Abstract
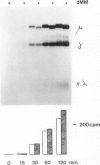
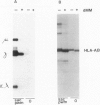
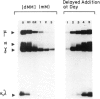
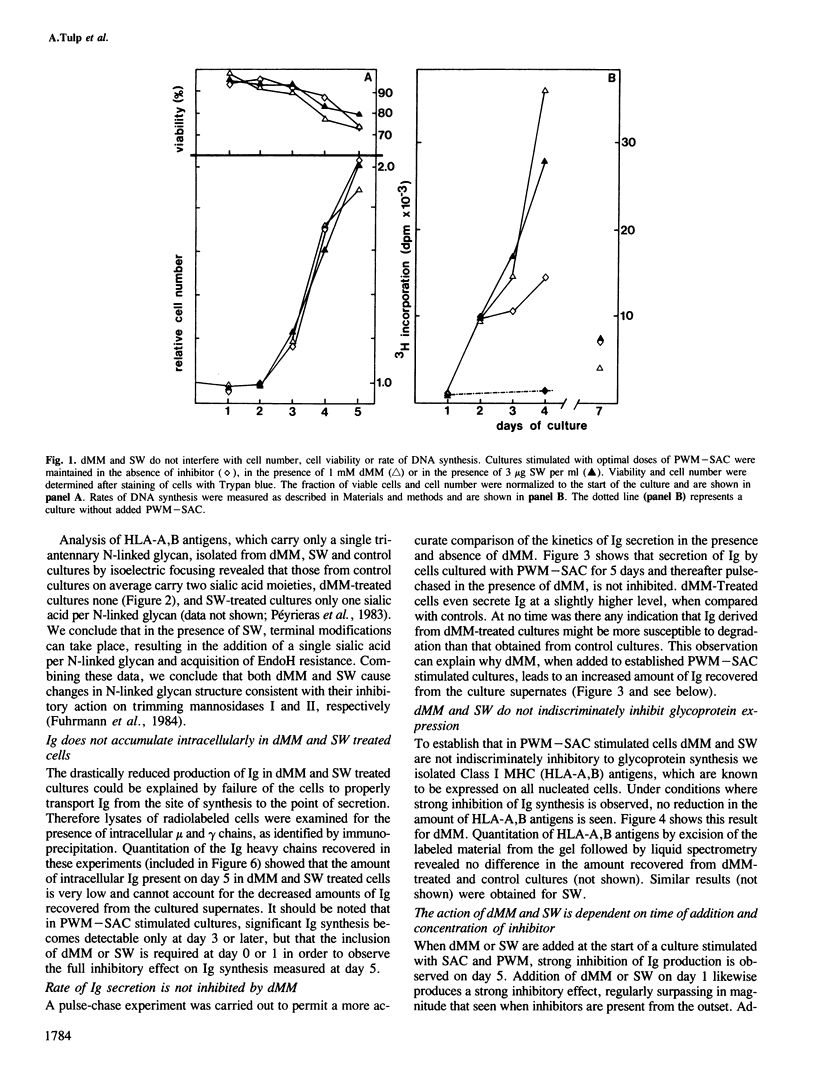
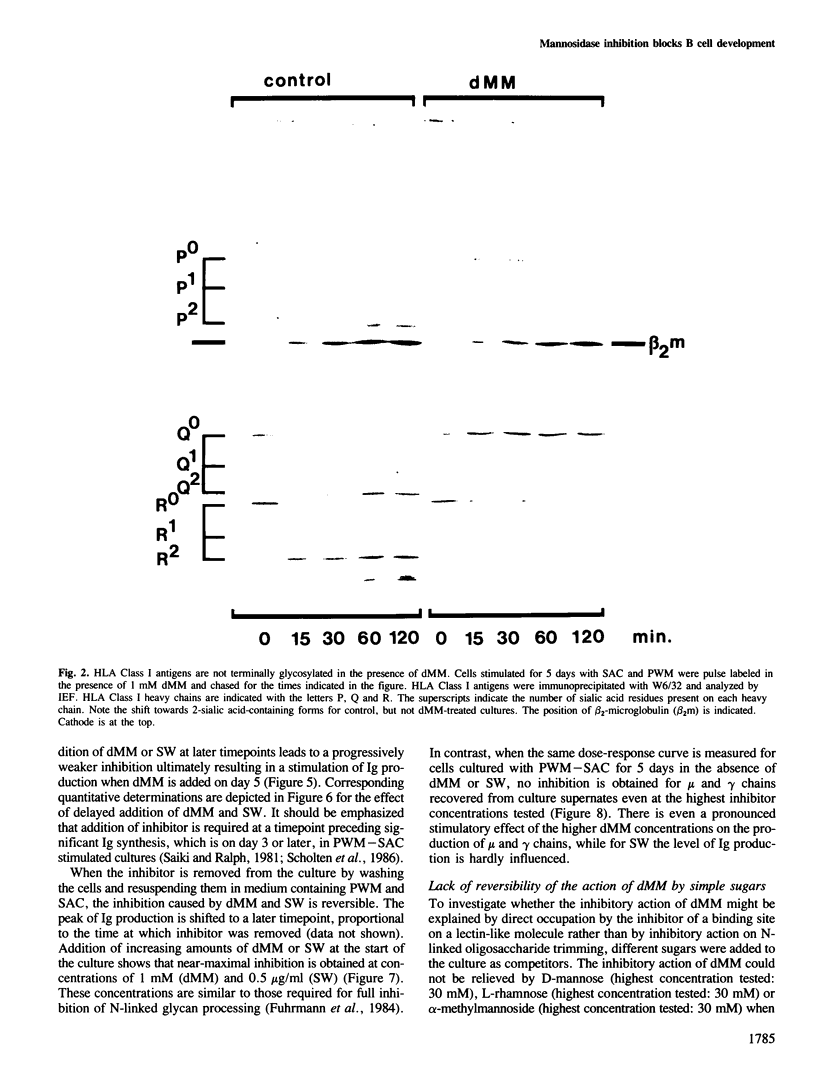
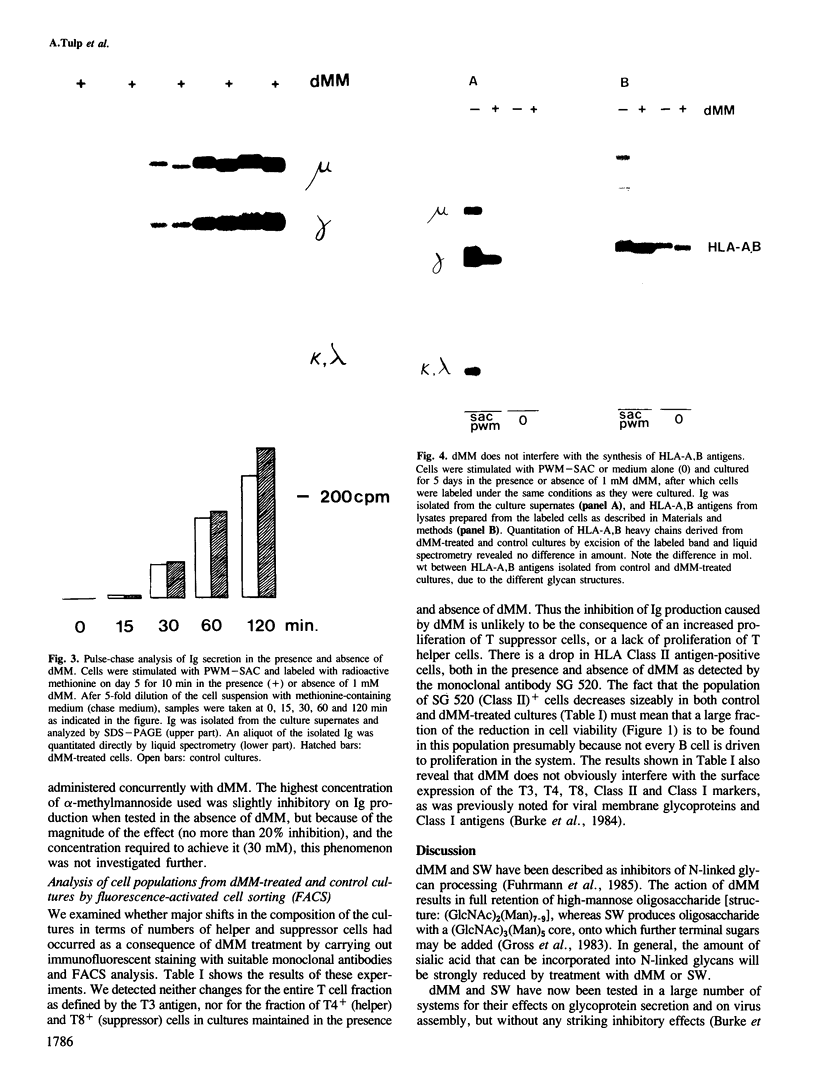
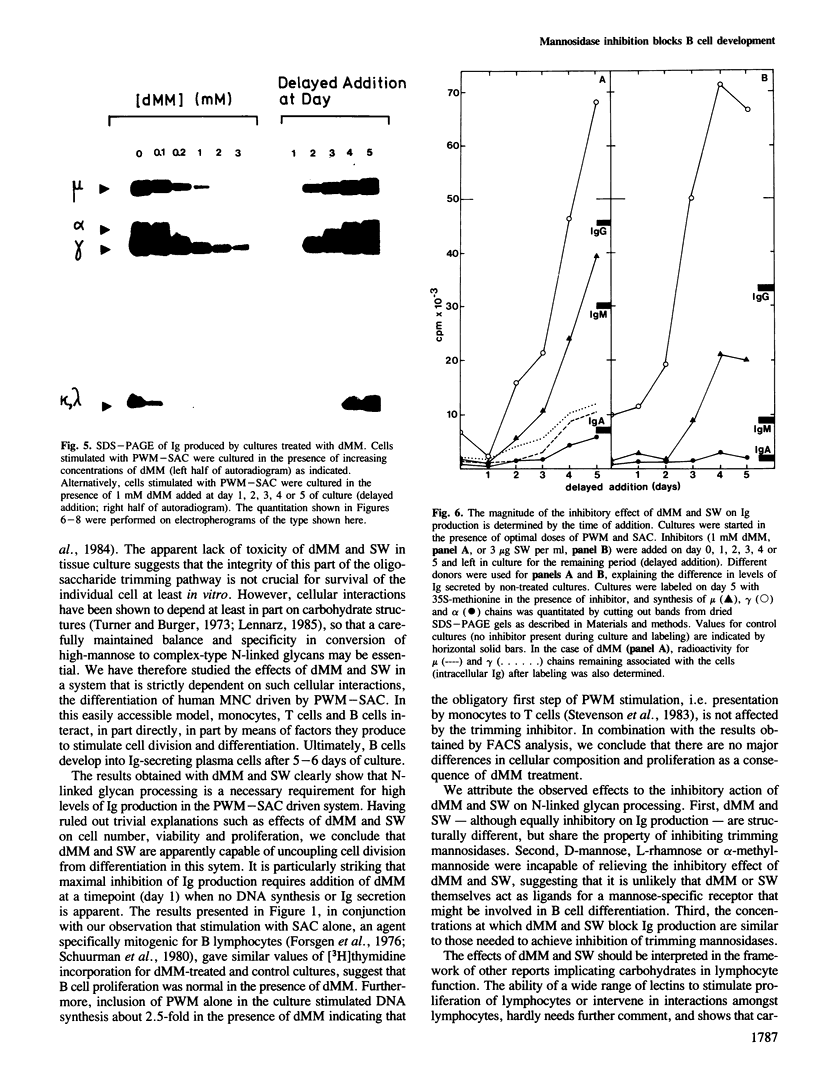
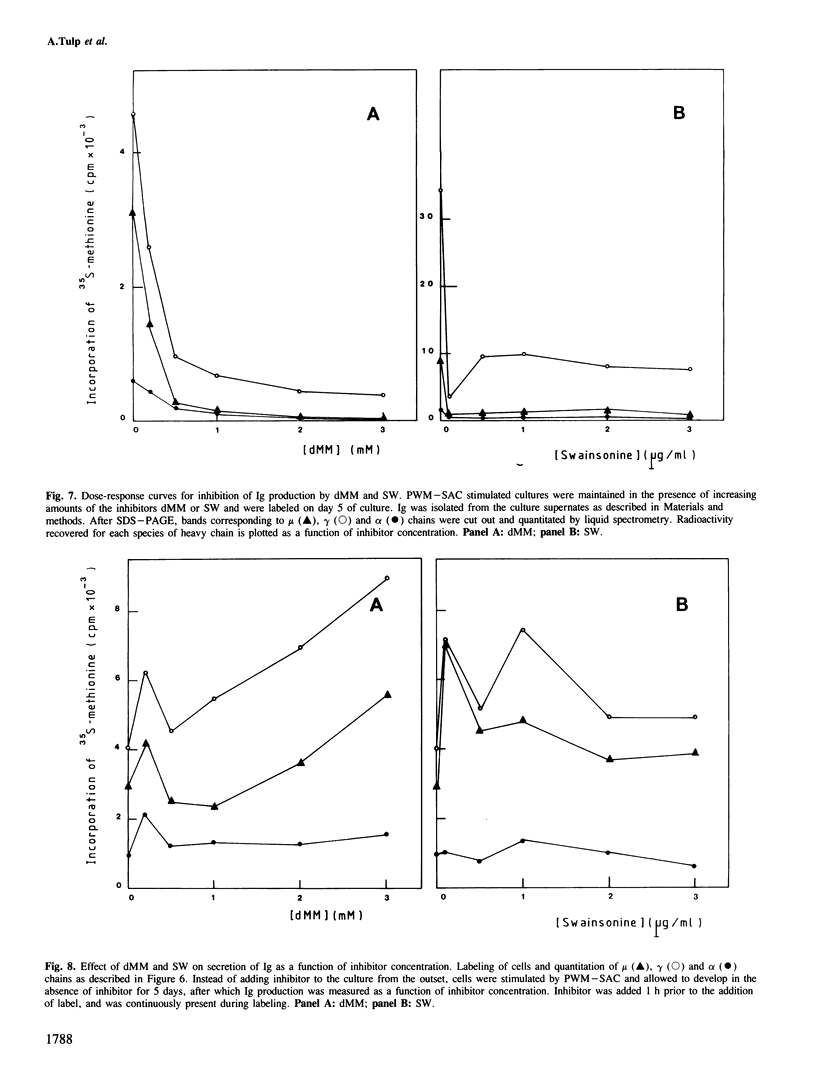
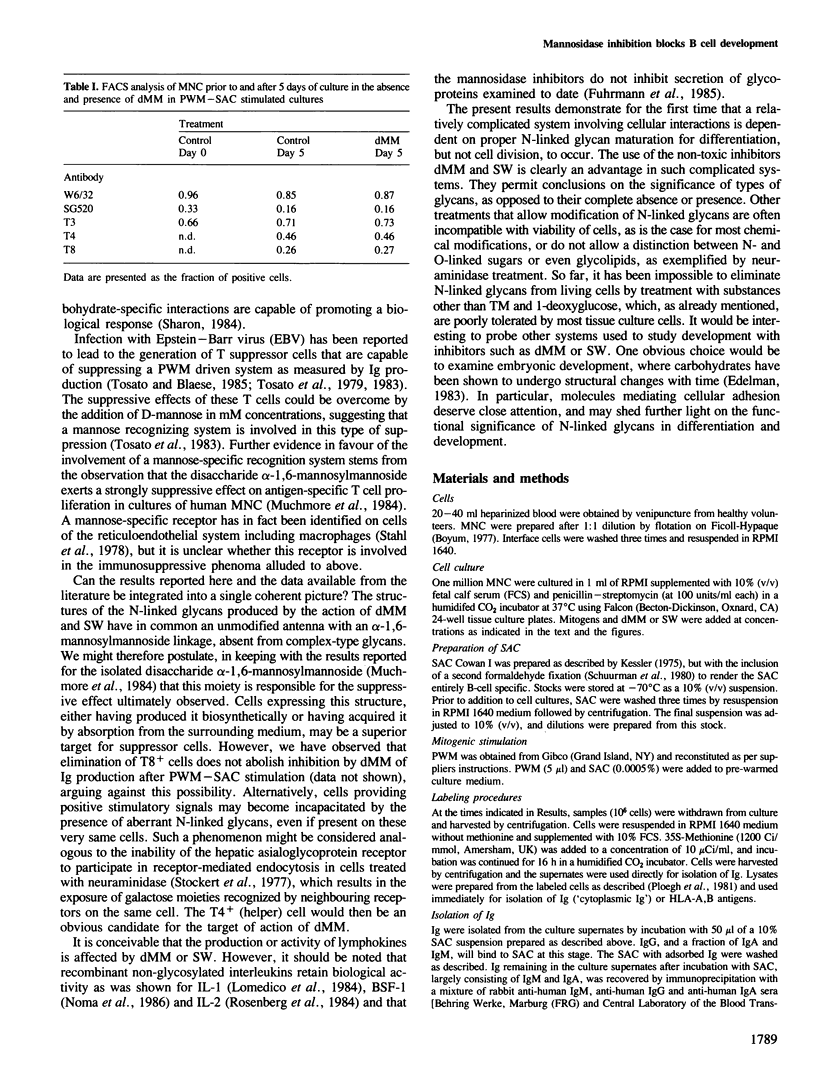
Deoxymannojirimycin (dMM) or swainsonine (SW), which block conversion of high-mannose to complex-type N-linked glycans, strongly inhibited the production of immunoglobulin (Ig) when added to cultures of human lymphocytes together with the polyclonal B cell activators pokeweed mitogen (PWM) and Staphylococcus aureus (SAC). To obtain the inhibitory effect, inhibitor had to be present during the first 36 h of culture. Addition at later timepoints was less effective and showed that neither inhibitor interfered with rate of production or secretion of Ig as such. Viability and proliferation of the lymphocytes, as defined by cell number and rate of DNA synthesis, were not influenced by the presence of dMM or SW, and no changes in the relative number of helper (T4+) or suppressor (T8+) cells were observed. Thus, for normal differentiation of human B lymphocytes into Ig secreting (plasma) cells in response to PWM and SAC, conversion of high-mannose to complex N-linked glycans is essential.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyum A. Separation of lymphocytes, lymphocyte subgroups and monocytes: a review. Lymphology. 1977 Jun;10(2):71–76. [PubMed] [Google Scholar]
