to hippocampal dysfunction, with additional contributions from the prefrontal cortex and basal forebrain ( Ito et al., 2018; Lalonde, 2002). In contrast, the spontaneous alternation task in the continuous Y-maze, which assesses working memory in the absence of environmental cues, exhibits reduced sensitivity for detecting memory impairments in Tg2576 mice, potentially masking stereotypic behaviors ( Stewart et al., 2011). The Morris water maze (MWM) test ( Vorhees & Williams, 2006), which evaluates spatial reference memory—the long-term recall of specific locations in a static environment—is associated with the hippocampal and parahippocampal regions but demonstrates lower sensitivity in Tg2576 mice compared to the T-maze forced-choice alternation test ( Cornwell et al., 2008; Stewart et al., 2011).
The Tg2576 model remains a crucial tool in preclinical drug testing due to its stable Aβ pathology and cognitive impairment profile ( Kastanenka et al., 2016). Specifically, Aβ oligomers (Aβo), known for their toxicity, are detectable at a very early age in Tg2576 mice, potentially disrupting memory functions even prior to the appearance of Aβ plaques ( Takahashi et al., 2004). However, certain pathological and behavioral features in Tg2576 mice deviate from clinical manifestations. For instance, plaques in Tg2576 mice exhibit a diffuse, dispersed morphology, contrasting with the dense, stable plaques typically observed in AD patients ( Jacobsen et al., 2006). Additionally, psychiatric symptoms, such as deficits in hippocampal-dependent contextual fear conditioning and alterations in sensorimotor activity, manifest at an early age in Tg2576 mice, differing from the clinical progression of AD symptoms ( D'Amelio et al., 2011; King et al., 1999).
The APP23: The APP23 model was designed to express human APP751 cDNA with the Swedish ( K670N/M671L) mutation inserted into the Thy-1 expression cassette, resulting in a 7-fold overexpression of mutant human APP ( Figure 1). Although APP23 shares the same mutation as Tg2576, it exhibits distinct Aβ pathology. Unlike the diffuse plaques observed in Tg2576, APP23 mice develop human-like congophilic plaques with a dense, fibrillar amyloid core ( Calhoun et al., 1998). This amyloidosis is believed to arise from stochastic formation of Aβ seeds that subsequently propagate and spread throughout the brain ( Eisenberg & Jucker, 2012). Interestingly, Aβ-containing brain extracts from 22- to 28-month-old APP23 transgenic mice can induce β-amyloidosis in young APP23 mice ( Eisele et al., 2009; Heilbronner et al., 2013), suggesting that established amyloid pathology can accelerate amyloid formation in younger animals. Moreover, the seeding activity of APP23 brain extracts reaches a plateau relatively early in the amyloidogenic process ( Ye et al., 2017).
Cerebral amyloid angiopathy (CAA), a prominent hallmark of AD, is characterized by the accumulation of Aβ protein within cerebral blood vessel walls. The APP23 mouse model demonstrates significant CAA pathology, supporting the hypothesis that neuronally derived Aβ can access and deposit in vessel walls ( Calhoun et al., 1999). This CAA pathology in APP23 mice is accompanied by cerebrovascular abnormalities and disruptions in blood flow, with cerebrovascular lesions appearing by 12 months of age, and flow voids detected at the internal carotid artery ( Beckmann et al., 2003; Meyer-Luehmann et al., 2008). By 20 months, these mice display flow disturbances in large arteries surrounding the circle of Willis, coupled with cerebral microbleeds ( Beckmann et al., 2003; Marazuela et al., 2022). Compared to younger APP23 mice or control groups, aged APP23 mice also show an elevated presence of megakaryocytes in bone marrow and pre-activated platelets, enhancing platelet propensity for thrombus formation under flow and thereby increasing the risk of arterial thrombosis ( Jarre et al., 2014). The CAA pathology observed in APP23 mice underscores a potential shared etiological origin between CAA and AD, highlighting the importance of vascular health in AD pathogenesis and pointing to the need for therapeutic strategies that target vascular components.
At 6 months of age, APP23 mice exhibit unique neuropathological features, including distorted neurites with hyperphosphorylated tau—a rare phenomenon among single-gene APP mutation models ( Sturchler-Pierrat et al., 1997). This observation highlights the potential of Aβ to trigger excitotoxicity through abnormal tau aggregation, supporting the amyloid cascade hypothesis ( Ittner et al., 2010, 2016). Motor and social behavior disturbances also emerge at this age, as evidenced by alterations in cage activity and ambulation in open-field tests ( Kelly et al., 2003). By 9 months, APP23 mice also display further signs of AD-related pathology, including immune responses, enlarged dystrophic synaptic boutons in the neuropil, and a breakdown of slow-wave brain activity ( Calhoun et al., 1999). An increase in complement component 1q (C1q) mRNA expression is first detected at 9 months, with extensive gliosis, characterized by hypertrophic astrocytes and activated microglia, closely associated with Aβ deposits by 12 months ( Sturchler-Pierrat et al., 1997). Significant neuritic degeneration and distortion of cholinergic fibers are also evident in regions near amyloid plaques by 9 months of age ( Sturchler-Pierrat et al., 1997).
Neuronal damage in APP23 mice progresses subsequent to amyloid plaque pathology, with initial observations in the hippocampal CA1 region occurring at 14 months ( Calhoun et al., 1998). By 24 months, an increasing number of neocortical neurons exhibit necrotic-apoptotic phenotypes ( Bondolfi et al., 2002). Interestingly, at 8 months, APP23 mice possess approximately 13%–15% more neocortical neurons than their wild-type counterparts ( Bondolfi et al., 2002), suggesting that these mice initially maintain elevated neuron counts before experiencing neuronal loss as cerebral amyloidogenesis advances. However, data on long-term potentiation (LTP) in APP23 mice are inconsistent. Roder et al. ( 2003) found no significant difference in LTP between hemizygous APP23 and wild-type littermates, while Huang et al. ( 2006) reported reduced LTP in 13–14-month-old APP23 mice, linked with reduced neprilysin activity. These contradictions pose challenges in assessing synaptic function using APP23 mice.
The rTg4510: The rTg4510 mouse model is produced by crossing the 4510-responder line, which carries human MAPT P301L cDNA, with an activator line regulated by the forebrain-specific CaMKIIα promoter. This cross results in an elevated expression of mutant human tau protein, approximately 13 times higher than that of endogenous mouse tau ( Ramsden et al., 2005) ( Figure 1).
Tau protein, encoded by the MAPT gene, plays a critical role in stabilizing neuronal microtubules. Under pathological conditions, hyperphosphorylated tau detaches from microtubules, leading to cytoskeletal destabilization and impaired neuronal function. The resulting accumulation of hyperphosphorylated tau into NFTs is neurotoxic and a hallmark of neurodegenerative disease. The P301L mutation in the MAPT gene, identified across FTD cases globally, poses an increased risk for neurodegeneration ( Borrego-Écija et al., 2017; Dumanchin et al., 1998; Gatto et al., 2017; Hutton et al., 1998; Moore et al., 2020; Palencia-Madrid et al., 2019). Although the precise mechanism by which P301L contributes to neurodegeneration remains unclear, studies suggest that the mutated protein may adopt different conformations, contributing to diverse neuropathological outcomes ( Daude et al., 2020). Hyperphosphorylated tau with the P301L mutation is more potent in inducing endoplasmic reticulum stress and cytoskeletal damage compared to non-phosphorylated forms, underscoring its role in neurodegenerative diseases ( Wang et al., 2023a).
In rTg4510 mice, tau pathology is detectable as early as 6–7 weeks of age, with phosphorylation at Ser202 and abnormal conformations evident in the hippocampus and cortex, identified by CP-13 and MC-1 antibodies, respectively ( Ramsden et al., 2005). By later stages, around 10% of cortical neurons become PHF1-positive, closely resembling human tauopathy ( Spires et al., 2006). Tau pathology is accompanied by gliosis at approximately 2.5 months of age, as indicated by increased immunoreactivity of GFAP and the microglial marker Iba1 ( Helboe et al., 2017). Between 2.5 and 4 months, rTg4510 mice develop a range of cognitive deficits, including impaired spatial memory in the MWM test ( Ramsden et al., 2005; Westerman et al., 2002; Yue et al., 2011), recognition memory deficits in the novel object recognition task ( Blackmore et al., 2017; Wes et al., 2014), compromised contextual fear memory in fear conditioning tests ( Cook et al., 2014), and hyperactivity in the open-field test ( Blackmore et al., 2017; Wes et al., 2014). LTP dysfunction can be observed from 4.5 months of age, followed by declined expression levels of pre- and post-synaptic proteins around 5.5 months, and substantial dendritic spine loss at approximately 9.5 months ( Helboe et al., 2017; Hoover et al., 2010; Kopeikina et al., 2013). At 5.5 months of age, hippocampal CA1 neurons show a 60% reduction ( Ramsden et al., 2005; Santacruz et al., 2005), while cortical cell loss occurs around 8.5 months ( Spires et al., 2006). The rTg4510 mice also exhibit progressive brain weight reduction, which correlates with general body weight loss but may not be specific to tau pathology ( Helboe et al., 2017).
Abnormal tau in rTg4510 mice demonstrates a notable capacity for propagation. Thioflavin-S staining reveals tau relocation from axons to soma and dendritic compartments, with spread beyond the entorhinal cortex, correlating with advanced NFT formation in neurons inside the entorhinal cortex ( Liu et al., 2012). Phosphorylated high-molecular-weight tau from rTg4510 brain extracts is internalized by neurons and transmitted across synaptically connected neurons ( Takeda et al., 2015). These findings highlight the potential of rTg4510 mice for investigating tau-driven neurodegeneration, offering insights into tau spread through neural circuits and degeneration induced by deafferentation.
The rTg4510 mouse model exhibits rapid progression of tau pathology, providing a compressed timeframe to study early tau-related events, including tau seeding. Unlike AD, where NFT pathology typically initiates in limbic structures, rTg4510 mice develop argyrophilic tangle-like inclusions as early as 2.5 months, affecting the cortex by 4 months and hippocampus by 5.5 months ( Ramsden et al., 2005; Santacruz et al., 2005). Although tau mutations are not a typical cause of AD, the rTg4510 model serves as a valuable tool for studying tauopathy without the confounding presence of Aβ pathology, offering insights into the specific mechanisms of tau-driven neurodegeneration.
The PS19: Compared to rTg4510 mice, PS19 mice exhibit slower pathological development ( Ramsden et al., 2005). The PS19 model expresses a transgene with the disease-associated P301S mutation, including all four microtubule-binding domains but only one N-terminal domain (4R/1N) ( Figure 1). The expression level of mutant human tau is approximately five-fold higher than that of endogenous mouse tau.
Tau seeding activity in PS19 mice is first detected at 1.5 months in the brainstem, neocortex, frontal lobe, and hippocampus, preceding detectable tau pathology by histological methods, suggesting that tau seeding may be an early pathological event ( Holmes et al., 2014). By 3 months, misfolded tau is reliably detected in the cortex and hippocampus based on the MC1 marker, while hyperphosphorylated tau becomes evident at 6 months based on the AT8 and PG5 markers ( Holmes et al., 2014). Between 3 and 6 months, soluble tau levels decline as insoluble tau deposits, predominantly composed of transgenic human tau, accumulate with age ( Yoshiyama et al., 2007). By 6 months, NFT-like inclusions are widely distributed across the neocortex, hippocampus, amygdala, brainstem, and spinal cord ( Yoshiyama et al., 2007). However, research has also reported a delayed appearance of those phenotypes, with prominent hyperphosphorylated tau inclusions and significant neuronal death only emerging at 12 months ( Zhang et al., 2012).
Microglial activation in PS19 mice is detectable as early as 3–4 months in the hippocampus without obvious gliosis, suggesting that microglial activation precedes astrogliosis ( Dejanovic et al., 2018; Yoshiyama et al., 2007). By 9 months, reactive microglia and astrocytes are prevalent in the hippocampus, associated with increased production of proinflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), and interleukin-1β (IL-1β) ( Litvinchuk et al., 2018). Additionally, PS19 mice show reduced synaptophysin immunoreactivity in the hippocampal CA3 region from 3 to 6 months ( Yoshiyama et al., 2007), likely resulting from increased microglial engulfment ( Litvinchuk et al., 2018). Consistent with this pathology, PS19 mice display reduced maximum field excitatory postsynaptic potential and paired-pulse facilitation by 6 months, indicative of impaired synaptic transmission ( Yoshiyama et al., 2007). No significant alternations in paired-pulse ratio and LTP are observed at 4 months, suggesting that synaptic impairments develop as tau pathology progresses ( Dejanovic et al., 2018).
PS19 mice exhibit significant behavioral deficits beginning at 6 months of age. Widespread cognitive impairments include deficits in spatial learning and memory in MWM tests ( Takeuchi et al., 2011; Zhang et al., 2014), associative learning and memory in cued and contextual fear conditioning tests and working memory in Y-maze tests ( Lasagna-Reeves et al., 2016; Takeuchi et al., 2011; Zhang et al., 2014). Motor impairments in the rotarod test are also evident in 6-month-old mice ( Caccamo et al., 2013), which progress to severe motor deficits between 7 and 10 months, characterized by a hunched-back posture and feeding difficulties ( Yoshiyama et al., 2007). PS19 mice also show reduced anxiety in open-field tests ( Caccamo et al., 2013).
PS19 mice begin to show signs of neuronal loss and brain atrophy by 8 months, with marked hippocampal degeneration at 9 months old ( Litvinchuk et al., 2018; Yoshiyama et al., 2007). Neurodegeneration extends to other brain regions, such as the neocortex, entorhinal cortex, and amygdala, by 12 months ( Yoshiyama et al., 2007). Significant reductions in neocortical and hippocampal volume are also evident by this stage ( Dejanovic et al., 2018; Yoshiyama et al., 2007).
Experimental inoculation of recombinant fibrils or tau strains into transgenic PS19 mice rapidly induces tau pathology, initiating within weeks and spreading to distant, synaptically connected regions ( Iba et al., 2013; Sanders et al., 2014). Notably, tau strains isolated from inoculated PS19 mice maintain seeding activity upon passage back into cell models ( Sanders et al., 2014). Unlike the spontaneously developed tangles in aged PS19 mice, fibril-induced tau inclusions resemble AD NFTs ( Iba et al., 2013). This feature establishes PS19 mice as a reasonable model for investigating the spread of tau pathology and its pathological consequences in AD.
Although not traditionally recognized as AD animal models, knockout of the App and Mapt genes reveals pathologies relevant to AD. APP knockout mice, first developed in 1995, exhibit reduced forelimb grip strength and decreased locomotor activity ( Zheng et al., 1995), accompanied by elevated reactive gliosis in the hippocampus and neocortex ( Zheng et al., 1995). Over time, these mice also display age-dependent iron accumulation in the brain ( Belaidi et al., 2018), resulting in cell loss within the substantia nigra ( Ayton et al., 2015). This pathology implicates APP in iron metabolism, suggesting that loss of APP function disrupts iron regulation and contributes to neurodegeneration ( Duce et al., 2010)—a hypothesis supported by successful iron chelation therapy in APP knockout mice ( Lei et al., 2017).
Several tau knockout models have been developed in the last few years ( Dawson et al., 2001; Harada et al., 1994; Tan et al., 2018; Tucker et al., 2001). While these mice are viable and present minimal phenotypes up to 6 months of age ( Roberson et al., 2007), by 12 months they exhibit age-dependent iron accumulation, neuronal loss, and brain atrophy, as well as cognitive and motor deficits, consistent with the features of AD ( Lei et al., 2012). In comparative studies, tau knockout mice on a C57BL6/SV129 background show deficits in the Y-maze cognition task, while those on a BL6 background do not ( Lei et al., 2014). Additionally, mice on a C57BL6/SV129 background exhibit olfactory deficits prior to behavioral symptoms ( Beauchamp et al., 2018), in accordance with early-stage symptoms seen in AD patients ( Murphy, 2019). Reductions in tau levels are also implicated in a variety of conditions associated with AD, including diabetes ( Mangiafico et al., 2023), traumatic brain injury ( Ding et al., 2024), stroke ( Bi et al., 2017; Tuo et al., 2017), and Parkinson’s disease ( Chen et al., 2023; Pan et al., 2022). These findings highlight the significance of tau in a variety of neurodegenerative and systemic conditions, providing valuable insights into the origins and progression of tau pathology in AD.
Double transgenic mouse models
In addition to mutations in APP, FAD studies have also identified disease-causing mutations in PSEN1 and PSEN2 genetic loci, which encode presenilin 1 (PS1) and presenilin 2 (PS2), respectively. Specifically, an in-frame deletion of exon 9 (PS1 delta9) ( Perez-Tur et al., 1995) within the PSEN1 gene has been found in families affected by FAD ( Figure 1). Both PS1 and PS2 affect APP processing ( Jiang et al., 2018), leading to the development of double transgenic mice expressing both mutant APP and PS1 as models of AD ( Jankowsky et al., 2001, 2004). Since then, various double transgenic mice models have been generated, ultimately becoming important tools for evaluating potential therapeutic interventions.
APPswe/PS1dE9: APP/PS1 double transgenic animal models are based on the Swedish mutation in APP combined with specific PSEN1 variants, such as M146L ( Duff et al., 1996), A246E ( Borchelt et al., 1996, 1997), and L166P ( Radde et al., 2006) ( Figure 1). By co-injecting two vectors, one encoding APP with the Swedish mutation and another encoding PSEN1 with the del-E9 mutation, Jankowsky et al. ( 2001) generated APPswe/PSEN1dE9 (line 85) mice on a C57BL/6/C3H genetic background. Comparative studies confirmed the superior onset time and intensity of the del-E9 mutation, establishing it as one of the most widely used strains. When further backcrossed with C57BL/6J mice, APPswe/PSEN1dE9 hemizygotes exhibit a high incidence of seizures, as detected by video-electroencephalography ( Minkeviciene et al., 2008, 2009).
APPswe/PSEN1dE9 mice display an early onset of plaque formation, in contrast to single APP-overexpressing models. By 3 months, small and rare plaques can be detected in the cortex and hippocampus ex vivo using thioflavin S or 3D6 ( Garcia-Alloza et al., 2006; Unger et al., 2016). At 6 months, levels of insoluble Aβ42 increase, with occasional deposits detectable in the cortex and hippocampus ( Garcia-Alloza et al., 2006). By 24 months, widespread Aβ deposition is evident throughout the brain, accompanied by heightened neuroinflammation marked by cortical IL-1β and TNF-α expression ( D’Angelo et al., 2021). In line with the APP23 model, APPswe/PSEN1dE9 mice exhibit prominent and compact congophilic Aβ deposits, with dense core plaques forming near, rather than inside, blood vessels, implying limited vascular involvement ( Meyer-Luehmann et al., 2008). APPswe/PSEN1dE9 mice also show elevated synaptic Aβ levels, contributing to presynaptic dysfunction ( Koffie et al., 2009; Roy et al., 2016; West et al., 2009). Both in vitro and in vivo studies have revealed that Aβ42 monomers and oligomers disrupt SNARE complex assembly in APPswe/PSEN1dE9 mice ( Yang et al., 2015), consistent with diminished SNARE complex formation observed in postmortem AD brains ( Sharma et al., 2012). Neurons in the hippocampal CA1 region of 6-month-old APPswe/PS1dE9 mice exhibit degeneration, shrinkage, and necrosis, with modest neuronal loss observed near plaques between 8 and 10 months, relative to more preserved neurons in distal regions ( Daniels et al., 2016; Shi et al., 2018).
