Abstract
Polycystic ovary syndrome (PCOS), as a common endocrine and metabolic disorder, is often regarded as a primary cause of anovulatory infertility in women. The pathogenesis of PCOS is complex and influenced by multiple factors. Emerging evidence highlights that energy metabolism dysfunction and oxidative stress in granulosa cells (GCs) are pivotal contributors to aberrant follicular development and impaired fertility in PCOS patients. Mitochondrial dysfunction, increased oxidative stress, and disrupted glucose metabolism are frequently observed in individuals with PCOS, collectively leading to compromised oocyte quality. This review delves into the mechanisms linking oxidative stress and energy metabolism abnormalities in PCOS, analyzing their adverse effects on reproductive function. Furthermore, potential therapeutic strategies to mitigate oxidative stress and metabolic disturbances are proposed, providing a theoretical basis for advancing clinical management of PCOS.
Keywords: PCOS, Mitochondrial dysfunction, Oxidative stress, Glycolysis, Energy metabolism
Background
Polycystic ovary syndrome (PCOS) is one of the most common ovarian disorders affecting female fertility, accounting for 80% of anovulatory infertile women [1]. Clinically, PCOS is characterized by hyperandrogenemia (HA), menstrual irregularities, and anovulation, along with reduced oocyte quality and functionality [2]. The pathogenesis of PCOS is influenced by a combination of genetic, environmental, and metabolic factors. Key contributors include HA, insulin resistance (IR), dysregulated glucose and lipid metabolism, oxidative stress (OS), and mitochondrial dysfunction [3–6]. Among these, impaired ovarian energy metabolism and OS are believed to be critical mechanisms underlying the reduced developmental competence of oocytes in PCOS patients.
OS results from an imbalance between oxidants and antioxidants, leading to the accumulation of reactive species that directly damage lipids, proteins, and DNA, ultimately causing cellular dysfunction and death [7]. OS plays a significant role in the onset and progression of PCOS, with an imbalance between oxidants and antioxidants in the blood and follicular fluid being a hallmark feature of the condition [8]. Mitochondria, as the primary site of reactive oxygen species (ROS) production and the central hub of energy metabolism, are considered key factors in the pathophysiology of PCOS [6].
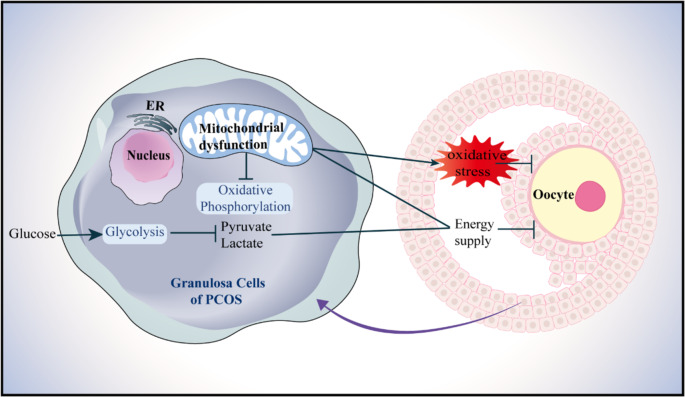
Energy metabolism is essential for follicular growth and development. Mitochondrial oxidative phosphorylation (OXPHOS) and glycolysis are the two primary energy pathways in granulosa cells (GCs), supporting follicular maturation. Targeted metabolomic studies have revealed energy metabolism imbalances in the follicular fluid of PCOS patients, including abnormalities in mitochondrial OXPHOS and glycolysis [9, 10]. These metabolic disruptions impair energy conversion processes, leading to decreased adenosine triphosphate (ATP) production and elevated ROS levels. Such changes are closely associated with reduced oocyte quality and lower pregnancy rates in PCOS patients (shown in Fig. 1) [11, 12].
Fig. 1.
Schematic diagram of PCOS oocyte damage induced by energy metabolism disorder and oxidative stress in GCs. Insufficient energy supply caused by mitochondrial dysfunction and glucose metabolism defects in ovarian GCs of PCOS patients may affect follicular development and ovulation
Thus, mitochondria play a pivotal role in the pathogenesis of PCOS not only by regulating OS but also by modulating energy metabolism. This study aims to comprehensively explore the relationship between PCOS, oxidative stress, and energy metabolism, with the goal of providing new perspectives and theoretical foundations for the development of future therapeutic strategies.