- Burke B., Matlin K., Bause E., Legler G., Peyrieras N., Ploegh H. Inhibition of N-linked oligosaccharide trimming does not interfere with surface expression of certain integral membrane proteins. EMBO J. 1984 Mar;3(3):551–556. doi: 10.1002/j.1460-2075.1984.tb01845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowing C., Chapdelaine J. M. T cells discriminate between Ia antigens expressed on allogeneic accessory cells and B cells: a potential function for carbohydrate side chains on Ia molecules. Proc Natl Acad Sci U S A. 1983 Oct;80(19):6000–6004. doi: 10.1073/pnas.80.19.6000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobberstein B., Garoff H., Warren G., Robinson P. J. Cell-free synthesis and membrane insertion of mouse H-2Dd histocompatibility antigen and beta 2-microglobulin. Cell. 1979 Aug;17(4):759–769. doi: 10.1016/0092-8674(79)90316-7. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Cell adhesion molecules. Science. 1983 Feb 4;219(4584):450–457. doi: 10.1126/science.6823544. [DOI] [PubMed] [Google Scholar]
- Elbein A. D., Solf R., Dorling P. R., Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkoff R. J., Zhu L. P., Fauci A. S. Separate signals for human B cell proliferation and differentiation in response to Staphylococcus aureus: evidence for a two-signal model of B cell activation. J Immunol. 1982 Jul;129(1):97–102. [PubMed] [Google Scholar]
- Forsgren A., Svedjelund A., Wigzell H. Lymphocyte stimulation by protein A of Staphylococcus aureus. Eur J Immunol. 1976 Mar;6(3):207–213. doi: 10.1002/eji.1830060312. [DOI] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Legler G., Ploegh H. Novel mannosidase inhibitor blocking conversion of high mannose to complex oligosaccharides. Nature. 1984 Feb 23;307(5953):755–758. doi: 10.1038/307755a0. [DOI] [PubMed] [Google Scholar]
- Fuhrmann U., Bause E., Ploegh H. Inhibitors of oligosaccharide processing. Biochim Biophys Acta. 1985 Jun 24;825(2):95–110. doi: 10.1016/0167-4781(85)90095-8. [DOI] [PubMed] [Google Scholar]
- Gallatin W. M., Weissman I. L., Butcher E. C. A cell-surface molecule involved in organ-specific homing of lymphocytes. Nature. 1983 Jul 7;304(5921):30–34. doi: 10.1038/304030a0. [DOI] [PubMed] [Google Scholar]
- Gross V., Tran-Thi T. A., Vosbeck K., Heinrich P. C. Effect of swainsonine on the processing of the asparagine-linked carbohydrate chains of alpha 1-antitrypsin in rat hepatocytes. Evidence for the formation of hybrid oligosaccharides. J Biol Chem. 1983 Mar 25;258(6):4032–4036. [PubMed] [Google Scholar]