Despite the dual genetic mutations, cognitive decline in APPswe/PSEN1dE9 mice does not manifest substantially earlier than in mice with single genetic mutations. APPswe/PSEN1dE9 mice start to exhibit memory impairment in the contextual fear conditioning task by 6 months ( Cramer et al., 2012; Kilgore et al., 2010), with deficits in spatial learning and passive avoidance acquisition becoming evident in the MWM and passive avoidance tests between 8 and 13 months ( Ding et al., 2008; Fang et al., 2019; Gimbel et al., 2010; Jo et al., 2014). Additionally, impairments in spatial learning and recognition memory are noted at 9 months in the radial arm water maze and object recognition tasks, coinciding with widespread plaque deposition in the hippocampus and cortex ( Cramer et al., 2012; Lewis et al., 2010; McClean et al., 2011).
Neuroinflammatory responses in APPswe/PSEN1dE9 mice have been extensively characterized, revealing plaque-associated reactive gliosis, primarily involving GFAP-positive reactive astrocytes within the cortex by 6 months ( Kamphuis et al., 2012). Microgliosis follows 1–2 months later, accompanied by a surge in proinflammatory cytokines. These mice exhibit elevated CD11b- and CD45-positive microglia and increased levels of IL-6 and TNF-α in isolated brain microglia ( Fang et al., 2019; Kim et al., 2009). Notably, astrocyte-microglia interactions are thought to influence amyloid pathology, potentially through complement activation mechanisms ( Lian et al., 2016).
In APPswe/PSEN1dE9 mice, the nucleotide-binding oligomerization domain-like receptor family pyrin domain containing 3 (NLRP3) inflammasome has also been identified as a crucial factor in sterile neuroinflammation ( Milner et al., 2021), mirroring observations in AD patients ( Rui et al., 2021). Inhibition of NLRP3 in these mice has been shown to reduce Aβ load and neuroinflammation and improve synaptic plasticity ( Heneka et al., 2013; Venegas et al., 2017), suggesting a role in modulating AD pathology. Based on these findings, novel NLRP3 inflammasome inhibitors have been developed for potential clinical application ( Coll et al., 2015).
APPswe/PSEN1dE9 mice serve as a valuable tool in AD research, particularly for investigating Aβ pathology, neuroinflammation, and memory decline. However, research on synaptic plasticity within AD mouse models has yielded contradictory findings. Specifically, some studies have reported impairments in high-frequency stimulation (HFS)-triggered hippocampal Schaffer collateral-CA1 LTP following amyloid plaque accumulation ( Fu et al., 2014; Ma et al., 2013), while other studies have suggested that Aβ accumulation during aging does not significantly change synaptic transmission or sustained LTP in hippocampal slices ( Volianskis et al., 2010). Considering that AD patients carrying both APP and PSEN1 mutations are rare, the pathology observed in this model may be artificial.
5xFAD: The 5xFAD mouse model exemplifies a rapidly progressing APP/PS1 double transgenic mouse model of AD that co-expresses three human APP mutations (Swedish ( K670N, M671L), Florida ( I716V), and London ( V717I) mutations) and two PSEN1 mutations ( M146L, L286V) ( Figure 1). This model is characterized by extensive amyloid pathology without notable tau accumulation, providing a robust system for investigating amyloid-driven AD pathology.
Amyloid accumulation in 5xFAD mice is detectable as early as 6 weeks, primarily driven by intraneuronal Aβ42 accumulation ( Oakley et al., 2006). By 2 months, extracellular amyloid deposition is observed in the subiculum and cortical layer V ( Oakley et al., 2006), progressively spreading to the outer cortical layers, hippocampal CA1 region, and eventually to the thalamus, brainstem, and olfactory bulb in an age-dependent manner ( Jawhar et al., 2012; Oakley et al., 2006; Youmans et al., 2012). PET imaging has revealed amyloid deposition from 4 months in several cortical regions, including the thalamus and motor cortex. Notably, 5xFAD mice exhibit sex-specific differences in plaque pathology, with females displaying a more pronounced plaque burden ( Sil et al., 2022), mirroring sex disparities in human AD.
Synaptic deterioration in 5xFAD mice accompanies plaque accumulation ( Oakley et al., 2006; Peretti et al., 2015). Beginning at 4 months, basal synaptic transmission and LTP in the hippocampal CA1 region deteriorate ( Crouzin et al., 2013; Kimura & Ohno, 2009). By 6 months, there is a marked loss of mushroom and dendritic spines in CA1 pyramidal and dentate gyrus (DG) granule neurons ( Busche et al., 2012). Neuronal density shows a substantial decrease in specific brain regions, such as the ventral horn, by 12 months, coinciding with motor impairment onset ( Jawhar et al., 2012). Among AD mouse models, 5xFAD mice demonstrate significant molecular overlap with human AD pathology, encompassing not only amyloid and tau but also neuroinflammation, endolysosomal function, and synapse formation, as revealed through transcriptomic analyses of microglia and bulk brain tissues ( Zhong et al., 2024). These findings suggest that the 5xFAD model closely reflects human AD, making it particularly valuable for studying neuroinflammation and endolysosomal dysfunction.
The 5xFAD model also recapitulates amyloid deposition in the spinal cord, paralleling findings in FAD cases ( Ogomori et al., 1989). In these mice, spinal cord amyloid pathology becomes evident at 3 months, initially in cervical and lumbar regions and progressively extending along the cord by approximately 5 months ( MacPherson et al., 2017).
The 5xFAD mouse model features rapid progression of cognitive impairment, with detectable deficits in spatial working memory in the Y-maze as early as 4–5 months ( Oakley et al., 2006) and further deterioration in memory performance in contextual-fear-conditioning ( Kimura & Ohno, 2009). By 6 months, behavioral impairments become pronounced, including deficits in spatial memory assessed via the MWM test ( Busche et al., 2012; Ceglia et al., 2015). Starting from 6 months, 5xFAD mice also exhibit abnormal social behaviors, such as excessive social engagement during home-cage observations, with increased sniffing, following, and mounting behaviors indicative of impaired social recognition ( Flanigan et al., 2014). Despite conflicting reports on anxiety levels in 5xFAD mice ( Flanigan et al., 2014; O'Leary et al., 2018), hyperactivity, an indicator of anxiety, is consistently observed in this model ( Merlini et al., 2019; Oblak et al., 2021).
Distinct from other AD models, 5xFAD mice are considered a reliable model for investigating sensory deficits, as they demonstrate reduced acoustic startle responses and impaired olfactory-guided behaviors as early as 3 to 4 months ( Ceglia et al., 2015; O'Leary et al., 2017). In addition, deficits in basal synaptic transmission in layer V of the somatosensory cortex emerge at 6 months ( Crouzin et al., 2013). Autoradiographic studies have further revealed alterations in glycolysis in 5xFAD mice compared to wild-type controls ( Oblak et al., 2021).
Triple-transgenic mouse models
The 3xTg-AD mouse model was developed to closely replicate human AD pathology by combining both Aβ and tau deposition, two key features that synergistically drive neurodegeneration ( Li et al., 2015; Shipton et al., 2022). In conventional rodent models overexpressing mutated APP, tau aggregation is rarely observed, except in the APP23 line, and transgenic tau models typically develop NFTs without amyloid plaques. To address this limitation, the 3xTg model incorporates three mutations associated with AD pathology, namely APP Swedish, MAPT P301L, and PSEN1 M146V ( Figure 1).
3xTg-AD mice were generated by injecting single-cell embryos from a mouse line carrying the PSEN1 M146V knock-in mutation with two human transgenes: APP with the Swedish mutation and MAPT with the P30IL mutation. Expression of these transgenes is driven by the Thy1.2 promoter, leading to predominant expression in the central nervous system, as confirmed through protein expression analyses ( Oddo et al., 2003b). By 4 months, 3xTg-AD mice exhibit a two-fold increase in tau and APP expression in the brain ( Oddo et al., 2003b).
Aβ accumulation in the 3xTg mice is age-dependent, with a pronounced elevation in Aβ42 levels. At 3 months, minimal Aβ immunoreactivity is detected in the hippocampal CA1 region, where Aβ primarily exists in a soluble, monomeric form ( Oddo et al., 2006; Yao et al., 2009). In this model, Aβ appears to migrate from intracellular to extracellular compartments, with intracellular Aβ immunoreactivity in cortical regions the first clear neuropathological manifestation, occurring between 3 and 4 months of age ( Oddo et al., 2003a). By 6 months, extracellular Aβ deposits appear in the frontal cortex, progressing to overt amyloid plaque formation by 12 months ( Oddo et al., 2003b, 2006; Yao et al., 2009).
Tau pathology in 3xTg-AD mice also follows an age-dependent progression. Initial signs of tau pathology are visible by 6 months, with HT7-positive tau immunoreactivity accumulating in the somatodendritic compartment of the hippocampal pyramidal neurons in the CA1 region, which intensifies by 12 months ( Oddo et al., 2003a, 2006). Misfolded tau, detectable with the PHF-1 antibody, emerges at about 18 months of age ( Oddo et al., 2003a), while neurons with hyperphosphorylated tau and elevated levels of phosphorylated and total tau in the hippocampus are readily apparent between 12 to 15 months ( Fang et al., 2019; Oddo et al., 2003a).
Microglial activation in 3xTg mice closely mirrors the age-related increase in amyloid burden, with 15-month-old mice developing intracellular aggregates that resemble small plaques and Aβ accumulation in lysosomal compartments ( Spangenberg et al., 2019). By 24 months, activated microglia colocalize with extraneuronal Aβ-immunopositive deposits in various brain regions ( Kitazawa et al., 2005). Inflammatory processes differentially affect Aβ and tau, potentially influencing tau pathology onset and development. Notably, IL-1β overexpression in 3xTg-AD mice significantly promotes tau phosphorylation and exacerbates tau pathology, emphasizing the complex interplay among inflammation, Aβ, and tau in AD ( Ghosh et al., 2013; Kitazawa et al., 2005). Levels of key inflammatory markers, such as CC chemokine ligand 4, CXCL1, and granulocyte-colony stimulating factor, markedly increase between 21 and 24 months ( Barber et al., 2024).
With its tau pathology, the 3xTg-AD model can be used to investigate the mechanistic links between Aβ and tau. Aβ pathology precedes tau pathology in these mice, consistent with the amyloid cascade hypothesis. Anatomical studies of the hippocampus in 3xTg-AD mice reveal early and prominent colocalization of Aβ and tau pathologies at CA1 synapses ( Takahashi et al., 2010). By 12 months, Aβ and tau immunoreactivity colocalize within the somatodendritic compartments of the same neurons, further supporting the theory that Aβ influences tau pathology ( Oddo et al., 2003a, 2006). Aβ oligomers also inhibit proteasome activity, exacerbating age-related accumulation of Aβ and tau ( Tseng et al., 2008). Targeted immunization against Aβ42 demonstrates potential in alleviating amyloid and tau aggregation ( Oddo et al., 2008; Rosenberg et al., 2018). Notably, while elevated tau levels and hyperphosphorylation do not directly affect Aβ pathology ( Oddo et al., 2007), tau appears to be essential for Aβ toxicity ( Peters et al., 2019). These findings partially substantiate the amyloid cascade hypothesis.
Roberson et al. ( 2007) developed a double transgenic model by crossing human APP transgenic mice with MAPT knock-out mice to study the interplay between tau and Aβ. They found that reducing tau did not impact Aβ plaque deposition, neuritic dystrophy, or aberrant sprouting, but it effectively prevented water maze deficits, behavioral abnormalities, and premature mortality in mice aged 4–7 months. Tau reduction also increased resistance to excitotoxin-induced seizures ( Ittner et al., 2010; Roberson et al., 2007).
Knock-in rodent animal models
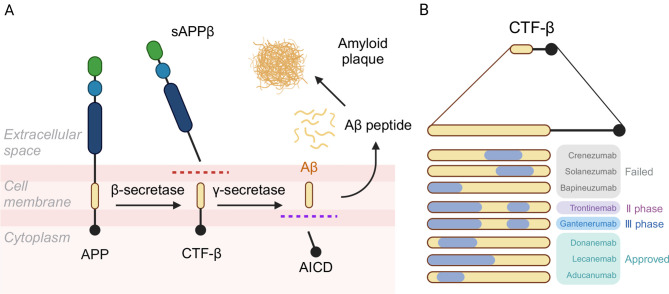
All APP-overexpressing mouse models generate elevated levels of C-terminal fragment β (CTF-β), a product of APP cleavage by β-secretase. However, the levels and distribution of these proteins in transgenic mice often do not accurately reflect human AD, limiting their translational relevance ( Saito et al., 2014). Moreover, CTF-β has been shown to be potentially more neurotoxic than Aβ itself ( Mitani et al., 2012). Due to shared epitopes near the amino terminus and mid-region of the sequence, CTF-β can bind to many therapeutic anti-Aβ antibodies ( Karran & De Strooper, 2022) ( Figure 2). Considering that human AD does not require excessive APP or tau protein levels, a model incorporating humanized protein sequences may better replicate disease mechanisms.
Figure 2.
Formation of CTF-β and antibody epitopes
A: After cleavage by β-secretase, APP forms the sAPPβ peptide (extracellular segment of APP in the generation of Aβ) and CTF-β peptide (intracellular segment of APP in the generation of Aβ). B: Correspondence between CTF-β and targets of major pipeline drugs aimed at amyloid plaques, showing that CTF-β can bind to all therapeutic anti-Aβ antibodies (https://clinicaltrials.gov/). APP: Amyloid precursor protein; AICD: APP intracellular domain; sAPPβ: Soluble peptide APPβ; CTF-β: Carboxy-terminal fragment β.
App knock-in mice: To address the above limitations, Saito et al. ( 2014) developed App knock-in mice by humanizing the murine Aβ sequence and sequentially introducing Swedish, Beyreuther/Iberian, and Arctic mutations, creating the App NL/NL, App NL-F/NL-F, and App NL-G-F/NL-G-F lines, respectively. These knock-in mice express APP and APP intracellular domain (AICD) in amounts comparable to wild-type mice but generate more Aβ42, resulting in a significantly elevated Aβ42/Aβ40 ratio ( Saito et al., 2014). This design makes App knock-in models particularly valuable for distinguishing genuine disease processes from artifacts often observed in transgenic App mice.
App knock-in mice exhibit progressive, age-dependent Aβ pathology, neuroinflammation, and cognitive decline. Aβ deposition begins at 6 months in the App NL-F/NL-F model ( Saito et al., 2014) but at 3 months in the App NL-G-F/NL-G-F model ( Chen et al., 2020; Saito et al., 2014). Both App NL-F/NL-F and App NL-G-F/NL-G-F mice demonstrate cognitive impairments, including reduced spatial reversal learning, enhanced impulsivity, and increased compulsivity ( Masuda et al., 2016; Saito et al., 2014). Consistent with extensive Aβ deposition, App NL-G-F/NL-G-F mice exhibit evident memory impairment by 6 months, while App NL-F/NL-F mice show similar abnormalities at 10 months ( Masuda et al., 2016). Memory impairments are observed in place avoidance learning tasks in both models, with enhanced compulsive behaviors detected in delay-discounting tasks ( Masuda et al., 2016). Notably, deficits in reversal learning and attention emerge later than compulsivity, indicating distinct regional brain involvement underlying these behavioral changes.
Tau seeding studies in App NL-F/NL-F mice have demonstrated the formation of NFTs and other tau pathologies following injection with AD-derived tau from a human case of AD. In 15-month-old AD- App NL-F/NL-F mice, initial tau pathology appears within one month post-infection, and progressively spreads across the hippocampus and various cortical regions over a period of one to six months. The pathology begins as tau deposits within neuronal processes and later develops into widespread NFTs, likely due to secondary seeding events that drive the expansion of tau aggregates ( He et al., 2018).
Given their humanized Aβ sequence, App NL-F/NL-F mice serve as an effective tool to investigate the molecular mechanisms underlying Aβ deposition ( Saito et al., 2014). For instance, a deficiency in autophagy-related gene 7 disrupts Aβ secretion, leading to intracellular Aβ accumulation and neurodegeneration in the absence of tau pathology ( Nilsson et al., 2013). This finding suggests the existence of a pathway that directly links intracellular Aβ to neurodegeneration, bypassing tauopathy.
APP knock-in rats: Gene manipulation in rats remains less advanced than in mice, resulting in fewer rat models of AD. However, due to their closer genomic similarities to humans, rats may provide a more relevant model for studying AD ( Pang et al., 2022). To leverage these advantages, App NL-G-F rats carrying three human FAD mutations have been developed. This model exhibits Aβ plaque deposition in key brain areas, accompanied by microglial activation and proliferation, progressive synaptic degeneration, and cognitive impairments, closely mirroring the symptoms of AD observed in humans. Interestingly, App NL-G-F rats also display tau pathology, neuronal cell death, and brain atrophy—phenotypes rarely observed in other APP-based models.
In App NL-G-F rats, Aβ plaques predominantly accumulate in the neocortex, followed by the thalamus, brainstem, striatum, and other brain regions, with minimal vascular-related deposition observed in early AD stages ( Chen & Zhang, 2022). Both apoptosis and necroptosis are evident in neuronal populations, resulting in severe cerebral cortex atrophy. This model is the first to demonstrate ventricular enlargement in an AD animal model. When directly compared to the App knock-in mice constructed by Saito et al. ( 2014), App NL-G-F rats show tau pathology, sustained neuronal death, and resulting brain atrophy, suggesting closer alignment with human AD pathology ( Pang et al., 2022).
The complex pathology displayed by App NL-G-F knock-in rats provides an opportunity to study interactions between different disease mechanisms. This model also enables direct comparisons of molecules targeting various pathways within a single preclinical system. Similar to APP23 mice, App NL-G-F rats are particularly useful for investigating the impacts of Aβ on tau pathology in AD, addressing the crucial question of whether tau aggregation is a consequence of Aβ deposition or an independent factor causing neurodegeneration. Furthermore, compared to mouse models, rats offer advantages in genetic homology and brain structure similarity, making them well-suited for drug testing, biomarker discovery, and omics-based research. However, App NL-G-F rats have yet to be widely distributed and evaluated across multiple research laboratories.