PCOS and OS
Oxidation and antioxidant imbalance in PCOS
PCOS patients commonly exhibit elevated OS levels and decreased antioxidant capacity [13]. The biological markers of OS in PCOS are presented in Table 1. In these patients, the Total Oxidant Status (TOS) is significantly increased, accompanied by elevated levels of oxidative damage markers such as malondialdehyde (MDA), homocysteine (HCY), 8-hydroxy-2’-deoxyguanosine (8-OHdG), advanced glycation end products (AGEs), hydrogen peroxide (H2O2), superoxide anion (SOA), and asymmetric dimethylarginine (ADMA), along with increased activity of xanthine oxidase (XO) [14–23]. As a terminal product of lipid peroxidation, MDA reflects the extent of oxidative damage. HCY exacerbates OS by inhibiting the expression and activity of antioxidant enzymes such as glutathione peroxidase (GPx) and superoxide dismutase (SOD) while promoting the expression of inducible nitric oxide synthase (iNOS). 8-OHdG, a biomarker of DNA oxidative damage, has been shown to negatively affect embryo transfer outcomes when present at high levels in follicular fluid [24]. AGEs bind to the receptor for advanced glycation end products (RAGE), activating the redox-sensitive transcription factor NF-κB, which triggers OS and promotes the production of inflammatory cytokines. This interaction contributes to disruptions in steroid hormone synthesis and secretion, as well as abnormalities in follicular development, ultimately impairing reproductive outcomes in PCOS patients [25].
Table 1.
Biological markers of oxidative stress in PCOS
| Markers | Sample types | Changes | References |
|---|---|---|---|
| 8-OHdG | Follicular fluid | ↑ | [12] |
| TOS | Follicular fluid, Serum | ↑ | [16, 17] |
| OSI | Serum | ↑ | [17] |
| H2O2 | Serum | ↑ | [18] |
| SOA | Serum | ↑ | [18] |
| GSSG | Serum | ↑ | [18] |
| MDA | Follicular fluid, Serum | ↑ | [13, 15, 17–20] |
| PC | Serum | ↑ | [21] |
| HCY | Serum | ↑ | [22] |
| ADMA | Serum | ↑ | [22] |
| XO | Serum | ↑ | [23] |
| NO | Serum | → | [22, 23] |
| AGEs | - | ↑ | [25] |
Note “↑” indicates an increase. “↓” indicates a decrease. “→” indicates no change
There is ongoing debate regarding changes in antioxidant biomarkers in PCOS. The alterations in antioxidant biomarkers observed in PCOS are summarized in Table 2. Most studies indicate that serum activities of SOD, catalase (CAT), and various glutathione-related enzymes are suppressed in PCOS. Additionally, levels of non-enzymatic antioxidants, such as vitamin C, vitamin E, retinol, and glutathione, show a decreasing trend [14, 18–20, 26]. Similarly, in PCOS animal models, significant reductions in glutathione peroxidase (GPx) and SOD activities have been observed in both serum and follicular fluid [27]. However, some studies have reported increased antioxidant enzyme activity in the blood and follicular fluid of PCOS patients [21, 22, 26]. Fatima Q and colleagues hypothesized that this increase might represent a compensatory response to OS in PCOS [22, 26, 28]. The body primarily counteracts excessive oxidative damage by utilizing non-enzymatic antioxidant systems, including glutathione, vitamins C and E, melatonin, polyphenols, β-carotene, and the trace element selenium, to directly neutralize ROS [29]. Glutathione (GSH) functions as a small molecule antioxidant scavenging excess free radicals and peroxides [30]. Vitamin C, serving as a redox catalyst, reduces and neutralizes ROS while maintaining redox equilibrium [31]. Vitamin E combats lipid peroxidation by neutralizing lipid free radicals and exerting antioxidant effects.
Table 2.