- Hart G. W. The role of asparagine-linked oligosaccharides in cellular recognition by thymic lymphocytes. Effects of tunicamycin on the mixed lymphocyte reaction. J Biol Chem. 1982 Jan 10;257(1):151–158. [PubMed] [Google Scholar]
- Kessler S. W. Rapid isolation of antigens from cells with a staphylococcal protein A-antibody adsorbent: parameters of the interaction of antibody-antigen complexes with protein A. J Immunol. 1975 Dec;115(6):1617–1624. [PubMed] [Google Scholar]
- Kiyohara T., Dennis J. W., Boegman R. J., Roder J. C. An exoglycosidase-sensitive triggering site on NK cells which is coupled to transmethylation of membrane phospholipids. J Immunol. 1985 Jul;135(1):659–664. [PubMed] [Google Scholar]
- Koszinowski U. H., Kramer M. Selective inhibition of T suppressor-cell function by a monosaccharide. Nature. 1981 Jan 15;289(5794):181–184. doi: 10.1038/289181a0. [DOI] [PubMed] [Google Scholar]
- Lomedico P. T., Gubler U., Hellmann C. P., Dukovich M., Giri J. G., Pan Y. C., Collier K., Semionow R., Chua A. O., Mizel S. B. Cloning and expression of murine interleukin-1 cDNA in Escherichia coli. 1984 Nov 29-Dec 5Nature. 312(5993):458–462. doi: 10.1038/312458a0. [DOI] [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M., Blaese R. M. Evidence that specific oligosaccharides block early events necessary for the expression of antigen-specific proliferation by human lymphocytes. J Immunol. 1980 Sep;125(3):1306–1311. [PubMed] [Google Scholar]
