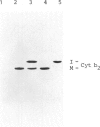
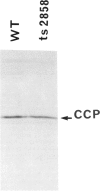
Abstract
The proteolytic processing of the mitochondrially encoded subunit II of cytochrome oxidase is prevented by the yeast mutation ts2858. We report that the mutant is, in addition, temperature sensitive for the processing of cytochrome b2, a protein encoded by nuclear DNA. Thus the same mutation affects the removal of pre-sequences from a mitochondrially encoded inner membrane protein and from an imported soluble protein located in the intermembrane space. The mutation blocks the second processing step of cytochrome b2. The cytochrome b2 intermediate accumulates in the mutant at 36 degrees C and assumes its enzyme activity. At 23 degrees C the conversion to the mature protein is considerably slower than in wild-type cells. The similarity of the cleavage sites Asn-Asp and Asn-Glu of the precursors for cytochrome oxidase subunit II and cytochrome b2, respectively, suggests a sequence-specific recognition by one protease or a factor activating a protease. On the other hand maturation of cytochrome c peroxidase, another enzyme of the intermembrane space, is not affected by the pet ts2858 mutation.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Böhni P. C., Daum G., Schatz G. Import of proteins into mitochondria. Partial purification of a matrix-located protease involved in cleavage of mitochondrial precursor polypeptides. J Biol Chem. 1983 Apr 25;258(8):4937–4943. [PubMed] [Google Scholar]
- Capaldi R. A., Malatesta F., Darley-Usmar V. M. Structure of cytochrome c oxidase. Biochim Biophys Acta. 1983 Jul 15;726(2):135–148. doi: 10.1016/0304-4173(83)90003-4. [DOI] [PubMed] [Google Scholar]
- Daum G., Böhni P. C., Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13028–13033. [PubMed] [Google Scholar]
- Daum G., Gasser S. M., Schatz G. Import of proteins into mitochondria. Energy-dependent, two-step processing of the intermembrane space enzyme cytochrome b2 by isolated yeast mitochondria. J Biol Chem. 1982 Nov 10;257(21):13075–13080. [PubMed] [Google Scholar]
- Felipo V., Grisolía S. Transport and regulation of polypeptide precursors of mature mitochondrial proteins. Curr Top Cell Regul. 1984;23:217–249. doi: 10.1016/b978-0-12-152823-2.50010-6. [DOI] [PubMed] [Google Scholar]
- GALZY P., SLONIMSKI P. P. Variations physiologiques de la levure au cours de la croissance sur l'acide lactique comme seule source de carbone. C R Hebd Seances Acad Sci. 1957 Dec 16;245(25):2423–2426. [PubMed] [Google Scholar]
- Gasser S. M., Ohashi A., Daum G., Böhni P. C., Gibson J., Reid G. A., Yonetani T., Schatz G. Imported mitochondrial proteins cytochrome b2 and cytochrome c1 are processed in two steps. Proc Natl Acad Sci U S A. 1982 Jan;79(2):267–271. doi: 10.1073/pnas.79.2.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guiard B., Buhler J. M. Yeast cytochrome b2 gene: isolation with antibody probes. Biochimie. 1984 Feb;66(2):151–158. doi: 10.1016/0300-9084(84)90204-9. [DOI] [PubMed] [Google Scholar]
- Guiard B., Lederer F. Baker's yeast flavocytochrome b2 (L-(+)-lactate dehydrogenase). An immunological study of structure and function. Eur J Biochem. 1976 Jun 1;65(2):537–542. doi: 10.1111/j.1432-1033.1976.tb10371.x. [DOI] [PubMed] [Google Scholar]
- Guiard B. Structure, expression and regulation of a nuclear gene encoding a mitochondrial protein: the yeast L(+)-lactate cytochrome c oxidoreductase (cytochrome b2). EMBO J. 1985 Dec 1;4(12):3265–3272. doi: 10.1002/j.1460-2075.1985.tb04076.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R., Böhni P., Gasser S. How mitochondria import proteins. Biochim Biophys Acta. 1984 Jan 27;779(1):65–87. doi: 10.1016/0304-4157(84)90004-2. [DOI] [PubMed] [Google Scholar]
- Heinrich P. C. Proteolytic processing of polypeptides during the biosynthesis of subcellular structures. Rev Physiol Biochem Pharmacol. 1982;93:115–187. doi: 10.1007/BFb0032670. [DOI] [PubMed] [Google Scholar]
- Jacq C., Lederer F. Cytochrome b2 from bakers' yeast (L-lactate dehydrogenase). A double-headed enzyme. Eur J Biochem. 1974 Jan 16;41(2):311–320. doi: 10.1111/j.1432-1033.1974.tb03271.x. [DOI] [PubMed] [Google Scholar]
- Kaput J., Goltz S., Blobel G. Nucleotide sequence of the yeast nuclear gene for cytochrome c peroxidase precursor. Functional implications of the pre sequence for protein transport into mitochondria. J Biol Chem. 1982 Dec 25;257(24):15054–15058. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labeyrie F., Baudras A., Lederer F. Flavocytochrome b 2 or L-lactate cytochrome c reductase from yeast. Methods Enzymol. 1978;53:238–256. doi: 10.1016/s0076-6879(78)53030-9. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maccecchini M. L., Rudin Y., Blobel G., Schatz G. Import of proteins into mitochondria: precursor forms of the extramitochondrially made F1-ATPase subunits in yeast. Proc Natl Acad Sci U S A. 1979 Jan;76(1):343–347. doi: 10.1073/pnas.76.1.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannhaupt G., Beyreuther K., Michaelis G. Cytochrome b, the var 1 protein, and subunits I and III of cytochrome c oxidase are synthesized without transient presequences in Saccharomyces cerevisiae. Eur J Biochem. 1985 Aug 1;150(3):435–439. doi: 10.1111/j.1432-1033.1985.tb09039.x. [DOI] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Pratje E., Mannhaupt G., Michaelis G., Beyreuther K. A nuclear mutation prevents processing of a mitochondrially encoded membrane protein in Saccharomyces cerevisiae. EMBO J. 1983;2(7):1049–1054. doi: 10.1002/j.1460-2075.1983.tb01544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid G. A., Yonetani T., Schatz G. Import of proteins into mitochondria. Import and maturation of the mitochondrial intermembrane space enzymes cytochrome b2 and cytochrome c peroxidase in intact yeast cells. J Biol Chem. 1982 Nov 10;257(21):13068–13074. [PubMed] [Google Scholar]
- SOMLO M. INDUCTION DES LACTICO-CYTOCHROME C REDUCTASES (D- ET L-) DE LA LEVURE AEROBIE PAR LES LACTATES (D- ET L-) Biochim Biophys Acta. 1965 Feb 15;97:183–201. [PubMed] [Google Scholar]
- Schatz G., Butow R. A. How are proteins imported into mitochondria? Cell. 1983 Feb;32(2):316–318. doi: 10.1016/0092-8674(83)90450-6. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velours J., Esparza M., Hoppe J., Sebald W., Guerin B. Amino acid sequence of a new mitochondrially synthesized proteolipid of the ATP synthase of Saccharomyces cerevisiae. EMBO J. 1984 Jan;3(1):207–212. doi: 10.1002/j.1460-2075.1984.tb01785.x. [DOI] [PMC free article] [PubMed] [Google Scholar]