Antioxidant biomarkers of PCOS
| Markers | Sample types | Changes | References |
|---|---|---|---|
| TAC | Follicular fluid, Serum | ↓ | [14, 16, 19–21] |
| SOD | Follicular fluid, Serum | ↓, ↑, → | [14, 18–22] |
| CAT | Serum | ↓ | [18, 21] |
| GPx | Follicular Fluid, Serum | ↓, ↑, → | [14, 18, 22, 26] |
| GR | Follicular Fluid, Serum | ↓, ↑ | [14, 18, 26] |
| GST | Serum | ↓, ↑ | [18, 26] |
| GCL | Serum | ↓ | [18] |
| PON1 | Follicular fluid, Serum | ↓ | [14, 16, 22] |
| Vitamin E | Serum | ↓, → | [19, 26] |
| Vitamin C | Follicular fluid, Serum | ↓ | [14, 18, 26] |
| GSH | Serum | ↓ | [19] |
| RET | Follicular fluid | ↓ | [14, 16] |
| Thiol groups | Follicular fluid | ↓ | [16] |
| NAD + | GCs | ↓ | [74] |
Note: “↑” indicates an increase. “↓” indicates a decrease. “→” indicates no change
The excessive production of ROS can lead to OS, with mitochondria being the primary source of ROS [32]. Another significant ROS source is the NADPH oxidase 2 (NOX2) complex. The NOX2 complex consists of six subunits, where p22phox binds to NOX2 in its inactive form at the cell membrane, while the other subunits, p40phox, p47phox, p67phox, and Rac, exist as a cytoplasmic complex. Upon activation, the cytoplasmic subunit p47phox translocates to gp91phox, activating NOX2 [33]. The presence of gp91phox and p47phox proteins suggests that the NOX2 pathway may contribute to ROS production in PCOS patients. The use of specific inhibitors for NOX2, such as diphenylene iodonium and apocynin, significantly diminishes ROS production in GCs of PCOS [34].
The regulation of OS relies heavily on the Kelch-like ECH-associated protein 1 (Keap1)/nuclear factor erythroid 2-related factor 2 (Nrf2) signaling pathway (Keap1-Nrf2/ARE). Under physiological conditions, Nrf2 primarily binds to its inhibitor, Keap1, and remains in an inactive state in the cytoplasm. Upon stimulation by ROS, Nrf2 dissociates from Keap1, translocates to the nucleus, and binds to antioxidant response elements (AREs). This process promotes the expression of antioxidant genes such as SOD, CAT, GPx, GCL, and heme oxygenase 1 (HO-1), thereby activating antioxidant defenses [35, 36]. In ovarian tissues of PCOS rat models, the activation of the Nrf2/HO-1 signaling pathway is suppressed, resulting in reduced activities of antioxidants, including SOD, GPx, CAT, and GSH [37]. Recent studies have identified the P21-activated kinase 2 (PAK2)/β-catenin/c-Myc/pyruvate kinase M2 (PKM2) axis as a newly discovered signaling pathway regulating oxidative stress. This pathway triggers OS in PCOS by inhibiting the Nrf2/HO-1 pathway, suggesting that PAK2 could serve as a potential therapeutic target for PCOS [38]. Furthermore, studies have shown that carnosol can improve PCOS phenotypes in mice by activating the Keap1-mediated Nrf2/HO-1 pathway [39]. ROS also interacts with the NF-κB signaling pathway through multiple mechanisms. The activity of NF-κB is regulated by ROS levels; under ROS-related stimulation, the phosphorylation of RelA (p65) modulates NF-κB activity, influencing the expression of downstream genes involved in inflammation, immunity, and cell growth. Additionally, NF-κB family members, including RelA (p65) and p50, directly regulate the transcription of antioxidant genes such as SOD, CAT, GST, HO-1, and GPx, thereby affecting intracellular ROS levels [40].
Factors associated with OS in PCOS
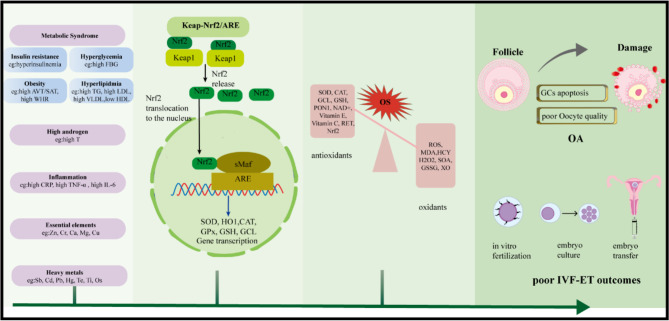
The mechanisms underlying ROS production in PCOS remain incompletely understood [41]. Mendelian randomization (MR) studies suggest that PCOS itself does not directly cause elevated OS levels. Instead, increased OS levels are closely associated with other underlying factors [42]. These factors commonly include metabolic syndrome, HA, inflammation, heavy metal exposure, and imbalances in trace elements. These factors may act independently or synergistically, ultimately exerting adverse effects on the reproductive function of individuals with PCOS (shown in Fig. 2).
Fig. 2.