- Muchmore A. V., Decker J. M., Blaese R. M., Nilsson B. Purification and characterization of a mannose-containing disaccharide obtained from human pregnancy urine. A new immunoregulatory saccharide. J Exp Med. 1984 Dec 1;160(6):1672–1685. doi: 10.1084/jem.160.6.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noma Y., Sideras P., Naito T., Bergstedt-Lindquist S., Azuma C., Severinson E., Tanabe T., Kinashi T., Matsuda F., Yaoita Y. Cloning of cDNA encoding the murine IgG1 induction factor by a novel strategy using SP6 promoter. Nature. 1986 Feb 20;319(6055):640–646. doi: 10.1038/319640a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Parker J. W. Oxidation-induced lymphocyte transformation. Cell. 1976 Jan;7(1):13–20. doi: 10.1016/0092-8674(76)90250-6. [DOI] [PubMed] [Google Scholar]
- Parham P., Barnstable C. J., Bodmer W. F. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C, antigens. J Immunol. 1979 Jul;123(1):342–349. [PubMed] [Google Scholar]
- Peyrieras N., Bause E., Legler G., Vasilov R., Claesson L., Peterson P., Ploegh H. Effects of the glucosidase inhibitors nojirimycin and deoxynojirimycin on the biosynthesis of membrane and secretory glycoproteins. EMBO J. 1983;2(6):823–832. doi: 10.1002/j.1460-2075.1983.tb01509.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ploegh H. L., Orr H. T., Strominger J. L. Major histocompatibility antigens: the human (HLA-A, -B, -C) and murine (H-2K, H-2D) class I molecules. Cell. 1981 May;24(2):287–299. doi: 10.1016/0092-8674(81)90318-4. [DOI] [PubMed] [Google Scholar]