MAPT knock-in mice: Although studies have noted an abnormal increase in 4R-tau isoforms in AD and tau pathologies, tau splicing analysis remains challenging in existing mouse models due to the lack of models expressing all human tau isoforms without overexpression. To address this, a MAPT knock-in mouse model was developed as early as 2005 through homologous recombination, replacing all murine tau isoforms with wild-type human tau-4R/2N to create Tau-KOKI mice ( Terwel et al., 2005). These mice express normal levels of tau protein, allowing researchers to investigate the underlying mechanisms of tau pathogenesis. For example, while tau-4R/2N in Tau-KOKI mice exhibits greater phosphorylation compared to tau in Tau-P301L mice, Tau-KOKI mice live a normal lifespan, showing only minor motor deficits in old age without significant tau pathology ( Terwel et al., 2005). This finding suggests that tau phosphorylation alone does not necessarily induce pathological changes; instead, conformational changes in tau may also contribute to the development of pathology. In P301S mice, tau exhibited abnormal dendritic mislocalization, whereas both MAPT- and P301L-knock-in mice maintain correct axonal localization, implying that excessive tau expression, rather than specific mutations, may drive mislocalization ( Benderradji et al., 2022; Hashimoto et al., 2019; Saito et al., 2019). Despite these insights, overt tau pathology is rarely observed in MAPT knock-in models. However, PLB2Tau mice, which express two tau mutations, show increased levels of phosphorylated tau, neuroinflammation, and early-set behavioral changes, including heightened anxiety, depressive/apathetic behavior, and reduced exploratory activity ( Koss et al., 2016). By 8 months, these mice also develop hyperglycemia, indicative of a diabetic phenotype ( Hull et al., 2020), similar to observations in the P301L-knock-in model with impaired glucose-stimulated insulin secretion in pancreatic β-cells ( Benderradji et al., 2022).
To investigate the interaction between Aβ and tau pathologies, Hashimoto et al. ( 2019) and Saito et al. ( 2019) developed double knock-in ( App NL-G-F/ MAPT dKI) mice by cross-breeding MAPT knock-in mice with single App NL-G-F mice and found that Aβ amyloidosis intensifies tau accumulation and is closely associated with dystrophic neurites, underscoring its role in tau pathology. Cognitive deficits, such as those in object-place association tasks, emerge as early as 4 months, accompanied by tau pathology in the medial temporal cortex and increased c-Fos activation, suggesting early perirhinal-entorhinal involvement. The pathology appears to progress through regions, including the claustrum, medial prefrontal cortex, and ultimately the retrosplenial hub, linking frontal and temporal brain areas ( Benskey et al., 2023).
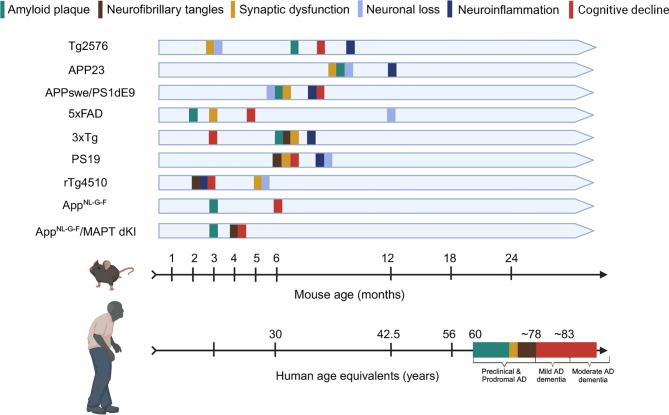
Overall, AD models with APP or tau mutations replicate essential AD characteristics, including neuroinflammation, synaptic damage, neuronal loss, and cognitive decline. Transgenic models based on APP and tau mutations exhibit clear early-onset symptoms and show evidence of protein seeding. However, there is no definitive pattern regarding the onset timing or specific morphology of these phenotypes, as shown in Figure 3. Intriguingly, although APP mutant mice rarely form tau tangles, elevated tau phosphorylation levels have been observed sporadically, with each model reflecting the pathologies associated with their respective mutations.
Figure 3.
Timeline of pathological progression in AD transgenic mouse models with reference to human age
Timeline illustrates age at which various pathological features emerge in different transgenic mouse models for AD. Specific transgenic models (Tg2576, APP23, APPswe/PSEN1dE9, 5xFAD, 3xTg, PS19, rTg4510, APP knock-in mice) are listed for clarity. Mouse Age (Months) vs. Human Age (Approximate Correspondence) ( Vermunt et al., 2019) (Preclinical & Prodromal AD; Mild AD dementia; Moderate AD dementia) ( Johnson et al., 2023).
Non-rodent genetic models
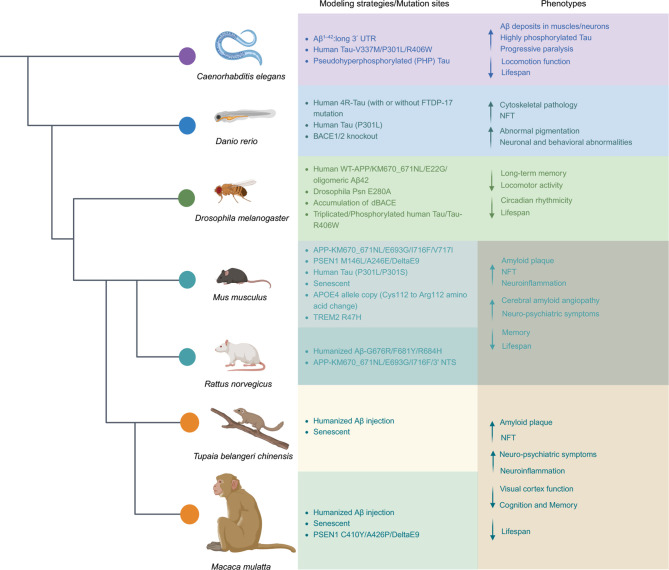
In addition to the widely used rodent models, classical model organisms such as Danio rerio (zebrafish), Drosophila melanogaster, and Caenorhabditis elegans offer valuable systems for mimicking AD ( Chen & Zhang, 2022). Their advantages, including low cost, short life span, and easy genetic manipulation, make them highly suitable for preliminary drug screening and investigations into AD pathogenesis ( Figure 4).
Figure 4.
Modeling strategies/mutation sites and main phenotypes of principal model organisms used in AD research
Principal model organisms include Caenorhabditis elegans, Danio rerio (Zebrafish), Drosophila melanogaster, Mus musculus (mice), Rattus norvegicus (rats), Tupaia belangeri chinensis (Chinese tree shrews), and Macaca mulatta (macaques). BACE1: beta-secretase 1; UTR: untranslated regions; FTDP-17: frontotemporal dementia with Parkinsonism-17; NFT: neurofibrillary tangle; TREM2: triggering receptor expressed in myeloid cells 2; PSEN1: presenilin 1.
Zebrafish have proven effective in drug screening due to their accessible delivery methods ( Kalueff et al., 2014) and exhibit AD-like behaviors, such as avoidance and impaired habituation to startling stimuli ( Best et al., 2008). Drosophila melanogaster is highly amenable to genetic manipulation, enabling the development of diverse AD models through mutations in genes such as APP, MAPT, and BACE1. For example, models co-overexpressing APP and BACE1 show age-dependent neurodegeneration and deposition of Aβ plaques ( Ogunsuyi et al., 2022). Caenorhabditis elegans, which retains functional synapses, demonstrates significant responses to external stimuli, making it a valuable model for studying neuronal decline. For instance, AD models overexpressing Aβ42 exhibit intracellular Aβ accumulation, chemotaxis deficits, and hypersensitivity to serotonin, mimicking aspects of AD pathology ( Link, 2006; Wu et al., 2006).
Despite their utility, these models have notable limitations due to significant differences in brain structures compared to humans. As lower-order species, their capacity to assess cognitive functions, a clinical endpoint of AD, remains limited. Consequently, these models are better suited for drug screening and mechanistic studies rather than for validating the clinical efficacy of therapeutic interventions. Nevertheless, they can serve as effective models for preliminary identification of potential therapeutic targets, providing important insights that can inform and guide subsequent research efforts.
ANIMAL MODELS FOR LATE-ONSET SPORADIC AD
Transgenic AD animal models commonly rely on the expression of alleles that induce Aβ aggregation, typically manifesting pathology during adolescence or early adulthood ( Sasaguri et al., 2017). However, AD is fundamentally an age-related disorder, characterized by the gradual accumulation of pathological changes over time, a core aspect of its etiology. Given the age equivalence between mice and humans, the early onset of cognitive impairment in these classical AD models does not align with the typical onset age of 65 years in most sporadic cases. Consequently, these models may not fully represent the complexity of the disease, particularly in elderly individuals, and fail to replicate critical aspects such as the inflammatory response and changes in neurotransmitter systems ( Krauthausen et al., 2015).
One approach to bridge this gap is to construct natural aging models through phenotype screening and selective breeding, which can better capture the delayed onset of cognitive decline associated with sporadic AD. NHPs may be particularly valuable in this area, offering closer physiological parallels to human aging. Simultaneously, advances in next-generation sequencing have uncovered novel AD risk factors and molecular pathways implicated in disease pathogenesis. With sophisticated genome-editing tools like CRISPR, researchers can now efficiently generate specific genetic mutations in mice, accelerating the production of models that better mirror the genetic complexity and gradual progression of late-onset sporadic AD.
APOE/TREM2 mouse models
In a landmark 1993 study, Strittmatter and colleagues identified a strong association between the APOE4 allele and an elevated risk of developing sporadic AD ( Corder et al., 1993), a discovery subsequently confirmed by multiple genome-wide association studies ( Beecham et al., 2009; Bertram et al., 2008; Coon et al., 2007; Grupe et al., 2007; Lambert et al., 2013; Li et al., 2008). Carriers of the APOE4 genotype often show signs of systemic metabolic dysfunction years before the emergence of AD. A single APOE4 allele, characterized by a Cys112-to-Arg112 amino acid substitution, increases AD risk by 3–4 times, while homozygous carriers face an 8–12 times greater risk ( Corder et al., 1993).
To investigate the effects of APOE in an AD context, researchers have developed mouse models with humanized APOE alleles inserted into the mouse genome ( Foley et al., 2022; Huynh et al., 2019; Mann et al., 2004), resulting in mice with distinct phenotypes related to neuronal function, mitochondrial dysfunction, and impaired insulin signaling. However, despite these phenotypic similarities to humans, these mice do not develop hallmark amyloid plaques or tau tangles ( Hauptmann et al., 2009; Johnson et al., 2017).
TREM2, a surface receptor expressed on microglia, initiates intracellular protein tyrosine phosphorylation pathways and plays a pivotal role in regulating microglial function. A rare mutation of TREM2, involving the substitution of histidine for arginine at position 47 ( R47H), is strongly associated with an increased risk of developing AD ( Guerreiro et al., 2013; Jonsson et al., 2013). TREM2 R47H knock-in mice exhibit reduced Trem2 expression and a decreased density of microglia in the hippocampus, indicating impaired microglial function ( Liu et al., 2020). Studies using siRNA-mediated Trem2 knockout in microglia have shown that TREM2 facilitates a shift in microglial activity from an inflammatory cytokine-producing phenotype to one that is phagocytic and anti-inflammatory ( Liu et al., 2020). This mechanism may underlie the heightened microglial density surrounding amyloid plaques observed in AD.
To better model sporadic, late-onset AD, researchers developed the B6.APOE4.Trem2*R47H mouse model, which features a humanized ApoE knock-in mutation, encoding for the E4 isoform, and a CRISPR/Cas9-generated R47H point mutation in the Trem2 gene. These mice exhibit decreased survival probability by 24 months ( Kotredes et al., 2021), with both B6.Trem2 *R47H and B6.APOE4.Trem2*R47H mice displaying disruptions in multiple immune-related processes at this age, highlighting the significant connection between aging, Trem2 function, and AD.
B6.APOE4.Trem2*R47H mice do not develop amyloid plaques or hyperphosphorylated tau, nor do they exhibit pronounced age-related behavioral changes ( Kotredes et al., 2021). However, between 4 to 24 months of age, they display progressive age-related deficits in locomotor activity, motor coordination, and wheel-running activity, while spatial working memory remains unaffected until 24 months.
Metabolic and functional imaging studies using 18F-FDG and 64Cu-PTSM have revealed that B6.APOE4.Trem2*R47H mice show reduced brain perfusion and increased glycolysis in key regions associated with sensory, cognitive, and motor functions compared to controls ( Kotredes et al., 2021). Molecular profiling has also identified genotype- and age-dependent alterations in cytokine expression, including variations in IL-6 and KC/GRO levels in both blood and brain tissues ( Kotredes et al., 2021). Given their characteristics, these mice may serve as valuable models for studying AD, lipoproteins, arteriosclerosis, and coronary heart disease, offering insights into age-related pathologies beyond classical AD hallmarks.
Thy1-ApoE4/C/EBPβ double transgenic mouse models
CCAAT/enhancer-binding protein β (C/EBPβ) is a crucial transcription factor in adipocyte differentiation and maturation, mediating the expression of inflammatory cytokines ( Cardinaux et al., 2000). Notably, its levels gradually increase in neurons with age ( Cortes-Canteli et al., 2011), suggesting a potential link in age-related neurodegenerative diseases. Recent research identified C/EBPβ as a key regulator of ApoE, modulating its mRNA levels in an age-dependent manner and selectively promoting the expression of ApoE4 in human neurons, with overexpression of C/EBPβ in 3xTg and 5xFAD mouse models shown to accelerate AD pathology ( Xia et al., 2021). These findings implicate C/EBPβ in the regulation of ApoE4 and its broader effects on tau and Aβ pathologies ( Xiong et al., 2023a).
Building on this understanding, researchers engineered the Thy1-ApoE4/C/EBPβ neuron-specific transgenic mouse model ( Qian et al., 2024). In this model, neuronal ApoE4 drives C/EBPβ expression, triggering a cascade of AD-like pathological changes. By 12 months, these mice exhibit hallmark features of AD, including memory deficits, brain volume reduction, Aβ and tau aggregation, and PET signal alterations. Thy1-ApoE4/C/EBPβ transgenic mice represent a promising tool for studying sporadic AD, providing valuable insights into the molecular mechanisms underlying disease progression and offering a platform for evaluating therapeutic interventions.
Senescence-accelerated mouse models
Senescence-accelerated mouse-prone 8 (SAMP8) is a naturally occurring mouse line characterized by accelerated aging, derived from the inbred AKR/J line. In the 1970s, Takeda and colleagues observed accelerated aging traits, including reduced activity, hair loss, and early mortality, in certain progeny. This led to the development of senescence-accelerated mice (SAM), categorized by survival curves and phenotypic features. SAMP8, which exhibits memory and learning disorders, emerged as a prominent outcome of this selective breeding process ( Takeda et al., 1991).
Age-related neuropathological changes in SAMP8 mice include the accumulation of periodic acid-Schiff (PAS)-positive granular structures within astrocytes in the hippocampus and brainstem, indicative of misfolded protein accumulations associated with amyloid plaques ( Akiyama et al., 1986). This is accompanied by prominent astrogliosis in the hippocampus as the mice age ( Han et al., 2010). Additionally, the brainstem reticular formation contains numerous vacuoles of varying sizes, surrounded by clusters of activated microglia ( Kawamata et al., 1998; Yagi et al., 1989). These features resemble changes in aging human brains and reflect specific glioneuronal reactions, where glial cells play an essential role in the brain’s response to neurodegeneration. Behavioral tests have revealed that SAMP8 mice experience marked declines in spatial working memory between 6 and 10 months of age, as assessed by the Y-maze test ( Del Valle et al., 2012; Lin et al., 2014; Pačesová et al., 2022). Moreover, SAMP8 mice show increased mobility with age compared to senescence-accelerated mouse resistant 1 (SAMR1) controls ( Sawano et al., 2013; Tsumagari et al., 2021; Yanai & Endo, 2016).
SAMP8 mice show elevated levels of phosphorylated tau at multiple epitopes, including Ser396, Thr231, and Ser214, by 6 to 9 months of age ( Pačesová et al., 2022). However, hallmark tau structures, such as NFTs, have not been conclusively observed in this model. These mice also demonstrate age-dependent increases in Aβ-like protein production, with some studies reporting the presence of amyloid granules containing both Aβ42 and Aβ40 peptides in the hippocampus of 6-month-old mice ( Del Valle et al., 2010). However, these findings are inconsistent across studies, as other antibody-based analyses have failed to confirm such deposits, and the observed Aβ increases are minor compared to transgenic mice. Notably, no evidence of Aβ deposition is evident in 12-month-old SAMP8 mice, indicating that plaque formation may not be crucial for amyloid-induced memory loss and brain damage ( Morley et al., 2012).
Chemically induced rodent models
Chemically induced rodent models have been established to investigate the role of specific neurotransmitter pathways in the pathophysiology of AD. These models, created by administering chemical agents, replicate certain symptoms of AD, such as impaired cholinergic function and deficits in learning ability ( Hampel et al., 2018). While these models effectively demonstrate cognitive defects, they do not replicate key pathological features of AD, such as amyloid plaques and NFTs, with the exception of the Aβ-injection model.
The Aβ-injection model, where Aβ monomers or oligomers are directly injected into the hippocampus, is widely used to investigate the mechanisms by which Aβ peptides contribute to neuronal dysfunction and cognitive decline. This model provides insights into the mechanisms underlying Aβ-induced toxicity and serves as a platform to evaluate the effectiveness of drugs aimed at reducing Aβ aggregation, preventing plaque formation, and alleviating associated neuroinflammation and neurodegeneration. Aβ injections in the hippocampus have been shown to induce memory impairment, impair long-term potentiation, and activate microglia in mice ( Hyun Yi et al., 2023), as well as impair cholinergic function and learning ability without progressive neurodegeneration ( Nakamura et al., 2001). Increasingly, this model is used to explore the interactions between Aβ and other prion proteins, such as tau and α-syn ( Bassil et al., 2020; Chen et al., 2024).
Other chemicals have also been used to induce AD-like pathologies by targeting specific neuronal or neurotransmitter pathways. For example, scopolamine impairs cholinergic function ( Wang et al., 2023b), L-methionine activates NMDA receptors ( Sain et al., 2011), and okadaic acid promotes tau phosphorylation ( Kamat et al., 2013). These compounds mimic aspects of AD-related dementia, providing tools to study specific mechanisms underlying cognitive impairment and evaluate potential therapeutics. However, these models cannot be considered true representations of AD, as they lack hallmark pathologies like amyloid plaques and NFTs, reflecting only partial disease phenotypes.
NHP MODELS WITH VALID PREDICTIVITY OF LATE-ONSET SPORADIC AD
The evolutionary divergence between rodents and humans results in significant genetic and neuroanatomical differences, hindering the translational applicability of rodent models to human AD ( Perlman, 2016). Behavioral assays widely used in mice often fail to predict clinically relevant outcomes in humans ( Wan et al., 2020), while species-specific variations in drug metabolism further complicate pharmacokinetics and therapeutic responses ( Seeley & MacDougald, 2021). These challenges highlight the need for more predictive models to bridge the gap between animal studies and human AD research.
NHPs
NHPs, owing to their closer genetic and neuroanatomical similarities to humans, provide an essential avenue for studying late-onset sporadic AD. Their well-developed brain structures, extended lifespans, and cognitive abilities make them uniquely suited for specific age-related neurodegeneration. Aging NHPs exhibit a broad range of cognitive, physical, and sensory declines, along with comorbid illnesses such as cancer, cardiovascular disease, and neurodegeneration.