Schematic diagram of OS and its negative effects on the reproductive system in PCOS. PCOS is characterized by elevated oxidative stress levels, which are closely associated with metabolic abnormalities (such as IR and MS), endocrine dysfunctions (including HA), exposure to heavy metals, and essential elements. Oxidative stress intensifies GCs apoptosis and impairs oocyte quality, leading to follicular atresia and a reduction in ovarian reserve. These detrimental effects ultimately result in anovulatory infertility and diminished success rates of assisted reproductive technologies, including IVF-ET
Mechanisms of OS associated with metabolic syndrome
PCOS patients often present with metabolic syndrome, characterized by disruptions in glucose and lipid metabolism as well as obesity [22, 43]. Approximately 50–70% of PCOS patients exhibit IR. HA in PCOS exacerbates inflammation and OS by increasing inflammatory markers and ROS levels, which damage pancreatic β-cells, impairing insulin secretion and further promoting IR [44–46]. When high concentrations of glucose and free fatty acids (FFAs) enter cells, they are metabolized into pyruvate and acetyl-CoA. These metabolites are oxidized in mitochondria, increasing NADH and FADH production, which activates the electron transport chain, leading to excessive ROS production and OS induction [47]. OS aggravates IR by impairing insulin receptor signaling, activating c-Jun N-terminal kinase (JNK), promoting serine phosphorylation of insulin receptor substrate1 (IRS-1), and reducing glucose transporter type 4 (GLUT4) transporter expression on the cell membrane, further exacerbating the progression of IR [48]. Thus, IR and OS are closely interconnected, significantly contributing to metabolic disturbances in PCOS patients.
Lipid metabolism disorders in PCOS are also closely linked to OS. Increased OS is associated with elevated levels of triglycerides (TG) and estradiol (E2), as well as reduced concentrations of apolipoprotein A1 (apoA1) [49]. This is manifested by reduced antioxidative activity of high-density lipoprotein (HDL) [50]. Conversion of high-density lipoprotein subtypes to smaller particles with weaker antioxidative capacity leads to an increase in levels of oxidized low-density lipoprotein (oxLDL) [51, 52]. Excess oxLDL induces autophagy in GCs through the oxLDL-oxidized low density lipoprotein receptor 1 (OLR1)-ROS pathway [53]. Elevated levels of free fatty acids (FFAs) and TG in the serum and follicular fluid of PCOS patients result in mitochondrial damage, endoplasmic reticulum stress, and hormonal synthesis imbalance in GCs [54, 55]. Increased FFAs inhibit glucose oxidation while enhancing FFA oxidation, leading to excessive ROS production via the tricarboxylic acid (TCA) cycle and electron transport chain. Arachidonic acid (AA), one of the most abundant FFAs in the body, significantly reduces total antioxidant capacity (TAC) and antioxidant enzyme activity in KGN cells while increasing levels of MDA, ROS, and SOA. AA also impairs mitochondrial function and secretion in KGN cells, inducing apoptosis [56].
Furthermore, elevated levels of palmitic acid (PA) have been detected in the serum and follicular fluid of PCOS patients [57]. In a PCOS theca cell model, PA disrupts mitochondrial function and induces ROS production, activating the ROS/p38 and JNK signaling pathways, which promote androgen production and ovarian fibrosis [58].
In patients with PCOS, an increase in waist-hip ratio (WHR) and visceral fat/subcutaneous fat ratio (VAT/SAT) is often associated with exacerbated oxidative stress [59]. Obesity-induced OS also impairs mitochondrial quality. Mouse models of obesity induced by high-fat, high-sugar diets have demonstrated a significant decline in oocyte mitochondrial quality [60]. As a result, weight loss is considered one of the most effective treatment strategies for obese PCOS patients. Research has shown that weight loss significantly reduces ROS production in leukocytes and decreases oxidative damage to lipids and proteins [61]. These findings underscore the crucial role of weight management in mitigating OS and its associated complications in PCOS patients.