- Powell L. D., Bause E., Legler G., Molyneux R. J., Hart G. W. Influence of asparagine-linked oligosaccharides on tumor cell recognition in the mixed lymphocyte reaction. J Immunol. 1985 Jul;135(1):714–724. [PubMed] [Google Scholar]
- Reinherz E. L., Schlossman S. F. The differentiation and function of human T lymphocytes. Cell. 1980 Apr;19(4):821–827. doi: 10.1016/0092-8674(80)90072-0. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Saiki O., Ralph P. Induction of human immunoglobulin secretion. I. Synergistic effect of B cell mitogen Cowan I plus T cell mitogens or factors. J Immunol. 1981 Sep;127(3):1044–1047. [PubMed] [Google Scholar]
- Scholten P., Schuurman R., Ploegh H. Activation of human B cells: involvement of surface immunoglobulin as evidenced by two biochemically distinct types of response to Staphylococcus aureus. Hum Immunol. 1986 May;16(1):1–13. doi: 10.1016/0198-8859(86)90031-5. [DOI] [PubMed] [Google Scholar]
- Schuurman R. K., Gelfand E. W., Dosch H. M. Polyclonal activation of human lymphocytes in vitro. I. Characterization of the lymphocyte response to a T cell-independent B cell mitogen. J Immunol. 