Nearly all studied NHP species develop amyloid-related pathology with age, although the onset, quantity, distribution, and appearance of amyloid pathology vary. Furthermore, given their similarity in brain structure, NHPs can be utilized to study the neocortical-to-allocortical expansion of AD-like pathology. While amyloid plaques are typically limited to the hippocampus in NHPs, their progressive spread within the brain mirrors aspects of human AD progression ( Poduri et al., 1994; Sani et al., 2003). However, the frequent sacrifice of NHPs for pathological testing prior to cognitive assessments poses a significant challenge in correlating pathological changes with cognition decline ( Stonebarger et al., 2021). Recent studies provide compelling evidence that aging NHPs may spontaneously develop AD-like conditions. Li et al. ( 2022b) reported that aged rhesus monkeys with cognitive impairments display hallmark clinical and pathological features of AD, including memory decline, intracellular NFTs, extracellular amyloid plaques, and significant neuronal loss. These findings suggest that NHPs may naturally recapitulate sporadic AD-like conditions upon aging, similar to humans.
However, rhesus macaques, one of the most common NHP species used in research, typically exhibit amyloid coverage limited to 5%–6% in regions of interest, even in advanced age ( Zhang et al., 2019). To enhance the utility of NHP models in AD research, various strategies have been engineered to accelerate the progression of AD pathology through targeted plaque and NFT induction in species with extended lifespans, such as rhesus macaques. Exogenous administration of Aβ oligomers has been shown to induce Aβ accumulation in key brain regions associated with higher-order cognition, such as the prefrontal cortex and hippocampus, closely mirroring human AD pathology ( Beckman et al., 2019). This approach has revealed hallmark AD features, including synaptic degeneration, heightened tau phosphorylation, and activation of astrocytes and microglia in Aβ-enriched macaque brain areas ( Forny-Germano et al., 2014). Additionally, studies on middle-aged female monkeys demonstrated that Aβ oligomers selectively target vulnerable dendritic spines in the neocortex and hippocampus, eliciting neuroinflammatory responses comparable to those observed in humans ( Beckman & Morrison, 2021). Genetic approaches have further advanced NHP modeling of AD. The introduction of mutated tau into rhesus monkey brains has been shown to trigger the spread of misfolded tau proteins, accompanied by TREM2-positive microglial activation and significant alterations in cerebrospinal fluid (CSF) and plasma biomarkers indicative of AD ( Beckman et al., 2021; Beckman & Morrison, 2021; Jiang et al., 2024). The transgenic cynomolgus macaque model expressing tau (P301L), developed through lentiviral embryo infection, shows age-related neurodegeneration, motor impairments, and spinal cord Aβ oligomer production induced by embryonic transgenic tau expression or stereotactic intracerebral injection of AAV-Tau ( Tu et al., 2023). This suggests that tau accumulation can trigger Aβ oligomer formation, demonstrating that tau plays a causal role in endogenous Aβ formation and toxicity. Moreover, since Aβ oligomers induced by mutated tau are stable in the spinal cords of NHPs, targeting this form of Aβ may be beneficial for treating pathologies induced by both tau and Aβ, at least in the spinal cord.
Longitudinal studies of neurocognitive functions in NHPs are necessary for understanding both healthy and pathological aging in humans. Short-lived primates, such as gray mouse lemurs and common marmosets, provide a unique opportunity to observe natural age-related changes in brain structure and behavior, offering potential predictors of neurogenerative diseases. These species serve as ideal primate models due to their naturally short lifespans, ease of handling, rapid reproduction, availability of neuroanatomy atlases, and brain organization comparable to larger NHPs and humans. At middle age (approximately 5 years old for gray mouse lemurs and 8 years for marmosets), these animals demonstrate a decline in psychomotor capacities and cognitive performance, respectively ( Chaudron et al., 2021; Rothwell et al., 2021). In certain instances, these animals exhibit spontaneous pathological aging, displaying age-related deficits similar to humans, including severe cognitive decline, brain atrophy, amyloidosis, and glucoregulatory imbalance ( Chaudron et al., 2021; Tardif et al., 2011). As such, they provide a suitable bridge between rodent and larger NHP models for neuroscience and aging research ( Tardif et al., 2011).
Recent advancements in genomics have enabled the development of genetically modified NHPs to model AD more effectively. Genetically engineered marmosets and cynomolgus monkeys ( Macaca fascicularis) carrying mutations associated with AD have been successfully established. For example, PSEN1 Δ9 founder marmosets have been generated ( Sato et al., 2020), as well as two founder lines carrying knock-in point mutations such as PSEN1 C410Y and A426P ( Rizzo et al., 2021). These mutations lead to a progressive increase in plasma Aβ42 levels and an elevated Aβ42-to-Aβ40 ratio with age. In 17-month-old marmosets with the homozygous PSEN1 C410Y mutation, Aβ42 (detected by NAB228 antibody) accumulates in both intracellular and extracellular compartments within various brain regions, including the sensorimotor cortex and globus pallidum ( Homanics et al., 2024). In cynomolgus monkeys, the dual-guide CRISPR/Cas9 system has been employed to successfully emulate the PSEN1-ΔE9 mutation through the genomic excision of exon 9, leading to a groundbreaking presentation of tau-related pathology, including phosphorylated tau in CSF. Blood transcriptome and proteome profiling of juvenile PSEN1 mutant cynomolgus monkeys has revealed early dysregulation of inflammatory and immune molecules, underscoring the complex interplay of molecular mechanisms in the early stages of AD ( Li et al., 2024b). These models are particularly promising for testing preventive therapies before the onset of overt symptoms.
In conclusion, NHPs represent powerful models for studying brain aging and AD-related pathology. Their genetic and physiological similarities to humans, combined with emerging genomic tools, provide critical insights into the mechanisms underlying neurodegeneration and offer platforms for evaluating novel therapeutic approaches.
Chinese tree shrews
Chinese tree shrews, small mammals of the order Scandentia, are phylogenetically close to NHPs. Recent genomic and nuclear DNA analyses place them within the Euarchonta branch, alongside primates and colugos. Their small size, brief reproductive cycle, relatively short lifespan, and low maintenance costs make them a promising alternative to NHPs in biomedical research ( Meng et al., 2016). The third edition of the tree shrew brain atlas, developed with single-cell RNA sequencing (scRNA-seq) and advanced multiomics technologies, provides detailed insights into brain region delineation and cellular composition, further affirming their close evolutionary relationship with NHPs ( Yao et al., 2024). Notably, their high brain-to-body mass ratio and developed frontal cortex make them suitable for studying clinical features related to advanced brain functions.
Chinese tree shrews have been used in various neurological disorder models, including depression ( Meng et al., 2016; Wang et al., 2012), glioma ( Tong et al., 2017), and Parkinson’s disease ( Wu et al., 2019). Genes involved in Aβ production and NFT pathways exhibit higher sequence identity with humans than those of rodents. In particular, the Aβ42 peptide sequence in tree shrews is identical to that of humans, unlike that in mice and rats, which differs by three residues ( Pawlik et al., 1999). Aging tree shrews display a substantial increase in 4G8-positive deposits and an elevated Aβ42-to-Aβ40 ratio in hippocampal and cortical tissues ( Li et al., 2024a). Behaviorally, tree shrews demonstrate superior memory performance compared to rodents, particularly in tasks involving novelty preference ( Khani & Rainer, 2012; Nair et al., 2014). They have also been utilized in preclinical drug development studies ( Shen et al., 2018; Wang et al., 2013).
Chinese tree shrews possess 131 AD-related genes, demonstrating a high degree of similarity to humans in both protein sequence identity and expression patterns ( Fan et al., 2018). Age-dependent increases in total Aβ levels have been documented in the cortical tissue, correlating with intracellular amyloid deposition that exhibits diffuse and dense patterns ( Fan et al., 2018; Yamashita et al., 2010, 2012). Astrocytes, identified through GFAP-positive staining, also show Aβ immunoreactivity ( Fan et al., 2018). While the manifestation of NFTs in the aging process is not yet well understood, key genes implicated in the NFT formation pathway share a high degree of protein sequence identity with their human counterparts. Elevated phosphorylated tau (pTau-181) levels in the hippocampus further underscore the similarity between Chinese tree shrews and humans in their respective NFT formation pathways ( Fan et al., 2018). In addition, aged tree shrews show progressive cognitive decline in maze-based tests, paralleling impairments observed in human AD patients.
Despite current limitations in the use of tree shrews for research, significant advancements have enhanced their utility. Publicly available resources, including brain anatomical MRI templates, atlases of white matter, striatum, pallidum, and cerebellum ( Dai et al., 2017; Ni et al., 2018a, 2018b), and fine anatomical features of the primary visual cortex and hippocampus ( Dai et al., 2018), provide essential tools for advanced investigations. Additionally, comprehensive maps of c-Fos expression in the forebrain and genome annotations derived from large-scale RNA sequencing have expanded the scope of genomic and transcriptomic studies in tree shrews ( Ni et al., 2020). The integration of advanced artificial intelligence (AI) and deep learning motion recognition technologies has further facilitated detailed behavioral studies, establishing the tree shrew as a valuable model for neuroscientific research ( Yao et al., 2024). These resources collectively enable further exploration of the Chinese tree shrew as a potential model for AD, supporting investigations into disease pathogenesis and potential therapeutic targets.
LIMITATIONS OF CURRENT ANIMAL MODELS
The development of animal models of AD faces significant challenges due to the complexity of the disease pathology and poorly understood pathogenesis. Currently, no animal model can fully replicate the intricate neurodegenerative processes observed in human AD brains.
Models incorporating mutations or knock-ins of pathogenic genes have inherent limitations. First, these models predominantly simulate early-onset FAD, which represents less than 5% of the AD population. The rapid and severe pathology observed in these models does not align with the slow and progressive nature of the disease in most AD patients. Second, while AD patients with diverse genomic variations often exhibit similar pathology, animal models with distinct genomic variations display inconsistent pathological features ( Sierksma et al., 2020). This genotype-phenotype discrepancy undermines the translatability of findings to clinical trials. Third, in AD patients with APP or PSEN1 mutations, tau pathology typically arises subsequent to Aβ pathology. However, existing animal models fail to recapitulate tau pathology under conditions of APP or PSEN1 overexpression.
Conversely, animal models without pathogenic gene mutations or knock-ins primarily simulate late-onset sporadic AD, which accounts for over 95% of the AD population. The multifactorial nature of sporadic AD involves genetic risk factors, aging, and environmental effects. Current models of sporadic AD predominantly focus on risk genes and the aging process, while neglecting environmental factors and their interactions with genes, such as the impacts of diet, exercise, and air quality ( Zhang et al., 2023)
NHPs exhibit greater similarity to human AD in brain anatomy, pathological features, and behavioral patterns. Their extended lifespan allows for the study of gradual cognitive decline, better mirroring the slow progression of AD. However, the limited reproductive capacity and high costs associated with NHPs significantly restrict their extensive use in research, a common challenge to many biomedical studies. A possible solution to address this issue involves using NHP models to identify key disease processes, which can be subsequently validated in rodent animals.
In addition to the respective limitations of each animal model, there are overarching challenges associated with using animal models for AD research. First, the heterogeneity observed among AD patients—including variations in genetics, sex, physical health, and lifestyle—profoundly affects disease progression and therapeutic responses. However, preclinical trials typically use male animals with a uniform genetic background and maintained on identical diets in identical pathogen-free environments. This lack of diversity may contribute to the limited predictive validity of these models in clinical settings. Second, aging, an important risk factor in AD, is often inadequately addressed. Many studies focus on a narrow age range, failing to account for the slow, progressive nature of the disease. Third, behavioral symptoms are frequently selected as primary therapeutic endpoints in preclinical studies. However, behavioral testing paradigms in animal models often yield inconsistent and unstable results, poorly predicting clinical trial outcomes ( Wan et al., 2020). Additionally, differences in drug metabolism between species lead to variable responses to therapeutic candidates, further complicating the translation of preclinical findings to human trials ( Seeley & MacDougald, 2021). Despite recent efforts to address these issues, many challenges remain unresolved, emphasizing the need for more refined and representative models to advance AD research.
FUTURE PERSPECTIVES
The fundamental biological differences between species contribute significantly to the poor predictive validity of animal models in preclinical trials. However, refining experimental design and leveraging novel technologies can help bridge this translational gap, providing more powerful tools for AD research.
To address the impact of heterogeneity within the AD population on preclinical testing, controlled variables, such as sex, age, and housing conditions, should be systematically varied ( Viaud-Delmon et al., 2011). Moreover, instead of increasing the number of animals in a single experiment, conducting parallel studies across multiple independent laboratories can introduce controlled variability. Although this approach may increase variability within individual studies, it can reduce variability across studies, potentially minimizing false-positive results and increasing reproducibility ( Viaud-Delmon et al., 2011).
The aging process, a critical factor in AD progression, remains challenging to replicate in short-lived animal models. Expanding age ranges or incorporating progressive pathological stages into experimental designs could improve the translational relevance of these models, even if the human aging process cannot be fully simulated. Additionally, investigating environmental factors and their interplay with genetic and pathological mechanisms requires a more diverse array of animal models and research methods. Developing crossover models that combine AD with comorbid conditions such as diabetes, cerebrovascular disease, and depression, or directly subjecting animal models to specific environmental factors under standardized conditions, represent viable avenues for future research.
In consideration of the fundamental biological differences between humans and animal models, in vitro human cell systems offer a promising strategy for AD research. Recent advances in induced pluripotent stem cell (iPSC) technology have enabled the generation of CNS cell types derived from healthy individuals or AD patients, facilitating the development of in vitro models ( Arber et al., 2017; Israel et al., 2012; Kwart et al., 2019). For example, organoid systems composed of iPSC-derived neurons with APP mutation replicate Aβ accumulation characteristic of amyloid plaques ( Choi et al., 2014; Park et al., 2018). Similarly, human cerebral organoid models with APP NL-G-F knock-in illustrate the sequential development of Aβ aggregates, tau pathology, astrogliosis, and cell death, closely resembling the full spectrum of pathological and pathophysiological events in the human AD brain ( Liu et al., 2024). Despite these advancements, replicating the complex microenvironment of the human brain within iPSC-derived human cell systems remains challenging. Emerging xenograft models, where iPSC-derived human cells are grafted into mouse brains, provide a novel platform for investigating human cell behavior in vivo ( Hasselmann et al., 2019). For instance, iPSC-derived human microglia grafted into mouse brains display distinct transcriptomic profiles compared to mouse microglia when exposed to AD-related amyloid pathology ( Mancuso et al., 2024). Additionally, neurons derived from fibroblasts of both healthy and AD patients exhibit signature AD traits. In neurons derived from patients with late-onset AD (LOAD), Aβ accumulation drives neurodegeneration, which can be alleviated by early secretase inhibitor treatment ( Sun et al., 2024). Single-cell analyses have further advanced our understanding of AD by unraveling gene expression and structural heterogeneity across various neural cell populations. These insights enhance our comprehension of AD pathogenesis, aiding marker gene identification for early diagnosis and timely intervention in neurodegenerative diseases with pathogenesis preceding clinical manifestation. One notable example is the discovery of disease-associated microglia (DAMs) through scRNA-seq in 5xFAD mice ( Keren-Shaul et al., 2017), which transition into activated response microglia (ARMs) and IFN response microglia (IRMs) in App NL-G-F/NL-G-F mice ( Keren-Shaul et al., 2017). Integrating data from multiple cohort studies on AD has also refined our understanding of disease trajectories, allowing for the stratification of subjects based on baseline gene expression and disease subtype. By comparing differentially expressed genes in 5xFAD and APP/PS1 mice, researchers identified significant enrichment patterns consistent with microglial changes observed in humans ( Wang et al., 2022).
Beyond transgenic mice, recent progress in single-cell and single-nucleus transcriptomic technologies have significantly expanded our understanding of AD pathogenesis. Notably, studies in NHPs and humans have provided critical insights into the cellular and molecular mechanisms underlying AD. For example, single-cell analyses of aged Macaca fascicularis identified a subpopulation of excitatory neurons (ExNs subpopulations) with heightened susceptibility to AD ( Li et al., 2022a). Similarly, a single-nucleus transcriptomic atlas of the human prefrontal cortex in elderly individuals revealed key cellular and molecular determinants of high cognitive function, dementia, and resistance to AD pathology ( Mathys et al., 2023). Further investigations into human microglia through single-nucleus transcriptomic and epigenomic profiling uncovered dynamic changes in microglial states across different pathological stages of AD, as well as the transcription factor networks regulating these transitions ( Sun et al., 2023). Additionally, analyses of the human brain revealed epigenomic erosion and loss of cellular identity in late-stage AD ( Xiong et al., 2023b). Comprehensive examination of six brain regions in aged individuals, with and without AD, demonstrated region-specific neuronal vulnerabilities and distinct genetic expression profiles, including a decrease in certain neuronal subtypes, an increase in reelin pathway-related genes, and alterations in astrocyte gene expression linked to cognitive resilience, underscoring their impact on AD susceptibility ( Mathys et al., 2024). These findings highlight the potential of single-cell molecular data to redefine existing paradigms of AD pathogenesis and uncover novel therapeutic avenues. Considering the accessibility of research subjects, further investigation of AD animal models is essential to translate these findings into meaningful advances in understanding and treatment.
Single-cell analysis primarily focuses on clustering and differential gene expression analyses, which rely heavily on similarities in expression profiles. However, it has limitations in accurately capturing developmental trajectories and lineage structure (or “fate”) of cells. In contrast, lineage tracing offers distinct advantages by enabling the tracking of cell fate through the labeling of stem and progenitor cells during embryonic stages. This is achieved by sequencing inherited DNA sequences or “barcodes” across subsequent stages ( Kalhor et al., 2018; McKenna et al., 2016). For example, MRI data from 71 patients showed that lower levels of Aβ42 and higher concentrations of total tau, pTau-181, sAPPβ, and their ratios with Aβ42 are closely linked to reduced brain myelin. These findings suggest that age-related myelin decline, influenced by CSF biomarkers, may serve as an early indicator of AD pathology in white matter, particularly in myelin content ( Dean III et al., 2017). Mouse models, such as APPswe/PS1dE9 and 5xFAD mice, exhibit demyelination phenotypes. Lineage tracing and cell labeling have further clarified the relationship between Aβ morphology, demyelination, and remyelination in APPswe/PS1dE9 mice, suggesting that soluble Aβ may not significantly alter myelin formation.
CONCLUSIONS
While AD animal models remain indispensable, acknowledging their limitations is crucial for advancing the field. Recognizing the species-specific nature of AD pathology has prompted a greater shift towards the use of NHPs in research. Their closer biological resemblance to humans offers a promising platform for exploring AD mechanisms and evaluating potential therapeutic strategies. By addressing these challenges through technological innovations and biologically relevant models, researchers can bridge the translational gap, improving the predictive accuracy of preclinical findings and accelerating the development of effective therapies for AD.
Acknowledgments
COMPETING INTERESTS
The authors declare that they have no competing interests.
AUTHORS’ CONTRIBUTIONS
P.L. provided ideas and outlines. Q.W. and B.T.Z. collected references and prepared the original manuscript and figures. P.L. supervised and revised the manuscript and figures. All authors read and approved the final version of the manuscript.