Mechanisms of OS associated with abnormal androgen levels
Approximately 60–80% of PCOS patients exhibit HA, characterized by elevated testosterone (T) levels, which lead to clinical manifestations such as acne, hirsutism, and alopecia. Notably, among the various phenotypes of PCOS, patients with the HA phenotype often experience more pronounced OS [50]. Studies have demonstrated a significant positive correlation between serum MDA levels and testosterone concentrations in PCOS patients [62]. Excess androgens exacerbate OS and inflammasome activation in GCs through multiple mechanisms. First, hyperandrogens activate leukocytes, enhancing their glucose sensitivity and promoting ROS production, which increases p47(phox) gene expression and plasma thiobarbituric acid reactive substances (TBARS) levels [63]. Second, hyperandrogens induce endoplasmic reticulum stress (ERS) and activate the inositol-requiring enzyme 1α (IRE1α)-thioredoxin-interacting protein (TXNIP)/ROS-NOD-like receptor family pyrin domain-containing 3 (NLRP3) signaling pathway, leading to increased ROS production in GCs and theca cells (TCs). Exercise has been shown to upregulate irisin, which inhibits IRE1α and its downstream targets, thereby improving ovarian function [64]. Additionally, elevated testosterone disrupts mitochondrial function and increases ROS levels via the androgen receptor (AR)-NADPH oxidase 4 (NOX4) signaling pathway in C2C12 cells. Treatment with the antioxidant N-acetylcysteine (NAC) reduces ROS production, restores mitochondrial function, and reverses IR [65]. Hyperandrogenism damages ovarian tissue and causes systemic abnormalities through OS-related mechanisms [66]. Resveratrol, by activating SIRT1 and inhibiting p66Shc, has been shown to prevent ovarian OS and fibrosis induced by HA [67]. Furthermore, HA exacerbate IR symptoms [68]. OS and HA are interrelated factors. In vitro studies suggest that OS promotes androgen production in PCOS by downregulating hepatocyte nuclear factor-4α (HNF-4α), thereby reducing the expression and secretion of sex hormone-binding globulin (SHBG) [69].
Unraveling the role of inflammation in OS
Chronic low-grade inflammation has been implicated in the pathogenesis of PCOS, with inflammatory markers such as TNF-α, CRP, IL-6, IL-8, and IL-18 significantly elevated in PCOS patients [70]. OS and inflammation are interlinked pathological mechanisms in PCOS, with NF-κB serving as a key mediator. In inflammatory states, increased expression of toll-like receptor 2 (TLR2) and other inflammatory factors in KGN cells induces OS. Resveratrol has been shown to downregulate TLR2 expression, thereby suppressing OS and restoring GCs function [71]. Inflammatory cytokines activate IL-1 receptor type 1 (IL-1R1) and TLR4 on GCs, triggering the release of the NF-κB complex. Once translocated to the nucleus, NF-κB activates RelA (p65) via phosphorylation, promoting the transcription of key components of the NLRP3 inflammasome, including NLRP3, ASC, and caspase-1. The assembled NLRP3 inflammasome leads to the caspase-1-dependent release of pro-inflammatory cytokines IL-1β and IL-18, perpetuating the inflammatory response [72]. Studies have also revealed that NLRP3 protein localizes to mitochondria in GCs and accumulates around the nucleus, where the inflammatory cascade exacerbates mitochondrial damage and induces ROS production [73]. Furthermore, inflammation reduces nicotinamide adenine dinucleotide (NAD) levels in PCOS GCs, leading to mitochondrial dysfunction. Nicotinamide riboside (NR) supplementation has been shown to restore NAD levels and alleviate mitochondrial dysfunction [74].
OS mediated by heavy metals and essential elements
In addition to endogenous factors, exposure to certain toxic metals, such as antimony (Sb), cadmium (Cd), lead (Pb), mercury (Hg), arsenic (As), tellurium (Te), thallium (Tl), and osmium (Os), can also induce OS in PCOS patients [18, 75]. For instance, subacute cadmium exposure disrupts hypothalamic-pituitary-gonadal (HPG) axis function, causing PCOS-like symptoms and OS in rats [76]. Heavy metals primarily impair antioxidant defenses by interacting with sulfhydryl groups in non-enzymatic antioxidant systems, forming organometallic complexes that reduce the body’s antioxidant capacity. Lead binds to protein sulfhydryl groups, depletes GSH, and enhances lipid peroxidation, while thallium damages mitochondrial function and increases ROS generation [77]. Moreover, lead exposure disrupts mitochondrial structure, activates the Nrf2/Keap1 pathway, and exacerbates OS, leading to reduced fertility in female mice [78]. These findings highlight the need for protective measures to minimize heavy metal exposure and prevent reproductive dysfunction.
Evidence suggests that alterations in essential element levels such as selenium (Se), zinc (Zn), chromium (Cr), calcium (Ca), magnesium (Mg), and copper (Cu) are associated with OS in PCOS patients [75]. However, discrepancies exist regarding serum trace element levels in PCOS patients. Some studies report reduced serum Zn levels, while others find no significant differences in Zn, iron (Fe), or Mg levels. Conversely, Cu concentrations are often elevated, and excessive Cu can catalyze ROS production and deplete GSH levels [76, 79]. Supplementation with trace elements such as Zn, Cr, Se, Ca, along with vitamins D and K, magnesium, and melatonin, has been shown to reduce OS and improve PCOS symptoms [75].