1980 Aug;125(2):820–826. [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson H. C., Miller P. J., Waxdal M. J., Haynes B. F., Thomas C. A., Fauci A. S. Interaction of pokeweed mitogen with monocytes in the activation of human lymphocytes. Immunology. 1983 Aug;49(4):633–640. [PMC free article] [PubMed] [Google Scholar]
- Stockert R. J., Morell A. G., Scheinberg I. H. Hepatic binding protein: the protective role of its sialic acid residues. Science. 1977 Aug 12;197(4304):667–668. doi: 10.1126/science.877581. [DOI] [PubMed] [Google Scholar]
- Tosato G., Blaese R. M. Epstein-Barr virus infection and immunoregulation in man. Adv Immunol. 1985;37:99–149. doi: 10.1016/s0065-2776(08)60339-9. [DOI] [PubMed] [Google Scholar]
- Tosato G., Magrath I., Koski I., Dooley N., Blaese M. Activation of suppressor T cells during Epstein-Barr-virus-induced infectious mononucleosis. N Engl J Med. 1979 Nov 22;301(21):1133–1137. doi: 10.1056/NEJM197911223012101. [DOI] [PubMed] [Google Scholar]
- Tosato G., Pike S. E., Blaese R. M. Reversal of infectious mononucleosis-associated suppressor T cell activity by D-mannose. J Exp Med. 1983 Oct 1;158(4):1048–1060. doi: 10.1084/jem.158.4.1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner R. S., Burger M. M. Involvement of a carbohydrate group in the active site for surface guided reassociation of animal cells. Nature. 1973 Aug 24;244(5417):509–510. doi: 10.1038/244509a0. [DOI] [PubMed] [Google Scholar]