Funding Statement
This work was supported by the National Key Research and Development Program of China (2021YFC2500100), Major Science & Technology Program of Sichuan Province (2022ZDZX0021), National Clinical Research Center for Geriatrics, West China Hospital, Sichuan University (Z2024JC007), Sichuan Science and Technology Program (2024YFHZ0010, 2024NSFSC1643), and West China Hospital 1.3.5 Project for Disciplines of Excellence (ZYYC23016)
References
- [Anonymous] 2024 Alzheimer's disease facts and figures. Alzheimer's & Dementia. 2024;20(5):3708–3821. doi: 10.1002/alz.13809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbas N, Bednar I, Mix E, et al Up-regulation of the inflammatory cytokines IFN-γ and IL-12 and down-regulation of IL-4 in cerebral cortex regions of APP SWE transgenic mice . Journal of Neuroimmunology. 2002;126(1-2):50–57. doi: 10.1016/S0165-5728(02)00050-4. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Kameyama M, Akiguchi I, et al Periodic acid-Schiff (PAS)-positive, granular structures increase in the brain of Senescence Accelerated Mouse (SAM) Acta Neuropathologica. 1986;72(2):124–129. doi: 10.1007/BF00685973. [DOI] [PubMed] [Google Scholar]
- Arber C, Lovejoy C, Wray S Stem cell models of Alzheimer's disease: progress and challenges. Alzheimer's Research & Therapy. 2017;9(1):42. doi: 10.1186/s13195-017-0268-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayton S, Lei P, Hare DJ, et al Parkinson's disease iron deposition caused by nitric oxide-induced loss of β-amyloid precursor protein. Journal of Neuroscience. 2015;35(8):3591–3597. doi: 10.1523/JNEUROSCI.3439-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber AJ, Del Genio CL, Swain AB, et al Age, sex and Alzheimer’s disease: a longitudinal study of 3xTg-AD mice reveals sex-specific disease trajectories and inflammatory responses mirrored in postmortem brains from Alzheimer’s patients. Alzheimer's Research & Therapy. 2024;16(1):134. doi: 10.1186/s13195-024-01492-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassil F, Brown HJ, Pattabhiraman S, et al. 2020. Amyloid-Beta (Aβ) plaques promote seeding and spreading of alpha-synuclein and tau in a mouse model of lewy body disorders with Aβ pathology. Neuron, 105(2): 260–275. e6.
- Beauchamp LC, Chan J, Hung LW, et al Ablation of tau causes an olfactory deficit in a murine model of Parkinson’s disease. Acta Neuropathologica Communications. 2018;6(1):57. doi: 10.1186/s40478-018-0560-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman D, Chakrabarty P, Ott S, et al A novel tau-based rhesus monkey model of Alzheimer's pathogenesis. Alzheimer’s & Dementia. 2021;17(6):933–945. doi: 10.1002/alz.12318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman D, Morrison JH Towards developing a rhesus monkey model of early Alzheimer's disease focusing on women's health. American Journal of Primatology. 2021;83(11):e23289. doi: 10.1002/ajp.23289. [DOI] [PubMed] [Google Scholar]
- Beckman D, Ott S, Donis-Cox K, et al Oligomeric Aβ in the monkey brain impacts synaptic integrity and induces accelerated cortical aging. Proceedings of the National Academy of Sciences of the United States of America. 2019;116(52):26239–26246. doi: 10.1073/pnas.1902301116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckmann N, Schuler A, Mueggler T, et al Age-dependent cerebrovascular abnormalities and blood flow disturbances in APP23 mice modeling Alzheimer's disease. Journal of Neuroscience. 2003;23(24):8453–8459. doi: 10.1523/JNEUROSCI.23-24-08453.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beecham GW, Martin ER, Li YJ, et al Genome-wide association study implicates a chromosome 12 risk locus for late-onset alzheimer disease. The American Journal of Human Genetics. 2009;84(1):35–43. doi: 10.1016/j.ajhg.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belaidi AA, Gunn AP, Wong BX, et al Marked age-related changes in brain iron homeostasis in amyloid protein precursor knockout mice. Neurotherapeutics. 2018;15(4):1055–1062. doi: 10.1007/s13311-018-0656-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benderradji H, Kraiem S, Courty E, et al Impaired glucose homeostasis in a tau knock-in mouse model. Frontiers in Molecular Neuroscience. 2022;15:841892. doi: 10.3389/fnmol.2022.841892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benskey MJ, Panoushek S, Saito T, et al Behavioral and neuropathological characterization over the adult lifespan of the human tau knock-in mouse. Frontiers in Aging Neuroscience. 2023;15:1265151. doi: 10.3389/fnagi.2023.1265151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger Z, Roder H, Hanna A, et al Accumulation of pathological tau species and memory loss in a conditional model of tauopathy. Journal of Neuroscience. 2007;27(14):3650–3662. doi: 10.1523/JNEUROSCI.0587-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Lange C, Mullin K, et al Genome-wide association analysis reveals putative Alzheimer's disease susceptibility loci in addition to APOE . The American Journal of Human Genetics. 2008;83(5):623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JD, Berghmans S, Hunt JJFG, et al Non-associative learning in larval zebrafish. Neuropsychopharmacology. 2008;33(5):1206–1215. doi: 10.1038/sj.npp.1301489. [DOI] [PubMed] [Google Scholar]
- Bi M, Gladbach A, Van Eersel J, et al Tau exacerbates excitotoxic brain damage in an animal model of stroke. Nature Communications. 2017;8(1):473. doi: 10.1038/s41467-017-00618-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackmore T, Meftah S, Murray TK, et al Tracking progressive pathological and functional decline in the rTg4510 mouse model of tauopathy. Alzheimer's Research & Therapy. 2017;9(1):77. doi: 10.1186/s13195-017-0306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondolfi L, Calhoun M, Ermini F, et al Amyloid-associated neuron loss and gliogenesis in the neocortex of amyloid precursor protein transgenic mice. Journal of Neuroscience. 2002;22(2):515–522. doi: 10.1523/JNEUROSCI.22-02-00515.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borchelt DR, Ratovitski T, Van Lare J, et al Accelerated amyloid deposition in the brains of transgenic mice coexpressing mutant presenilin 1 and amyloid precursor proteins. Neuron. 1997;19(4):939–945. doi: 10.1016/S0896-6273(00)80974-5. [DOI] [PubMed] [Google Scholar]
- Borchelt DR, Thinakaran G, Eckman CB, et al Familial Alzheimer's disease-linked presenilin 1 variants elevate Aβ1–42/1–40 ratio in vitro and in vivo. Neuron. 1996;17(5):1005–1013. doi: 10.1016/S0896-6273(00)80230-5. [DOI] [PubMed] [Google Scholar]
- Borrego-Écija S, Morgado J, Palencia-Madrid L, et al Frontotemporal dementia caused by the P301L mutation in the MAPT gene: clinicopathological features of 13 cases from the same geographical origin in Barcelona, Spain . Dementia and Geriatric Cognitive Disorders. 2017;44(3-4):213–221. doi: 10.1159/000480077. [DOI] [PubMed] [Google Scholar]
- Bugiani O, Murrell JR, Giaccone G, et al Frontotemporal dementia and corticobasal degeneration in a family with a P301S mutation in Tau . Journal of Neuropathology & Experimental Neurology. 1999;58(6):667–677. doi: 10.1097/00005072-199906000-00011. [DOI] [PubMed] [Google Scholar]
- Busche MA, Chen XW, Henning HA, et al Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(22):8740–8745. doi: 10.1073/pnas.1206171109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caccamo A, Magrì A, Medina DX, et al mTOR regulates tau phosphorylation and degradation: implications for Alzheimer's disease and other tauopathies. Aging Cell. 2013;12(3):370–380. doi: 10.1111/acel.12057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun ME, Burgermeister P, Phinney AL, et al Neuronal overexpression of mutant amyloid precursor protein results in prominent deposition of cerebrovascular amyloid. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(24):14088–14093. doi: 10.1073/pnas.96.24.14088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calhoun ME, Wiederhold KH, Abramowski D, et al Neuron loss in APP transgenic mice. Nature. 1998;395(6704):755–756. doi: 10.1038/27351. [DOI] [PubMed] [Google Scholar]
- Cardinaux JR, Allaman I, Magistretti PJ Pro-inflammatory cytokines induce the transcription factors C/EBPβ and C/EBPδ in astrocytes. Glia. 2000;29(1):91–97. doi: 10.1002/(SICI)1098-1136(20000101)29:1<91::AID-GLIA9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Ceglia I, Reitz C, Gresack J, et al APP intracellular domain-WAVE1 pathway reduces amyloid-β production. Nature Medicine. 2015;21(9):1054–1059. doi: 10.1038/nm.3924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudron Y, Pifferi F, Aujard F Overview of age-related changes in psychomotor and cognitive functions in a prosimian primate, the gray mouse lemur ( Microcebus murinus): recent advances in risk factors and antiaging interventions . American Journal of Primatology. 2021;83(11):e23337. doi: 10.1002/ajp.23337. [DOI] [PubMed] [Google Scholar]
- Chen K, Tang F, Du B, et al Leucine-rich repeat kinase 2 (LRRK2) inhibition upregulates microtubule-associated protein 1B to ameliorate lysosomal dysfunction and parkinsonism. MedComm. 2023;4(6):e429. doi: 10.1002/mco2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WT, Lu A, Craessaerts K, et al. 2020. Spatial transcriptomics and in situ sequencing to study Alzheimer's disease. Cell, 182(4): 976–991. e19 .
- Chen Y, Song SH, Parhizkar S, et al. 2024. APOE3ch alters microglial response and suppresses Aβ-induced tau seeding and spread. Cell, 187(2): 428–445. e20.
- Chen ZY, Zhang Y Animal models of Alzheimer's disease: applications, evaluation, and perspectives. Zoological Research. 2022;43(6):1026–1040. doi: 10.24272/j.issn.2095-8137.2022.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi BR, Cho WH, Kim J, et al Increased expression of the receptor for advanced glycation end products in neurons and astrocytes in a triple transgenic mouse model of Alzheimer's disease. Experimental & Molecular Medicine. 2014;46(2):e75. doi: 10.1038/emm.2013.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark RF, Hutton M, Fuldner M, et al The structure of the presenilin 1 ( S182) gene and identification of six novel mutations in early onset AD families . Nature Genetics. 1995;11(2):219–222. doi: 10.1038/ng1095-219. [DOI] [PubMed] [Google Scholar]
- Coll RC, Robertson AAB, Chae JJ, et al A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nature Medicine. 2015;21(3):248–255. doi: 10.1038/nm.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook C, Dunmore JH, Murray ME, et al Severe amygdala dysfunction in a MAPT transgenic mouse model of frontotemporal dementia . Neurobiology of Aging. 2014;35(7):1769–1777. doi: 10.1016/j.neurobiolaging.2013.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coon KD, Myers AJ, Craig DW, et al A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer's disease . The Journal of Clinical Psychiatry. 2007;68(4):613–618. doi: 10.4088/JCP.v68n0419. [DOI] [PubMed] [Google Scholar]
- Corder EH, Saunders AM, Strittmatter WJ, et al Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science. 1993;261(5123):921–923. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- Cornwell BR, Johnson LL, Holroyd T, et al Human hippocampal and parahippocampal theta during goal-directed spatial navigation predicts performance on a virtual Morris water maze. Journal of Neuroscience. 2008;28(23):5983–5990. doi: 10.1523/JNEUROSCI.5001-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes-Canteli M, Aguilar-Morante D, Sanz-Sancristobal M, et al Role of C/EBPβ transcription factor in adult hippocampal neurogenesis. PLoS One. 2011;6(10):e24842. doi: 10.1371/journal.pone.0024842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramer PE, Cirrito JR, Wesson DW, et al ApoE-directed therapeutics rapidly clear β-amyloid and reverse deficits in AD mouse models. Science. 2012;335(6075):1503–1506. doi: 10.1126/science.1217697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crouzin N, Baranger K, Cavalier M, et al Area-specific alterations of synaptic plasticity in the 5XFAD mouse model of Alzheimer's disease: dissociation between somatosensory cortex and hippocampus. PLoS One. 2013;8(9):e74667. doi: 10.1371/journal.pone.0074667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amelio M, Cavallucci V, Middei S, et al Caspase-3 triggers early synaptic dysfunction in a mouse model of Alzheimer's disease. Nature Neuroscience. 2011;14(1):69–76. doi: 10.1038/nn.2709. [DOI] [PubMed] [Google Scholar]
- D’Angelo C, Costantini E, Salvador N, et al nAChRs gene expression and neuroinflammation in APPswe/PS1dE9 transgenic mouse. Scientific Reports. 2021;11(1):9711. doi: 10.1038/s41598-021-89139-x. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Dai JK, Wang SX, Shan D, et al A diffusion tensor imaging atlas of white matter in tree shrew. Brain Structure and Function. 2017;222(4):1733–1751. doi: 10.1007/s00429-016-1304-z. [DOI] [PubMed] [Google Scholar]
- Dai JK, Wang SX, Shan D, et al Super-resolution track-density imaging reveals fine anatomical features in tree shrew primary visual cortex and hippocampus. Neuroscience Bulletin. 2018;34(3):438–448. doi: 10.1007/s12264-017-0199-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels MJD, Rivers-Auty J, Schilling T, et al Fenamate NSAIDs inhibit the NLRP3 inflammasome and protect against Alzheimer's disease in rodent models. Nature Communications. 2016;7:12504. doi: 10.1038/ncomms12504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daude N, Kim C, Kang SG, et al Diverse, evolving conformer populations drive distinct phenotypes in frontotemporal lobar degeneration caused by the same MAPT-P301L mutation . Acta Neuropathologica. 2020;139(6):1045–1070. doi: 10.1007/s00401-020-02148-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson HN, Ferreira A, Eyster MV, et al Inhibition of neuronal maturation in primary hippocampal neurons from tau deficient mice. Journal of Cell Science. 2001;114(6):1179–1187. doi: 10.1242/jcs.114.6.1179. [DOI] [PubMed] [Google Scholar]
- Deacon RMJ, Rawlins JNP T-maze alternation in the rodent. Nature Protocols. 2006;1(1):7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- Dean III DC, Hurley SA, Kecskemeti SR, et al Association of amyloid pathology with myelin alteration in preclinical Alzheimer disease. JAMA Neurology. 2017;74(1):41–49. doi: 10.1001/jamaneurol.2016.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dejanovic B, Huntley MA, De Mazière A, et al. 2018. Changes in the synaptic proteome in tauopathy and rescue of tau-induced synapse loss by C1q antibodies. Neuron, 100(6): 1322–1336. e7.
- Del Valle J, Bayod S, Camins A, et al Dendritic spine abnormalities in hippocampal CA1 pyramidal neurons underlying memory deficits in the SAMP8 mouse model of Alzheimer's disease. Journal of Alzheimer's Disease. 2012;32(1):233–240. doi: 10.3233/JAD-2012-120718. [DOI] [PubMed] [Google Scholar]
- Del Valle J, Duran-Vilaregut J, Manich G, et al Early amyloid accumulation in the hippocampus of SAMP8 mice. Journal of Alzheimer's Disease. 2010;19(4):1303–1315. doi: 10.3233/JAD-2010-1321. [DOI] [PubMed] [Google Scholar]
- Ding XL, Cao SQ, Wang Q, et al DNALI1 promotes neurodegeneration after traumatic brain injury via inhibition of autophagosome-lysosome fusion. Advanced Science. 2024;11(15):2306399. doi: 10.1002/advs.202306399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding Y, Qiao AM, Wang ZQ, et al Retinoic acid attenuates β-amyloid deposition and rescues memory deficits in an Alzheimer's disease transgenic mouse model. Journal of Neuroscience. 2008;28(45):11622–11634. doi: 10.1523/JNEUROSCI.3153-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodart JC, Meziane H, Mathis C, et al Behavioral disturbances in transgenic mice overexpressing the V717F Β-amyloid precursor protein. Behavioral Neuroscience. 1999;113(5):982–990. doi: 10.1037/0735-7044.113.5.982. [DOI] [PubMed] [Google Scholar]
- Duce JA, Tsatsanis A, Cater MA, et al Iron-export ferroxidase activity of β-amyloid precursor protein is inhibited by zinc in Alzheimer's disease. Cell. 2010;142(6):857–867. doi: 10.1016/j.cell.2010.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duff K, Eckman C, Zehr C, et al Increased amyloid-β42(43) in brains of mice expressing mutant presenilin 1. Nature. 1996;383(6602):710–713. doi: 10.1038/383710a0. [DOI] [PubMed] [Google Scholar]
- Duffy ÁM, Morales-Corraliza J, Bermudez-Hernandez KM, et al Entorhinal cortical defects in Tg2576 mice are present as early as 2–4 months of age. Neurobiology of Aging. 2015;36(1):134–148. doi: 10.1016/j.neurobiolaging.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumanchin C, Camuzat A, Campion D, et al Segregation of a missense mutation in the microtubule-associated protein tau gene with familial frontotemporal dementia and parkinsonism. Human Molecular Genetics. 1998;7(11):1825–1829. doi: 10.1093/hmg/7.11.1825. [DOI] [PubMed] [Google Scholar]
- Eisele YS, Bolmont T, Heikenwalder M, et al Induction of cerebral β-amyloidosis: intracerebral versus systemic Aβ inoculation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(31):12926–12931. doi: 10.1073/pnas.0903200106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg D, Jucker M The amyloid state of proteins in human diseases. Cell. 2012;148(6):1188–1203. doi: 10.1016/j.cell.2012.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan Y, Luo RC, Su LY, et al. 2018. Does the genetic feature of the Chinese tree shrew ( Tupaia belangeri chinensis) support its potential as a viable model for Alzheimer's disease research? Journal of Alzheimer's Disease, 61(3): 1015–1028.
- Fang EF, Hou YJ, Palikaras K, et al Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer's disease. Nature Neuroscience. 2019;22(3):401–412. doi: 10.1038/s41593-018-0332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanigan TJ, Xue Y, Kishan Rao S, et al Abnormal vibrissa-related behavior and loss of barrel field inhibitory neurons in 5xFAD transgenics. Genes Brain and Behavior. 2014;13(5):488–500. doi: 10.1111/gbb.12133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley KE, Hewes AA, Garceau DT, et al The APOE ε3/ε4 genotype drives distinct gene signatures in the cortex of young mice . Frontiers in Aging Neuroscience. 2022;14:838436. doi: 10.3389/fnagi.2022.838436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forny-Germano L, Lyra e Silva NM, Batista AF, et al Alzheimer's disease-like pathology induced by amyloid-β oligomers in nonhuman primates. Journal of Neuroscience. 2014;34(41):13629–13643. doi: 10.1523/JNEUROSCI.1353-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frautschy SA, Yang FS, Irrizarry M, et al Microglial response to amyloid plaques in APPsw transgenic mice. The American Journal of Pathology. 1998;152(1):307–317. [PMC free article] [PubMed] [Google Scholar]
- Fu AKY, Hung KW, Huang HQ, et al Blockade of EphA4 signaling ameliorates hippocampal synaptic dysfunctions in mouse models of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(27):9959–9964. doi: 10.1073/pnas.1405803111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, et al Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature. 1995;373(6514):523–527. doi: 10.1038/373523a0. [DOI] [PubMed] [Google Scholar]
- Garcia-Alloza M, Robbins EM, Zhang-Nunes SX, et al Characterization of amyloid deposition in the APPswe/PS1dE9 mouse model of Alzheimer disease. Neurobiology of Disease. 2006;24(3):516–524. doi: 10.1016/j.nbd.2006.08.017. [DOI] [PubMed] [Google Scholar]
- Gatto EM, Allegri RF, Da Prat G, et al. 2017. Intrafamilial variable phenotype including corticobasal syndrome in a family with p. P301L mutation in the MAPT gene: first report in South America. Neurobiology of Aging, 53: 195. e11–195. e17.