The abnormal energy metabolism in PCOS
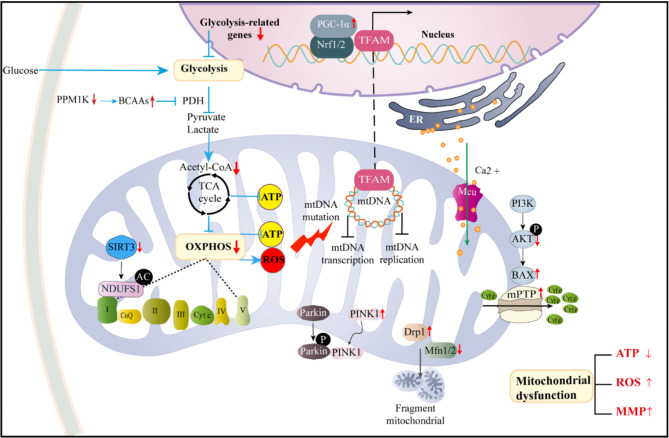
In the ovaries of PCOS rat models, ATP levels and lactate concentrations are significantly reduced, indicating disruptions in ovarian energy metabolism. Mitochondrial function and glucose metabolism are critical for bidirectional signaling between GCs and oocytes. Mitochondrial dysfunction and glucose metabolic defects in GCs from PCOS patients lead to insufficient energy supply, directly impairing oocyte development and reducing pregnancy rates (shown in Fig. 3) [11].
Fig. 3.
The mechanism of mitochondrial dysfunction and limited glycolysis leading to abnormal energy metabolism in PCOS. In PCOS GCs, impaired glycolytic metabolism and mitochondrial dysfunction are the main reasons for the lack of sufficient energy substrates in oocytes and GCs. The mitochondrial dysfunction of PCOS granulosa cells may be caused by a variety of factors, including mitochondrial DNA mutations, decreased mitochondrial biogenesis, impaired mitochondrial dynamics, abnormal mitochondrial autophagy, oxidative phosphorylation dysfunction, enhanced oxidative stress or calcium homeostasis imbalance. In addition, impaired glycolysis metabolism can also lead to impaired mitochondrial OXPHOS function, exacerbating the abnormal energy metabolism of PCOS GCs. This imbalance of energy metabolism leads to the inability to maintain ATP synthesis, but produces excessive ROS, which further aggravates mitochondrial damage and oxidative stress
Disordered glycolytic activity
GCs play a critical role in supporting oocyte development by producing pyruvate and lactate through glycolysis, which serve as the primary energy sources for oocyte maturation. Normal follicle development accompanied by increased GC glycolysis [80]. However, studies have shown that glycolytic function is impaired in GCs from PCOS patients, as evidenced by reduced levels of pyruvate and lactate in follicular fluid and significantly downregulated expression of glycolysis-related enzyme genes such as glucose transporter type 1 (GLUT1), Lactate dehydrogenase A (LDHA), and Phosphofructokinase (PFKP) [12]. This indicates that the decreased glycolytic rate in PCOS is driven by the downregulation of rate-limiting enzymes in the glycolytic pathway. In addition, GCs in PCOS patients exhibit mitochondrial abnormalities, including altered morphology, number, and localization, reduced mitochondrial membrane potential (MMP), decreased ATP production, elevated mitochondrial ROS, increased oxidative stress, and insufficient OXPHOS [81]. These findings indicate that mitochondrial dysfunction and glycolytic limitations together contribute to the energy metabolism abnormalities in the ovarian microenvironment of PCOS. Sirtuin3 (SIRT3), predominantly localized in mitochondria, regulates mitochondrial function through deacetylation. In PCOS, SIRT3 deficiency may induce abnormal activation of the Phosphoinositide 3-kinase/protein kinase B (PI3K/Akt) signaling pathway via mitochondrial oxidative stress, disrupting insulin-dependent glucose metabolism [81]. Additionally, SIRT2 modulates the acetylation and activity of key glycolytic enzymes such as LDHA, PKM2, and hexokinase 2 (HK2) in GCs. Resveratrol has been shown to upregulate SIRT2, thereby promoting the expression of rate-limiting glycolytic enzymes, increasing lactate and ATP levels, restoring glycolysis, and improving ovarian energy metabolism in PCOS rat models [82]. Combination therapy with Diane-35 and metformin has also been found to restore glycolytic pathways and improve ovarian energy metabolism by regulating glycolysis-related rate-limiting enzymes such as PKM2 and LDHA [83]. Meanwhile, elevated concentrations of branched-chain amino acids (BCAAs) have been observed in both the blood and follicular fluid of PCOS patients [84]. High levels of BCAAs inhibit the activity of the pyruvate dehydrogenase complex (PDH), interfering with mitochondrial pyruvate utilization and thereby suppressing glucose metabolism [85]. The Protein phosphatase Mg2+/Mn2+-dependent 1 K(PPM1K)gene, a genetic driver of BCAA catabolism in PCOS, promotes the diversion of GCs glycolysis into the pentose phosphate pathway, further inhibiting mitochondrial OXPHOS and exacerbating the mitochondrial energy burden [86].