- Ghosh S, Wu MD, Shaftel SS, et al Sustained interleukin-1β overexpression exacerbates tau pathology despite reduced amyloid burden in an Alzheimer's mouse model. Journal of Neuroscience. 2013;33(11):5053–5064. doi: 10.1523/JNEUROSCI.4361-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbel DA, Nygaard HB, Coffey EE, et al Memory impairment in transgenic Alzheimer mice requires cellular prion protein. Journal of Neuroscience. 2010;30(18):6367–6374. doi: 10.1523/JNEUROSCI.0395-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J, Yong KXX, Apostolova LG, et al New insights into atypical Alzheimer's disease in the era of biomarkers. The Lancet Neurology. 2021;20(3):222–234. doi: 10.1016/S1474-4422(20)30440-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grupe A, Abraham R, Li YH, et al Evidence for novel susceptibility genes for late-onset Alzheimer's disease from a genome-wide association study of putative functional variants. Human Molecular Genetics. 2007;16(8):865–873. doi: 10.1093/hmg/ddm031. [DOI] [PubMed] [Google Scholar]
- Guerreiro R, Wojtas A, Bras J, et al TREM2 variants in Alzheimer's disease . New England Journal of Medicine. 2013;368(2):117–127. doi: 10.1056/NEJMoa1211851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo TT, Zhang DH, Zeng YZ, et al Molecular and cellular mechanisms underlying the pathogenesis of Alzheimer's disease. Molecular Neurodegeneration. 2020;15(1):40. doi: 10.1186/s13024-020-00391-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel H, Mesulam MM, Cuello AC, et al The cholinergic system in the pathophysiology and treatment of Alzheimer's disease. Brain. 2018;141(7):1917–1933. doi: 10.1093/brain/awy132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S, Rudd JA, Hu ZY, et al Analysis of neuronal nitric oxide synthase expression and increasing astrogliosis in the brain of senescence-accelerated-prone 8 mice. International Journal of Neuroscience. 2010;120(9):602–608. doi: 10.3109/00207454.2010.503911. [DOI] [PubMed] [Google Scholar]
- Harada A, Oguchi K, Okabe S, et al Altered microtubule organization in small-calibre axons of mice lacking tau protein . Nature. 1994;369(6480):488–491. doi: 10.1038/369488a0. [DOI] [PubMed] [Google Scholar]
- Hartman RE, Izumi Y, Bales KR, et al Treatment with an amyloid-β antibody ameliorates plaque load, learning deficits, and hippocampal long-term potentiation in a mouse model of Alzheimer's disease. Journal of Neuroscience. 2005;25(26):6213–6220. doi: 10.1523/JNEUROSCI.0664-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto S, Matsuba Y, Kamano N, et al Tau binding protein CAPON induces tau aggregation and neurodegeneration. Nature Communications. 2019;10(1):2394. doi: 10.1038/s41467-019-10278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmann J, Coburn MA, England W, et al. 2019. Development of a chimeric model to study and manipulate human microglia in vivo. Neuron, 103(6): 1016–1033. e10.
- Hauptmann S, Scherping I, Dröse S, et al Mitochondrial dysfunction: an early event in Alzheimer pathology accumulates with age in AD transgenic mice. Neurobiology of Aging. 2009;30(10):1574–1586. doi: 10.1016/j.neurobiolaging.2007.12.005. [DOI] [PubMed] [Google Scholar]
- He ZH, Guo JL, McBride JD, et al Amyloid-β plaques enhance Alzheimer's brain tau-seeded pathologies by facilitating neuritic plaque tau aggregation. Nature Medicine. 2018;24(1):29–38. doi: 10.1038/nm.4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronner G, Eisele YS, Langer F, et al Seeded strain-like transmission of β-amyloid morphotypes in APP transgenic mice. EMBO Reports. 2013;14(11):1017–1022. doi: 10.1038/embor.2013.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helboe L, Egebjerg J, Barkholt P, et al Early depletion of CA1 neurons and late neurodegeneration in a mouse tauopathy model. Brain Research. 2017;1665:22–35. doi: 10.1016/j.brainres.2017.04.002. [DOI] [PubMed] [Google Scholar]
- Heneka MT, Kummer MP, Stutz A, et al NLRP3 is activated in Alzheimer's disease and contributes to pathology in APP/PS1 mice. Nature. 2013;493(7434):674–678. doi: 10.1038/nature11729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes BB, Furman JL, Mahan TE, et al Proteopathic tau seeding predicts tauopathy in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(41):E4376–E4385. doi: 10.1073/pnas.1411649111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homanics GE, Park JE, Bailey L, et al Early molecular events of autosomal-dominant Alzheimer's disease in marmosets with PSEN1 mutations. Alzheimer's & Dementia. 2024;20(5):3455–3471. doi: 10.1002/alz.13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover BR, Reed MN, Su JJ, et al Tau mislocalization to dendritic spines mediates synaptic dysfunction independently of neurodegeneration. Neuron. 2010;68(6):1067–1081. doi: 10.1016/j.neuron.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsia AY, Masliah E, McConlogue L, et al Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proceedings of the National Academy of Sciences of the United States of America. 1999;96(6):3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao K, Chapman P, Nilsen S, et al Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science. 1996;274(5284):99–103. doi: 10.1126/science.274.5284.99. [DOI] [PubMed] [Google Scholar]
- Hsieh H, Boehm J, Sato C, et al AMPAR removal underlies Aβ-induced synaptic depression and dendritic spine loss. Neuron. 2006;52(5):831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang SM, Mouri A, Kokubo H, et al Neprilysin-sensitive synapse-associated amyloid-β peptide oligomers impair neuronal plasticity and cognitive function. Journal of Biological Chemistry. 2006;281(26):17941–17951. doi: 10.1074/jbc.M601372200. [DOI] [PubMed] [Google Scholar]
- Hull C, Dekeryte R, Koss DJ, et al Knock-in of mutated hTAU causes insulin resistance, inflammation and proteostasis disturbance in a mouse model of frontotemporal dementia . Molecular Neurobiology. 2020;57(1):539–550. doi: 10.1007/s12035-019-01722-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton M, Lendon CL, Rizzu P, et al Association of missense and 5'-splice-site mutations in tau with the inherited dementia FTDP-17 . Nature. 1998;393(6686):702–705. doi: 10.1038/31508. [DOI] [PubMed] [Google Scholar]
- Huynh TPV, Wang C, Tran AC, et al Lack of hepatic apoE does not influence early Aβ deposition: observations from a new APOE knock-in model . Molecular Neurodegeneration. 2019;14(1):37. doi: 10.1186/s13024-019-0337-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyun Yi J, Cho E, Lee S, et al Aβ dissociation by pectolinarin may counteract against Aβ-induced synaptic dysfunction and memory impairment. Biochemical Pharmacology. 2023;216:115792. doi: 10.1016/j.bcp.2023.115792. [DOI] [PubMed] [Google Scholar]
- Iba M, Guo JL, McBride JD, et al Synthetic tau fibrils mediate transmission of neurofibrillary tangles in a transgenic mouse model of Alzheimer's-like tauopathy. Journal of Neuroscience. 2013;33(3):1024–1037. doi: 10.1523/JNEUROSCI.2642-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel MA, Yuan SH, Bardy C, et al Probing sporadic and familial Alzheimer's disease using induced pluripotent stem cells. Nature. 2012;482(7384):216–220. doi: 10.1038/nature10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito HT, Moser EI, Moser MB. 2018. Supramammillary nucleus modulates spike-time coordination in the prefrontal-thalamo-hippocampal circuit during navigation. Neuron, 99(3): 576–587. e5.
- Ittner A, Chua SW, Bertz J, et al Site-specific phosphorylation of tau inhibits amyloid-β toxicity in Alzheimer's mice. Science. 2016;354(6314):904–908. doi: 10.1126/science.aah6205. [DOI] [PubMed] [Google Scholar]
- Ittner LM, Ke YD, Delerue F, et al Dendritic function of tau mediates amyloid-β toxicity in Alzheimer's disease mouse models. Cell. 2010;142(3):387–397. doi: 10.1016/j.cell.2010.06.036. [DOI] [PubMed] [Google Scholar]
- Jacobsen JS, Wu CC, Redwine JM, et al Early-onset behavioral and synaptic deficits in a mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(13):5161–5166. doi: 10.1073/pnas.0600948103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Fadale DJ, Anderson J, et al Mutant presenilins specifically elevate the levels of the 42 residue β-amyloid peptide in vivo: evidence for augmentation of a 42-specific γ secretase . Human Molecular Genetics. 2004;13(2):159–170. doi: 10.1093/hmg/ddh019. [DOI] [PubMed] [Google Scholar]
- Jankowsky JL, Slunt HH, Ratovitski T, et al Co-expression of multiple transgenes in mouse CNS: a comparison of strategies. Biomolecular Engineering. 2001;17(6):157–165. doi: 10.1016/S1389-0344(01)00067-3. [DOI] [PubMed] [Google Scholar]
- Jarre A, Gowert NS, Donner L, et al Pre-activated blood platelets and a pro-thrombotic phenotype in APP23 mice modeling Alzheimer's disease. Cellular Signalling. 2014;26(9):2040–2050. doi: 10.1016/j.cellsig.2014.05.019. [DOI] [PubMed] [Google Scholar]
- Jawhar S, Trawicka A, Jenneckens C, et al. 2012. Motor deficits, neuron loss, and reduced anxiety coinciding with axonal degeneration and intraneuronal Aβ aggregation in the 5XFAD mouse model of Alzheimer's disease. Neurobiology of Aging, 33(1): 196. e29–196. e40.
- Jiang HW, Jayadev S, Lardelli M, et al A review of the familial Alzheimer's Disease locus PRESENILIN 2 and its relationship to PRESENILIN 1. Journal of Alzheimer's Disease. 2018;66(4):1323–1339. doi: 10.3233/JAD-180656. [DOI] [PubMed] [Google Scholar]
- Jiang ZQ, Wang J, Qin YP, et al A nonhuman primate model with Alzheimer's disease-like pathology induced by hippocampal overexpression of human tau. Alzheimer's Research & Therapy. 2024;16(1):22. doi: 10.1186/s13195-024-01392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jo S, Yarishkin O, Hwang YJ, et al GABA from reactive astrocytes impairs memory in mouse models of Alzheimer's disease. Nature Medicine. 2014;20(8):886–896. doi: 10.1038/nm.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ECB, Bian SJ, Haque RU, et al Cerebrospinal fluid proteomics define the natural history of autosomal dominant Alzheimer's disease. Nature Medicine. 2023;29(8):1979–1988. doi: 10.1038/s41591-023-02476-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson NR, Condello C, Guan SH, et al Evidence for sortilin modulating regional accumulation of human tau prions in transgenic mice. Proceedings of the National Academy of Sciences of the United States of America. 2017;114(51):E11029–E11036. doi: 10.1073/pnas.1717193114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Stefansson H, Steinberg S, et al Variant of TREM2 associated with the risk of Alzheimer's disease . The New England Journal of Medicine. 2013;368(2):107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalhor R, Kalhor K, Mejia L, et al Developmental barcoding of whole mouse via homing CRISPR. Science. 2018;361(6405):893. doi: 10.1126/science.aat9804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalueff AV, Stewart AM, Gerlai R Zebrafish as an emerging model for studying complex brain disorders. Trends in Pharmacological Sciences. 2014;35(2):63–75. doi: 10.1016/j.tips.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamat PK, Rai S, Swarnkar S, et al Okadaic acid-induced Tau phosphorylation in rat brain: role of NMDA receptor. Neuroscience. 2013;238:97–113. doi: 10.1016/j.neuroscience.2013.01.075. [DOI] [PubMed] [Google Scholar]
- Kamphuis W, Mamber C, Moeton M, et al GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease. PLoS One. 2012;7(8):e42823. doi: 10.1371/journal.pone.0042823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karran E, De Strooper B The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nature Reviews Drug Discovery. 2022;21(4):306–318. doi: 10.1038/s41573-022-00391-w. [DOI] [PubMed] [Google Scholar]
- Kastanenka KV, Bussiere T, Shakerdge N, et al Immunotherapy with Aducanumab Restores Calcium Homeostasis in Tg2576 Mice. Journal of Neuroscience. 2016;36(50):12549–12558. doi: 10.1523/JNEUROSCI.2080-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamata T, Akiguchi I, Maeda K, et al Age-related changes in the brains of Senescence-Accelerated Mice (SAM): association with glial and endothelial reactions. Microscopy Research and Technique. 1998;43(1):59–67. doi: 10.1002/(SICI)1097-0029(19981001)43:1<59::AID-JEMT9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Kawarabayashi T, Younkin LH, Saido TC, et al Age-dependent changes in brain, CSF, and plasma amyloid β protein in the Tg2576 transgenic mouse model of Alzheimer's disease. Journal of Neuroscience. 2001;21(2):372–381. doi: 10.1523/JNEUROSCI.21-02-00372.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly PH, Bondolfi L, Hunziker D, et al Progressive age-related impairment of cognitive behavior in APP23 transgenic mice. Neurobiology of Aging. 2003;24(2):365–378. doi: 10.1016/S0197-4580(02)00098-2. [DOI] [PubMed] [Google Scholar]
- Keren-Shaul H, Spinrad A, Weiner A, et al. 2017. A unique microglia type associated with restricting development of Alzheimer's disease. Cell, 169(7): 1276–1290. e17.
- Khani A, Rainer G Recognition memory in tree shrew ( Tupaia belangeri) after repeated familiarization sessions . Behavioural Processes. 2012;90(3):364–371. doi: 10.1016/j.beproc.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Kilgore M, Miller CA, Fass DM, et al Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer's disease. Neuropsychopharmacology. 2010;35(4):870–880. doi: 10.1038/npp.2009.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura R, Ohno M Impairments in remote memory stabilization precede hippocampal synaptic and cognitive failures in 5XFAD Alzheimer mouse model. Neurobiology of Disease. 2009;33(2):229–235. doi: 10.1016/j.nbd.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Castellano JM, Jiang H, et al Overexpression of low-density lipoprotein receptor in the brain markedly inhibits amyloid deposition and increases extracellular Aβ clearance. Neuron. 2009;64(5):632–644. doi: 10.1016/j.neuron.2009.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King DL, Arendash GW, Crawford F, et al Progressive and gender-dependent cognitive impairment in the APP SW transgenic mouse model for Alzheimer's disease . Behavioural Brain Research. 1999;103(2):145–162. doi: 10.1016/S0166-4328(99)00037-6. [DOI] [PubMed] [Google Scholar]
- Kitazawa M, Oddo S, Yamasaki TR, et al Lipopolysaccharide-induced inflammation exacerbates tau pathology by a cyclin-dependent kinase 5-mediated pathway in a transgenic model of Alzheimer's disease. Journal of Neuroscience. 2005;25(39):8843–8853. doi: 10.1523/JNEUROSCI.2868-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knopman DS, Amieva H, Petersen RC, et al Alzheimer disease. Nature Reviews Disease Primers. 2021;7(1):33. doi: 10.1038/s41572-021-00269-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koffie RM, Meyer-Luehmann M, Hashimoto T, et al Oligomeric amyloid β associates with postsynaptic densities and correlates with excitatory synapse loss near senile plaques. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(10):4012–4017. doi: 10.1073/pnas.0811698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopeikina KJ, Wegmann S, Pitstick R, et al Tau causes synapse loss without disrupting calcium homeostasis in the rTg4510 model of tauopathy. PLoS One. 2013;8(11):e80834. doi: 10.1371/journal.pone.0080834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss DJ, Robinson L, Drever BD, et al Mutant Tau knock-in mice display frontotemporal dementia relevant behaviour and histopathology. Neurobiology of Disease. 2016;91(21):105–123. doi: 10.1016/j.nbd.2016.03.002. [DOI] [PubMed] [Google Scholar]
- Kotredes KP, Oblak A, Pandey RS, et al Uncovering disease mechanisms in a novel mouse model expressing humanized APOEε4 and Trem2*R47H. Frontiers in Aging Neuroscience. 2021;13:735524. doi: 10.3389/fnagi.2021.735524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauthausen M, Kummer MP, Zimmermann J, et al CXCR3 promotes plaque formation and behavioral deficits in an Alzheimer's disease model. The Journal of Clinical Investigation. 2015;125(1):365–378. doi: 10.1172/JCI66771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwart D, Gregg A, Scheckel C, et al. 2019. A large panel of isogenic APP and PSEN1 mutant human ipsc neurons reveals shared endosomal abnormalities mediated by APP β-CTFs, Not Aβ. Neuron, 104(2): 256–270. e5.
- Lalonde R The neurobiological basis of spontaneous alternation. Neuroscience & Biobehavioral Reviews. 2002;26(1):91–104. doi: 10.1016/s0149-7634(01)00041-0. [DOI] [PubMed] [Google Scholar]
- Lambert JC, Ibrahim-Verbaas CA, Harold D, et al Meta-analysis of 74, 046 individuals identifies 11 new susceptibility loci for Alzheimer's disease. Nature Genetics. 2013;45(12):1452–1458. doi: 10.1038/ng.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lasagna-Reeves CA, de Haro M, Hao S, et al Reduction of Nuak1 decreases tau and reverses phenotypes in a tauopathy mouse model. Neuron. 2016;92(2):407–418. doi: 10.1016/j.neuron.2016.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei P, Ayton S, Appukuttan AT, et al Lithium suppression of tau induces brain iron accumulation and neurodegeneration. Molecular Psychiatry. 2017;22(3):396–406. doi: 10.1038/mp.2016.96. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Finkelstein DI, et al Tau deficiency induces parkinsonism with dementia by impairing APP-mediated iron export. Nature Medicine. 2012;18(2):291–295. doi: 10.1038/nm.2613. [DOI] [PubMed] [Google Scholar]
- Lei P, Ayton S, Moon S, et al Motor and cognitive deficits in aged tau knockout mice in two background strains. Molecular Neurodegeneration. 2014;9:29. doi: 10.1186/1750-1326-9-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy E, Carman MD, Fernandez-Madrid IJ, et al Mutation of the Alzheimer's disease amyloid gene in hereditary cerebral hemorrhage, Dutch type. Science. 1990;248(4959):1124–1126. doi: 10.1126/science.2111584. [DOI] [PubMed] [Google Scholar]
- Lewis TL, Cao DF, Lu HL, et al Overexpression of human apolipoprotein A-I preserves cognitive function and attenuates neuroinflammation and cerebral amyloid angiopathy in a mouse model of Alzheimer disease. Journal of Biological Chemistry. 2010;285(47):36958–36968. doi: 10.1074/jbc.M110.127829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Wetten S, Li L, et al. 2008. Candidate single-nucleotide polymorphisms from a genomewide association study of Alzheimer disease. Archives of Neurolog 65(1): 45–53.
- Li HL, Xiang BL, Li X, et al Cognitive deficits and Alzheimer's disease-like pathologies in the aged Chinese tree shrew. Molecular Neurobiology. 2024a;61(4):1892–1906. doi: 10.1007/s12035-023-03663-7. [DOI] [PubMed] [Google Scholar]
- Li ML, Wu SH, Song B, et al Single-cell analysis reveals transcriptomic reprogramming in aging primate entorhinal cortex and the relevance with Alzheimer's disease. Aging Cell. 2022a;21(11):e13723. doi: 10.1111/acel.13723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MQ, Guan MF, Lin JB, et al Early blood immune molecular alterations in cynomolgus monkeys with a PSEN1 mutation causing familial Alzheimer's disease. Alzheimer’s & Dementia. 2024b;20(8):5492–5510. doi: 10.1002/alz.14046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, Lei P, Tuo Q, et al Enduring elevations of hippocampal amyloid precursor protein and iron are features of β-amyloid toxicity and are mediated by tau. Neurotherapeutics. 2015;12(4):862–873. doi: 10.1007/s13311-015-0378-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZH, He XP, Wu SH, et al. 2022b. Naturally occurring Alzheimer’s disease in rhesus monkeys. BioRxiv.
- Lian H, Litvinchuk A, Chiang ACA, et al Astrocyte-microglia cross talk through complement activation modulates amyloid pathology in mouse models of Alzheimer's disease. Journal of Neuroscience. 2016;36(2):577–589. doi: 10.1523/JNEUROSCI.2117-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin N, Pan XD, Chen AQ, et al Tripchlorolide improves age-associated cognitive deficits by reversing hippocampal synaptic plasticity impairment and NMDA receptor dysfunction in SAMP8 mice. Behavioural Brain Research. 2014;258:8–18. doi: 10.1016/j.bbr.2013.10.010. [DOI] [PubMed] [Google Scholar]
- Link CD C. elegans models of age-associated neurodegenerative diseases: lessons from transgenic worm models of Alzheimer's disease . Experimental Gerontology. 2006;41(10):1007–1013. doi: 10.1016/j.exger.2006.06.059. [DOI] [PubMed] [Google Scholar]
- Litvinchuk A, Wan YW, Swartzlander DB, et al. 2018. Complement C3aR inactivation attenuates tau pathology and reverses an immune network deregulated in tauopathy models and Alzheimer's disease. Neuron, 100(6): 1337–1353. e5.
- Liu H, Mei F, Ye RR, et al APOE3ch alleviates Aβ and tau pathology and neurodegeneration in the human APP NL-G-F cerebral organoid model of Alzheimer's disease . Cell Research. 2024;34(6):451–454. doi: 10.1038/s41422-024-00957-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Drouet V, Wu JW, et al Trans-synaptic spread of tau pathology in vivo . PLoS One. 2012;7(2):e31302. doi: 10.1371/journal.pone.0031302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu WF, Taso O, Wang R, et al Trem2 promotes anti-inflammatory responses in microglia and is suppressed under pro-inflammatory conditions . Human Molecular Genetics. 2020;29(19):3224–3248. doi: 10.1093/hmg/ddaa209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T, Trinh MA, Wexler AJ, et al Suppression of eIF2α kinases alleviates Alzheimer's disease-related plasticity and memory deficits. Nature Neuroscience. 2013;16(9):1299–1305. doi: 10.1038/nn.3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson KP, Sompol P, Kannarkat GT, et al Peripheral administration of the soluble TNF inhibitor XPro1595 modifies brain immune cell profiles, decreases beta-amyloid plaque load, and rescues impaired long-term potentiation in 5xFAD mice. Neurobiology of Disease. 2017;102:81–95. doi: 10.1016/j.nbd.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso R, Fattorelli N, Martinez-Muriana A, et al Xenografted human microglia display diverse transcriptomic states in response to Alzheimer's disease-related amyloid-β pathology. Neurobiology of Disease. 2024;27(5):886–900. doi: 10.1038/s41593-024-01600-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manczak M, Anekonda TS, Henson E, et al Mitochondria are a direct site of Aβ accumulation in Alzheimer's disease neurons: implications for free radical generation and oxidative damage in disease progression. Human Molecular Genetics. 2006;15(9):1437–1449. doi: 10.1093/hmg/ddl066. [DOI] [PubMed] [Google Scholar]
- Mangiafico SP, Tuo QZ, Li XL, et al Tau suppresses microtubule-regulated pancreatic insulin secretion. Molecular Psychiatry. 2023;28(9):3982–3993. doi: 10.1038/s41380-023-02267-w. [DOI] [PubMed] [Google Scholar]
- Mann KM, Thorngate FE, Katoh-Fukui Y, et al. 2004. Independent effects of APOE on cholesterol metabolism and brain Aβ levels in an Alzheimer disease mouse model. Human Molecular Genetics 13(17): 1959–1968.
- Marazuela P, Paez-Montserrat B, Bonaterra-Pastra A, et al Impact of cerebral amyloid angiopathy in two transgenic mouse models of cerebral β-amyloidosis: a neuropathological study. International Journal of Molecular Sciences. 2022;23(9):4972. doi: 10.3390/ijms23094972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda A, Kobayashi Y, Kogo N, et al Cognitive deficits in single App knock-in mouse models. Neurobiology of Learning and Memory. 2016;135:73–82. doi: 10.1016/j.nlm.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Mathys H, Boix CA, Akay LA, et al Single-cell multiregion dissection of Alzheimer's disease. Nature. 2024;632(8026):858–868. doi: 10.1038/s41586-024-07606-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathys H, Peng ZY, Boix CA, et al. 2023. Single-cell atlas reveals correlates of high cognitive function, dementia, and resilience to Alzheimer's disease pathology. Cell, 186(20): 4365–4385. e27.
- McClean PL, Parthsarathy V, Faivre E, et al The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer's disease. Journal of Neuroscience. 2011;31(17):6587–6594. doi: 10.1523/JNEUROSCI.0529-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna A, Findlay GM, Gagnon JA, et al Whole-organism lineage tracing by combinatorial and cumulative genome editing. Science. 2016;353(6298):aaf7907. doi: 10.1126/science.aaf7907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng XL, Shen F, Li CL, et al Depression-like behaviors in tree shrews and comparison of the effects of treatment with fluoxetine and carbetocin. Pharmacology Biochemistry and Behavior. 2016;145:1–8. doi: 10.1016/j.pbb.2016.03.006. [DOI] [PubMed] [Google Scholar]
- Merlini M, Rafalski VA, Rios Coronado PE, et al. 2019. Fibrinogen induces microglia-mediated spine elimination and cognitive impairment in an Alzheimer's disease model. Neuron, 101(6): 1099–1108. e6.
- Meyer-Luehmann M, Spires-Jones TL, Prada C, et al Rapid appearance and local toxicity of amyloid-β plaques in a mouse model of Alzheimer's disease. Nature. 2008;451(7179):720–724. doi: 10.1038/nature06616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner MT, Maddugoda M, Götz J, et al The NLRP3 inflammasome triggers sterile neuroinflammation and Alzheimer's disease. Current Opinion in Immunology. 2021;68:116–124. doi: 10.1016/j.coi.2020.10.011. [DOI] [PubMed] [Google Scholar]
- Minkeviciene R, Ihalainen J, Malm T, et al Age-related decrease in stimulated glutamate release and vesicular glutamate transporters in APP/PS1 transgenic and wild-type mice. Journal of Neurochemistry. 2008;105(3):584–594. doi: 10.1111/j.1471-4159.2007.05147.x. [DOI] [PubMed] [Google Scholar]
- Minkeviciene R, Rheims S, Dobszay MB, et al Amyloid β-induced neuronal hyperexcitability triggers progressive epilepsy. Journal of Neuroscience. 2009;29(11):3453–3462. doi: 10.1523/JNEUROSCI.5215-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani Y, Yarimizu J, Saita K, et al Differential effects between γ-secretase inhibitors and modulators on cognitive function in amyloid precursor protein-transgenic and nontransgenic mice. Journal of Neuroscience. 2012;32(6):2037–2050. doi: 10.1523/JNEUROSCI.4264-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore KM, Nicholas J, Grossman M, et al Age at symptom onset and death and disease duration in genetic frontotemporal dementia: an international retrospective cohort study. The Lancet Neurology. 2020;19(2):145–156. doi: 10.1016/S1474-4422(19)30394-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley JE, Farr SA, Kumar VB, et al The SAMP8 mouse: a model to develop therapeutic interventions for Alzheimer's disease. Current Pharmaceutical Design. 2012;18(8):1123–1130. doi: 10.2174/138161212799315795. [DOI] [PubMed] [Google Scholar]
- Murphy C Olfactory and other sensory impairments in Alzheimer disease. Nature Reviews Neurology. 2019;15(1):11–24. doi: 10.1038/s41582-018-0097-5. [DOI] [PubMed] [Google Scholar]
- Nair J, Topka M, Khani A, et al Tree shrews ( Tupaia belangeri) exhibit novelty preference in the novel location memory task with 24-h retention periods . Frontiers in Psychology. 2014;5:303. doi: 10.3389/fpsyg.2014.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S, Takahashi T, Yamashita H, et al Nicotinic acetylcholine receptors and neurodegenerative disease. Alcohol. 2001;24(2):79–81. doi: 10.1016/S0741-8329(01)00150-1. [DOI] [PubMed] [Google Scholar]
- Ni RJ, Huang ZH, Luo PH, et al The tree shrew cerebellum atlas: systematic nomenclature, neurochemical characterization, and afferent projections. Journal of Comparative Neurology. 2018a;526(17):2744–2775. doi: 10.1002/cne.24526. [DOI] [PubMed] [Google Scholar]
- Ni RJ, Huang ZH, Shu YM, et al Atlas of the striatum and globus pallidus in the tree shrew: comparison with rat and mouse. Neuroscience Bulletin. 2018b;34(3):405–418. doi: 10.1007/s12264-018-0212-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni RJ, Tian Y, Dai XY, et al Social avoidance behavior in male tree shrews and prosocial behavior in male mice toward unfamiliar conspecifics in the laboratory. Zoological Research. 2020;41(3):258–272. doi: 10.24272/j.issn.2095-8137.2020.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson P, Loganathan K, Sekiguchi M, et al Aβ secretion and plaque formation depend on autophagy. Cell Reports. 2013;5(1):61–69. doi: 10.1016/j.celrep.2013.08.042. [DOI] [PubMed] [Google Scholar]
- Nobili A, Latagliata EC, Viscomi MT, et al Dopamine neuronal loss contributes to memory and reward dysfunction in a model of Alzheimer's disease. Nature Communications. 2017;8(1):14727. doi: 10.1038/ncomms14727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, et al Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. Journal of Neuroscience. 2006;26(40):10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oblak AL, Lin PB, Kotredes KP, et al Comprehensive evaluation of the 5XFAD mouse model for preclinical testing applications: a MODEL-AD study. Frontiers in Aging Neuroscience. 2021;13:713726. doi: 10.3389/fnagi.2021.713726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Cheng D, et al Genetically augmenting tau levels does not modulate the onset or progression of Aβ pathology in transgenic mice. Journal of Neurochemistry. 2007;102(4):1053–1063. doi: 10.1111/j.1471-4159.2007.04607.x. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Kitazawa M, et al Amyloid deposition precedes tangle formation in a triple transgenic model of Alzheimer's disease. Neurobiology of Aging. 2003a;24(8):1063–1070. doi: 10.1016/j.neurobiolaging.2003.08.012. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Shepherd JD, et al Triple-transgenic model of Alzheimer's disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron. 2003b;39(3):409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tran L, et al Temporal profile of amyloid-β (Aβ) oligomerization in an in vivo model of Alzheimer disease-A link between Aβ and tau pathology . Journal of Biological Chemistry. 2006;281(3):1599–1604. doi: 10.1074/jbc.M507892200. [DOI] [PubMed] [Google Scholar]
- Oddo S, Caccamo A, Tseng B, et al Blocking Aβ 42 accumulation delays the onset and progression of tau pathology via the c terminus of heat shock protein70-interacting protein: a mechanistic link between Aβ and tau pathology . Journal of Neuroscience. 2008;28(47):12163–12175. doi: 10.1523/JNEUROSCI.2464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogomori K, Kitamoto T, Tateishi J, et al Beta-protein amyloid is widely distributed in the central nervous system of patients with Alzheimer's disease. The American Journal of Pathology. 1989;134(2):243–251. [PMC free article] [PubMed] [Google Scholar]
- Ogunsuyi OB, Olasehinde TA, Oboh G Neuroprotective properties of solanum leaves in transgenic Drosophila melanogaster model of Alzheimer's disease . Biomarkers. 2022;27(6):587–598. doi: 10.1080/1354750X.2022.2077446. [DOI] [PubMed] [Google Scholar]
- O'Leary TP, Robertson A, Chipman PH, et al Motor function deficits in the 12 month-old female 5xFAD mouse model of Alzheimer's disease. Behavioural Brain Research. 2018;337:256–263. doi: 10.1016/j.bbr.2017.09.009. [DOI] [PubMed] [Google Scholar]
- O'Leary TP, Shin S, Fertan E, et al. 2017. Reduced acoustic startle response and peripheral hearing loss in the 5xFAD mouse model of Alzheimer's disease. Genes, Brain and Behavior, 16(5): 554–563.
- Pačesová A, Holubová M, Hrubá L, et al Age-related metabolic and neurodegenerative changes in SAMP8 mice. Aging. 2022;14(18):7300–7327. doi: 10.18632/aging.204284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmanabhan P, Götz J Clinical relevance of animal models in aging-related dementia research. Nature Aging. 2023;3(5):481–493. doi: 10.1038/s43587-023-00402-4. [DOI] [PubMed] [Google Scholar]
- Palencia-Madrid L, Sánchez-Valle R, Fernández De Retana I, et al. 2019. A unique common ancestor introduced P301L mutation in MAPT gene in frontotemporal dementia patients from Barcelona (Baix Llobregat, Spain). Neurobiology of Aging, 84: 236. e9–236. e15.
- Pan LN, Li CR, Meng LX, et al Tau accelerates α-synuclein aggregation and spreading in Parkinson's disease. Brain. 2022;145(10):3454–3471. doi: 10.1093/brain/awac171. [DOI] [PubMed] [Google Scholar]
- Pang KL, Jiang RC, Zhang W, et al An App knock-in rat model for Alzheimer's disease exhibiting Aβ and tau pathologies, neuronal death and cognitive impairments . Cell Research. 2022;32(2):157–175. doi: 10.1038/s41422-021-00582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park BN, Lim TS, Yoon JK, et al In vivo tracking of intravenously injected mesenchymal stem cells in an Alzheimer's animal model. Cell Transplantation. 2018;27(8):1203–1209. doi: 10.1177/0963689718788067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlik M, Fuchs E, Walker LC, et al Primate-like amyloid-βsequence but no cerebral amyloidosis in aged tree shrews. Neurobiology of Aging. 1999;20(1):47–51. doi: 10.1016/S0197-4580(99)00017-2. [DOI] [PubMed] [Google Scholar]
- Peretti D, Bastide A, Radford H, et al RBM3 mediates structural plasticity and protective effects of cooling in neurodegeneration. Nature. 2015;518(7538):236–239. doi: 10.1038/nature14142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Tur J, Froelich S, Prihar G, et al A mutation in Alzheimer's disease destroying a splice acceptor site in the presenilin-1 gene. NeuroReport. 1995;7(1):297–301. doi: 10.1097/00001756-199512000-00071. [DOI] [PubMed] [Google Scholar]
- Perlman RL. 2016. Mouse models of human disease: an evolutionary perspective. Evolution, Medicine, and Public Health, 2016(1): 170–176.
- Peters F, Salihoglu H, Pratsch K, et al Tau deletion reduces plaque-associated BACE1 accumulation and decelerates plaque formation in a mouse model of Alzheimer's disease. The EMBO Journal. 2019;38(23):e102345. doi: 10.15252/embj.2019102345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poduri A, Gearing M, Rebeck GW, et al Apolipoprotein E4 and beta amyloid in senile plaques and cerebral blood vessels of aged rhesus monkeys. The American Journal of Pathology. 1994;144(6):1183–1187. [PMC free article] [PubMed] [Google Scholar]
- Qian ZJ, Wang ZH, Li BW, et al Thy1-ApoE4/C/EBPβ double transgenic mice act as a sporadic model with Alzheimer's disease. Molecular Psychiatry. 2024;29(10):3040–3055. doi: 10.1038/s41380-024-02565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radde R, Bolmont T, Kaeser SA, et al Aβ42-driven cerebral amyloidosis in transgenic mice reveals early and robust pathology. EMBO Reports. 2006;7(9):940–946. doi: 10.1038/sj.embor.7400784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsden M, Kotilinek L, Forster C, et al Age-dependent neurofibrillary tangle formation, neuron loss, and memory impairment in a mouse model of human tauopathy (P301L) Journal of Neuroscience. 2005;25(46):10637–10647. doi: 10.1523/JNEUROSCI.3279-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo SJS, Homanics GE, Park JE, et al Establishing the marmoset as a non-human primate model of Alzheimer’s disease. Alzheimer's & Dementia. 2021;17(S3):e049952. [Google Scholar]
- Roberson ED, Scearce-Levie K, Palop JJ, et al Reducing endogenous tau ameliorates amyloid ß-induced deficits in an Alzheimer's disease mouse model. Science. 2007;316(5825):750–754. doi: 10.1126/science.1141736. [DOI] [PubMed] [Google Scholar]
- Roder S, Danober L, Pozza MF, et al Electrophysiological studies on the hippocampus and prefrontal cortex assessing the effects of amyloidosis in amyloid precursor protein 23 transgenic mice. Neuroscience. 2003;120(3):705–720. doi: 10.1016/S0306-4522(03)00381-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg RN, Fu M, Lambracht-Washington D Active full-length DNA Aβ 42 immunization in 3xTg-AD mice reduces not only amyloid deposition but also tau pathology . Alzheimer's Research & Therapy. 2018;10(1):115. doi: 10.1186/s13195-018-0441-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell ES, Freire-Cobo C, Varghese M, et al The marmoset as an important primate model for longitudinal studies of neurocognitive aging. American Journal of Primatology. 2021;83(11):e23271. doi: 10.1002/ajp.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy DS, Arons A, Mitchell TI, et al Memory retrieval by activating engram cells in mouse models of early Alzheimer's disease. Nature. 2016;531(7595):508–512. doi: 10.1038/nature17172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui WJ, Xiao H, Fan Y, et al Systemic inflammasome activation and pyroptosis associate with the progression of amnestic mild cognitive impairment and Alzheimer's disease. Journal of Neuroinflammation. 2021;18(1):280. doi: 10.1186/s12974-021-02329-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sain H, Sharma B, Jaggi AS, et al Pharmacological investigations on potential of peroxisome proliferator-activated receptor-gamma agonists in hyperhomocysteinemia-induced vascular dementia in rats. Neuroscience. 2011;192:322–333. doi: 10.1016/j.neuroscience.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Saito T, Matsuba Y, Mihira N, et al Single App knock-in mouse models of Alzheimer's disease . Nature Neuroscience. 2014;17(5):661–663. doi: 10.1038/nn.3697. [DOI] [PubMed] [Google Scholar]
- Saito T, Mihira N, Matsuba Y, et al Humanization of the entire murine Mapt gene provides a murine model of pathological human tau propagation . Journal of Biological Chemistry. 2019;294(34):12754–12765. doi: 10.1074/jbc.RA119.009487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders DW, Kaufman SK, Devos SL, et al Distinct tau prion strains propagate in cells and mice and define different tauopathies. Neuron. 2014;82(6):1271–1288. doi: 10.1016/j.neuron.2014.04.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sani S, Traul D, Klink A, et al Distribution, progression and chemical composition of cortical amyloid-β deposits in aged rhesus monkeys: similarities to the human. Acta Neuropathologica. 2003;105(2):145–156. doi: 10.1007/s00401-002-0626-5. [DOI] [PubMed] [Google Scholar]
- Santacruz K, Lewis J, Spires T, et al Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaguri H, Nilsson P, Hashimoto S, et al APP mouse models for Alzheimer's disease preclinical studies. The EMBO Journal. 2017;36(17):2473–2487. doi: 10.15252/embj.201797397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki A, Shoji M, Harigaya Y, et al Amyloid cored plaques in Tg2576 transgenic mice are characterized by giant plaques, slightly activated microglia, and the lack of paired helical filament-typed, dystrophic neurites. Virchows Archiv. 2002;441(4):358–367. doi: 10.1007/s00428-002-0643-8. [DOI] [PubMed] [Google Scholar]
- Sato K, Sasaguri H, Kumita W, et al. 2020. A non-human primate model of familial Alzheimer’s disease. BioRxiv.