Lipid metabolism dysfunction in PCOS
In PCOS, lipid metabolism pathways, including glycerophospholipid metabolism, fatty acid degradation, fatty acid biosynthesis, and ether lipid metabolism, are significantly affected. Alterations in fatty acid degradation and biosynthesis pathways in PCOS patients may disrupt energy balance and hormone levels, as evidenced by changes in acylcarnitine and other fatty acid metabolism-related metabolites [87]. Oocytes regulate the intrafollicular microenvironment through bidirectional signaling with surrounding cells, such as GCs, which provide essential support for oocyte developmental competence, including meiotic progression, fertilization, and embryogenesis. Thus, the composition of fatty acids in follicular fluid and GCs directly influences oocyte quality [88].
In obese women with PCOS, levels of palmitic acid and oleic acid are significantly elevated in both serum and follicular fluid. Treatment of KGN cells and primary GCs from PCOS patients with oleic acid suppresses the phosphorylation of AMPKα, suggesting that elevated fatty acid levels in follicular fluid may reduce fatty acid oxidation in GCs by inhibiting AMPKα phosphorylation, thereby further impairing oocyte development [89].
Lipid metabolism in GCs primarily involves fatty acid and cholesterol metabolism to produce steroid hormones. GCs regulate hormonal changes within the follicle by processing steroid hormones, thus controlling oocyte development. Fatty acid β-oxidation serves as a vital source of ATP for oocyte maturation. Carnitine is indispensable in fatty acid metabolism as it facilitates the transport of long-chain free fatty acids into the mitochondrial matrix for β-oxidation. Supplementing culture media with carnitine enhances β-oxidation in follicles, promotes lipid utilization, increases energy availability, and positively impacts oocyte and embryo developmental competence [90]. However, both obese and non-obese PCOS women exhibit reduced serum carnitine levels [91], potentially inhibiting fatty acid β-oxidation. Despite these findings, the precise mechanisms by which GCs lipid metabolism regulates energy metabolism remain to be elucidated.
Transcriptomic studies of PCOS GCs have revealed interactions between ovarian steroidogenesis and GC lipid metabolism, including fatty acid biosynthesis, and their effects on follicle growth and ovulation in vitro. Genes encoding enzymes involved in fatty acid biosynthesis, such as fatty acid synthase (FASN), stearoyl-CoA desaturase (SCD), and fatty acid desaturase (FADS), are downregulated in GCs from PCOS patients, potentially altering the fatty acid profile in GCs and leading to insufficient energy supply for oocyte maturation. Additionally, the expression of genes involved in ovarian steroidogenesis, such as 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR), is reduced, resulting in disordered steroid hormone metabolism, decreased estrogen levels, and increased androgen and progesterone levels. In mice treated with dehydroepiandrosterone (DHEA), the expression of key genes in androgen and estrogen biosynthesis pathways, including Cytochrome P450 17A1 (CYP17A1) and Cytochrome P450 19A1 (CYP19A1), is significantly reduced, leading to lower estradiol (E2) levels, which negatively affect follicle development and ovulation [3].
In summary, lipid metabolism dysregulation in PCOS manifests in various aspects, including abnormal fatty acid metabolic pathways, altered fatty acid composition in follicular fluid and GCs, inhibited fatty acid oxidation, and disrupted steroid hormone metabolism. These factors contribute to ovarian energy metabolism abnormalities, adversely affecting oocyte development and follicular function.