- Sawano E, Negishi T, Aoki T, et al Alterations in local thyroid hormone signaling in the hippocampus of the SAMP8 mouse at younger ages: association with delayed myelination and behavioral abnormalities. Journal of Neuroscience Research. 2013;91(3):382–392. doi: 10.1002/jnr.23161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott HA, Gebhardt FM, Mitrovic AD, et al. 2011. Glutamate transporter variants reduce glutamate uptake in Alzheimer's disease. Neurobiology of Aging, 32(3): 553. e11.
- Seeley RJ, MacDougald OA Mice as experimental models for human physiology: when several degrees in housing temperature matter. Nature Metabolism. 2021;3(4):443–445. doi: 10.1038/s42255-021-00372-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevigny J, Chiao P, Bussière T, et al The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature. 2016;537(7618):50–56. doi: 10.1038/nature19323. [DOI] [PubMed] [Google Scholar]
- Sharma M, Burré J, Südhof TC Proteasome inhibition alleviates SNARE-dependent neurodegeneration. Science Translational Medicine. 2012;4(147):147ra113. doi: 10.1126/scitranslmed.3004028. [DOI] [PubMed] [Google Scholar]
- Shen F, Qi KK, Duan Y, et al Differential effects of clomipramine on depression-like behaviors induced by the chronic social defeat paradigm in tree shrews. Journal of Psychopharmacology. 2018;32(10):1141–1149. doi: 10.1177/0269881118793560. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhang XK, Ni JN, et al. 2018. The influence of GAPT extraction on synapse loss of APPswe/PS1dE9 transgenic mice via adjusting Bcl-2/Bax balance. Alzheimer's & Dementia: Translational Research & Clinical Interventions, 4(1): 724–736.
- Shipton OA, Tang CS, Paulsen O, et al Differential vulnerability of hippocampal CA3-CA1 synapses to Aβ. Acta Neuropathologica Communications. 2022;10(1):45. doi: 10.1186/s40478-022-01350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierksma A, Lu A, Mancuso R, et al Novel Alzheimer risk genes determine the microglia response to amyloid-β but not to TAU pathology. EMBO Molecular Medicine. 2020;12(3):e10606. doi: 10.15252/emmm.201910606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sil A, Erfani A, Lamb N, et al Sex differences in behavior and molecular pathology in the 5XFAD model. Journal of Alzheimer's Disease. 2022;85(2):755–778. doi: 10.3233/JAD-210523. [DOI] [PubMed] [Google Scholar]
- Sims JR, Zimmer JA, Evans CD, et al Donanemab in early symptomatic alzheimer disease: the TRAILBLAZER-ALZ 2 randomized clinical trial. JAMA. 2023;330(6):512–527. doi: 10.1001/jama.2023.13239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spangenberg E, Severson PL, Hohsfield LA, et al Sustained microglial depletion with CSF1R inhibitor impairs parenchymal plaque development in an Alzheimer's disease model. Nature Communications. 2019;10(1):3758. doi: 10.1038/s41467-019-11674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spires TL, Orne JD, Santacruz K, et al Region-specific dissociation of neuronal loss and neurofibrillary pathology in a mouse model of tauopathy. The American Journal of Pathology. 2006;168(5):1598–1607. doi: 10.2353/ajpath.2006.050840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S, Cacucci F, Lever C Which memory task for my mouse? A systematic review of spatial memory performance in the Tg2576 Alzheimer's mouse model. Journal of Alzheimer's Disease. 2011;26(1):105–126. doi: 10.3233/JAD-2011-101827. [DOI] [PubMed] [Google Scholar]
- Stonebarger GA, Bimonte-Nelson HA, Urbanski HF The rhesus macaque as a translational model for neurodegeneration and Alzheimer's disease. Frontiers in Aging Neuroscience. 2021;13:734173. doi: 10.3389/fnagi.2021.734173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Abramowski D, Duke M, et al Two amyloid precursor protein transgenic mouse models with Alzheimer disease-like pathology. Proceedings of the National Academy of Sciences of the United States of America. 1997;94(24):13287–13292. doi: 10.1073/pnas.94.24.13287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun N, Victor MB, Park YP, et al. 2023. Human microglial state dynamics in Alzheimer's disease progression. Cell, 186(20): 4386–4403. e29.
- Sun Z, Kwon JS, Ren YD, et al Modeling late-onset Alzheimer's disease neuropathology via direct neuronal reprogramming. Science. 2024;385(6708):adl2992. doi: 10.1126/science.adl2992. [DOI] [PubMed] [Google Scholar]
- Takahashi RH, Almeida CG, Kearney PF, et al Oligomerization of Alzheimer's β-amyloid within processes and synapses of cultured neurons and brain. Journal of Neuroscience. 2004;24(14):3592–3599. doi: 10.1523/JNEUROSCI.5167-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi RH, Capetillo-Zarate E, Lin MT, et al Co-occurrence of Alzheimer's disease β-amyloid and tau pathologies at synapses. The Journal of Neuroscience. 2010;31(7):1145–1152. doi: 10.1016/j.neurobiolaging.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda S, Wegmann S, Cho H, et al Neuronal uptake and propagation of a rare phosphorylated high-molecular-weight tau derived from Alzheimer's disease brain. Nature Communications. 2015;6(1):8490. doi: 10.1038/ncomms9490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T, Hosokawa M, Higuchi K Senescence-Accelerated Mouse (SAM): a novel murine model of accelerated senescence. Journal of the American Geriatrics Society. 1991;39(9):911–919. doi: 10.1111/j.1532-5415.1991.tb04460.x. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Iba M, Inoue H, et al P301S mutant human tau transgenic mice manifest early symptoms of human tauopathies with dementia and altered sensorimotor gating. PLoS One. 2011;6(6):e21050. doi: 10.1371/journal.pone.0021050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan DCS, Yao S, Ittner A, et al Generation of a new tau knockout (tau Δex1) line using CRISPR/cas9 genome editing in mice . Journal of Alzheimer's Disease. 2018;62(2):571–578. doi: 10.3233/JAD-171058. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, et al The marmoset as a model of aging and age-related diseases. ILAR Journal. 2011;52(1):54–65. doi: 10.1093/ilar.52.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terwel D, Lasrado R, Snauwaert J, et al Changed conformation of mutant Tau-P301L underlies the moribund tauopathy, absent in progressive, nonlethal axonopathy of Tau-4R/2N transgenic mice. Journal of Biological Chemistry. 2005;280(5):3963–3973. doi: 10.1074/jbc.M409876200. [DOI] [PubMed] [Google Scholar]
- Tong YH, Hao JJ, Tu Q, et al A tree shrew glioblastoma model recapitulates features of human glioblastoma. Oncotarget. 2017;8(11):17897–17907. doi: 10.18632/oncotarget.15225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng BP, Green KN, Chan JL, et al Aβ inhibits the proteasome and enhances amyloid and tau accumulation. Neurobiology of Aging. 2008;29(11):1607–1618. doi: 10.1016/j.neurobiolaging.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumagari R, Maruo K, Nakao T, et al Motor dyscoordination and alteration of functional correlation between DGKγ and PKCγ in senescence-accelerated mouse prone 8 (SAMP8) Frontiers in Aging Neuroscience. 2021;13:573966. doi: 10.3389/fnagi.2021.573966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu ZC, Yan S, Han BF, et al Tauopathy promotes spinal cord-dependent production of toxic amyloid-beta in transgenic monkeys. Signal Transduction and Targeted Therapy. 2023;8(1):358. doi: 10.1038/s41392-023-01601-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KL, Meyer M, Barde YA Neurotrophins are required for nerve growth during development. Nature Neuroscience. 2001;4(1):29–37. doi: 10.1038/82868. [DOI] [PubMed] [Google Scholar]
- Tuo QZ, Lei P, Jackman KA, et al Tau-mediated iron export prevents ferroptotic damage after ischemic stroke. Molecular Psychiatry. 2017;22(11):1520–1530. doi: 10.1038/mp.2017.171. [DOI] [PubMed] [Google Scholar]
- Unger MS, Marschallinger J, Kaindl J, et al Early changes in hippocampal neurogenesis in transgenic mouse models for Alzheimer's disease. Molecular Neurobiology. 2016;53(8):5796–5806. doi: 10.1007/s12035-016-0018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyck CH, Swanson CJ, Aisen P, et al Lecanemab in early Alzheimer’s disease. New England Journal of Medicine. 2023;388(1):9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- Venegas C, Kumar S, Franklin BS, et al Microglia-derived ASC specks cross-seed amyloid-β in Alzheimer's disease. Nature. 2017;552(7685):355–361. doi: 10.1038/nature25158. [DOI] [PubMed] [Google Scholar]
- Vermunt L, Sikkes SaM, Van Den Hout A, et al Duration of preclinical, prodromal, and dementia stages of Alzheimer's disease in relation to age, sex, and APOE genotype . Alzheimer's & Dementia. 2019;15(7):888–898. doi: 10.1016/j.jalz.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viaud-Delmon I, Venault P, Chapouthier G Behavioral models for anxiety and multisensory integration in animals and humans. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2011;35(6):1391–1399. doi: 10.1016/j.pnpbp.2010.09.016. [DOI] [PubMed] [Google Scholar]
- Volianskis A, Køstner R, Mølgaard M, et al Episodic memory deficits are not related to altered glutamatergic synaptic transmission and plasticity in the CA1 hippocampus of the APPswe/PS1ΔE9-deleted transgenic mice model of β-amyloidosis. Neurobiology of Aging. 2010;31(7):1173–1187. doi: 10.1016/j.neurobiolaging.2008.08.005. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nature Protocols. 2006;1(2):848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YW, Al-Ouran R, Mangleburg CG, et al Meta-analysis of the Alzheimer's disease human brain transcriptome and functional dissection in mouse models. Cell Reports. 2020;32(2):107908. doi: 10.1016/j.celrep.2020.107908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Chai AP, Zhou QX, et al Chronic clomipramine treatment reverses core symptom of depression in subordinate tree shrews. PLoS One. 2013;8(12):e80980. doi: 10.1371/journal.pone.0080980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang KW, Zhang G, Kuo MH Frontotemporal dementia P301L mutation potentiates but is not sufficient to cause the formation of cytotoxic fibrils of tau. International Journal of Molecular Sciences. 2023a;24(19):14996. doi: 10.3390/ijms241914996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang MH, Song WM, Ming C, et al Guidelines for bioinformatics of single-cell sequencing data analysis in Alzheimer’s disease: review, recommendation, implementation and application. Molecular Neurodegeneration. 2022;17(1):17. doi: 10.1186/s13024-022-00517-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Yang C, Su JJ, et al Factors influencing long-term hepatitis B virus infection of the tree shrew ( Tupaia belangeri chinensis) as an in vivo model of chronic hepatitis B . Chinese Journal of Hepatology. 2012;20(9):654–658. doi: 10.3760/cma.j.issn.1007-3418.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zhang X, Guo YJ, et al Scopolamine causes delirium-like brain network dysfunction and reversible cognitive impairment without neuronal loss. Zoological Research. 2023b;44(4):712–724. doi: 10.24272/j.issn.2095-8137.2022.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wes PD, Easton A, Corradi J, et al Tau overexpression impacts a neuroinflammation gene expression network perturbed in Alzheimer's disease. PLoS One. 2014;9(8):e106050. doi: 10.1371/journal.pone.0106050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ, Bach G, Søderman A, et al Synaptic contact number and size in stratum radiatum CA1 of APP/PS1ΔE9 transgenic mice . Neurobiology of Aging. 2009;30(11):1756–1776. doi: 10.1016/j.neurobiolaging.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Westerman MA, Cooper-Blacketer D, Mariash A, et al The relationship between Aβ and memory in the Tg2576 mouse model of Alzheimer's disease. Journal of Neuroscience. 2002;22(5):1858–1867. doi: 10.1523/JNEUROSCI.22-05-01858.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu YJ, Wu ZX, Butko P, et al Amyloid-β-induced pathological behaviors are suppressed by Ginkgo biloba extract EGb 761 and ginkgolides in transgenic Caenorhabditis elegans . Journal of Neuroscience. 2006;26(50):13102–13113. doi: 10.1523/JNEUROSCI.3448-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu ZC, Gao JH, Du TF, et al Alpha-synuclein is highly prone to distribution in the hippocampus and midbrain in tree shrews, and its fibrils seed Lewy body-like pathology in primary neurons. Experimental Gerontology. 2019;116:37–45. doi: 10.1016/j.exger.2018.12.008. [DOI] [PubMed] [Google Scholar]
- Xia YY, Wang ZH, Zhang JC, et al C/EBPβ is a key transcription factor for APOE and preferentially mediates ApoE4 expression in Alzheimer's disease . Molecular Psychiatry. 2021;26(10):6002–6022. doi: 10.1038/s41380-020-00956-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong J, Zhang ZT, Ye KQ C/EBPβ/AEP signaling drives Alzheimer's disease pathogenesis. Neuroscience Bulletin. 2023a;39(7):1173–1185. doi: 10.1007/s12264-023-01025-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong XS, James BT, Boix CA, et al. 2023b. Epigenomic dissection of Alzheimer's disease pinpoints causal variants and reveals epigenome erosion. Cell, 186(20): 4422–4437. e21.
- Yagi H, Irino M, Matsushita T, et al Spontaneous spongy degeneration of the brain stem in SAM-P/8 mice, a newly developed memory-deficient strain. Journal of Neuropathology & Experimental Neurology. 1989;48(5):577–590. doi: 10.1097/00005072-198909000-00008. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Fuchs E, Taira M, et al Amyloid beta (Aβ) protein- and amyloid precursor protein (APP)- immunoreactive structures in the brains of aged tree shrews. Current Aging Science. 2010;3(3):230–238. doi: 10.2174/1874609811003030230. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Fuchs E, Taira M, et al Somatostatin-immunoreactive senile plaque-like structures in the frontal cortex and nucleus accumbens of aged tree shrews and Japanese macaques. Journal of Medical Primatology. 2012;41(3):147–157. doi: 10.1111/j.1600-0684.2012.00540.x. [DOI] [PubMed] [Google Scholar]
- Yanai S, Endo S Early onset of behavioral alterations in senescence-accelerated mouse prone 8 (SAMP8) Behavioural Brain Research. 2016;308:187–195. doi: 10.1016/j.bbr.2016.04.026. [DOI] [PubMed] [Google Scholar]
- Yang Y, Kim J, Kim HY, et al Amyloid-β oligomers may impair SNARE-mediated exocytosis by direct binding to syntaxin 1a. Cell Reports. 2015;12(8):1244–1251. doi: 10.1016/j.celrep.2015.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J, Irwin RW, Zhao LQ, et al Mitochondrial bioenergetic deficit precedes Alzheimer's pathology in female mouse model of Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(34):14670–14675. doi: 10.1073/pnas.0903563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao YG, Lu L, Ni RJ, et al Study of tree shrew biology and models: A booming and prosperous field for biomedical research. Zoological Research. 2024;45(4):877–909. doi: 10.24272/j.issn.2095-8137.2024.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye L, Rasmussen J, Kaeser SA, et al Aβ seeding potency peaks in the early stages of cerebral β-amyloidosis. EMBO Reports. 2017;18(9):1536–1544. doi: 10.15252/embr.201744067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshiyama Y, Higuchi M, Zhang B, et al Synapse loss and microglial activation precede tangles in a P301S tauopathy mouse model. Neuron. 2007;53(3):337–351. doi: 10.1016/j.neuron.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Youmans KL, Tai LM, Nwabuisi-Heath E, et al APOE4-specific changes in Aβ accumulation in a new transgenic mouse model of Alzheimer disease . Journal of Biological Chemistry. 2012;287(50):41774–41786. doi: 10.1074/jbc.M112.407957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue M, Hanna A, Wilson J, et al Sex difference in pathology and memory decline in rTg4510 mouse model of tauopathy. Neurobiology of Aging. 2011;32(4):590–603. doi: 10.1016/j.neurobiolaging.2009.04.006. [DOI] [PubMed] [Google Scholar]
- Zhang B, Carroll J, Trojanowski JQ, et al The microtubule-stabilizing agent, epothilone d, reduces axonal dysfunction, neurotoxicity, cognitive deficits, and Alzheimer-like pathology in an interventional study with aged tau transgenic mice. Journal of Neuroscience. 2012;32(11):3601–3611. doi: 10.1523/JNEUROSCI.4922-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chen BA, Lu J, et al Brains of rhesus monkeys display Aβ deposits and glial pathology while lacking Aβ dimers and other Alzheimer's pathologies. Aging Cell. 2019;18(4):e12978. doi: 10.1111/acel.12978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Chen SD, Deng YT, et al Identifying modifiable factors and their joint effect on dementia risk in the UK Biobank. Nature Human Behaviour. 2023;7(7):1185–1195. doi: 10.1038/s41562-023-01585-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ma RH, Li XC, et al Silencing I 2 PP2A rescues tau pathologies and memory deficits through rescuing PP2A and inhibiting GSK-3β signaling in human tau transgenic mice . Frontiers in Aging Neuroscience. 2014;6:123. doi: 10.3389/fnagi.2014.00123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H, Jiang MH, Trumbauer ME, et al β-amyloid precursor protein-deficient mice show reactive gliosis and decreased locomotor activity. Cell. 1995;81(4):525–531. doi: 10.1016/0092-8674(95)90073-X. [DOI] [PubMed] [Google Scholar]
- Zhong MZ, Peng T, Duarte ML, et al Updates on mouse models of Alzheimer's disease. Molecular Neurodegeneration. 2024;19(1):23. doi: 10.1186/s13024-024-00712-0. [DOI] [PMC free article] [PubMed] [Google Scholar]