Mitochondrial abnormalities in energy metabolism of PCOS
Mitochondrial dysfunction represents another critical factor contributing to the abnormal energy metabolism of GCs. In PCOS, ovarian GCs exhibit structural abnormalities and impaired mitochondrial function [92]. This dysfunction directly compromises GC functionality, subsequently affecting oocyte quality and ovarian reserve capacity, ultimately leading to adverse pregnancy outcomes [93].
Mitochondrial oxidative phosphorylation
Mitochondria are the primary site of ROS production within cells. Under normal physiological conditions, intracellular ROS act as critical signaling molecules, regulating essential physiological processes. However, when tissues are subjected to damage, mitochondrial ROS levels can increase sharply, triggering oxidative stress. NDUFS1, a subunit of mitochondrial respiratory chain complex I, is regulated by SIRT3 through deacetylation, which influences mitochondrial oxidative phosphorylation and ROS production. In GCs of PCOS, SIRT3 deficiency may lead to mitochondrial dysfunction and elevated OS levels, ultimately impairing oocyte quality [81]. Mitochondria-targeted antioxidants, such as coenzyme Q10, have been shown to regulate folliculogenesis and redox signaling pathways in granulosa cells of PCOS mouse models, providing a promising therapeutic avenue for PCOS treatment [16].
Mitochondrial morphology
Mitochondrial morphology is closely linked to its bioenergetic function, and transmission electron microscopy (TEM) is widely used to examine structural changes in mitochondria. The PI3K/Akt signaling pathway plays a protective role by reducing the opening of mitochondrial permeability transition pore (mPTP), stabilizing mitochondrial membrane potential, and preventing membrane damage. Mitochondrial membrane injury can lead to swelling, increased cytochrome C release, and exacerbated apoptosis [94]. In GCs from PCOS patients and mouse models, mitochondrial swelling, cristae loss, and membrane damage have been observed. Concurrently, elevated cytoplasmic cytochrome C levels, upregulated mitochondrial BAX (BCL2-associated X protein) expression, and downregulated p-Akt expression were noted. Melatonin protects GCs from mitochondrial membrane damage by enhancing SIRT1 expression and activating the PDK1/Akt signaling pathway [95].
Mitochondrial DNA mutations
Mitochondrial DNA (mtDNA) encodes key proteins and components of the mitochondrial respiratory chain complexes, playing a crucial role in regulating mitochondrial energy metabolism. Due to its proximity to the electron transport chain (ETC) and the absence of histone protection and corresponding repair mechanisms, mtDNA is vulnerable to mutations caused by ROS attacks [96]. Specifically, mtDNA mutations lead to mitochondrial dysfunction, disrupting OXPHOS, reducing ATP production, and increasing ROS generation, which ultimately trigger apoptosis and genomic damage [97]. Meta-analyses have revealed decreased mtDNA copy numbers in PCOS female patients, along with genetic variations in both coding and non-coding regions of mtDNA, which are believed to contribute to abnormal protein synthesis of mitochondrial components, leading to OS in cells, triggering autophagy [98]. Notably, these variations involve mutations in several OXPHOS complex components and tRNA genes [99, 100]. For example, the ND1T3394C mutation affects the stability of ND1 mRNA, the assembly and activity of Complex I, and results in decreased ATP levels, reduced MMP, and increased ROS production [101]. Similarly, mutations in tRNA-encoding genes, such as tRNALeu(UUR) C3275T, tRNAGln T4363C, and tRNALys A8343G, alter tRNA secondary structures, leading to increased ROS levels, reduced MMP, diminished ATP production, and lower mtDNA copy numbers [99]. PCOS patients with IR carrying mt-tRNA mutations exhibit significant OS and mitochondrial dysfunction [102]. These mutations impair OXPHOS complexes and mitochondrial protein synthesis, reducing ATP production in granulosa cells and leading to insufficient energy supply [103]. MtDNA copy number serves as an essential indicator of mitochondrial metabolic function. Studies have shown that the mtDNA copy number in follicular GCs correlates with embryo quality in in vitro fertilization (IVF) [104]. These findings suggest that mtDNA genetic variations may lead to mitochondrial dysfunction, contributing to the pathogenesis of PCOS.
Mitochondrial quality control
The mitochondrial quality control system, encompassing mitochondrial biogenesis, dynamics, and mitophagy, is essential for maintaining mitochondrial homeostasis and function. Mitochondrial biogenesis acts as a regenerative mechanism to sustain mitochondrial quantity. In GCs of PCOS patients, the expression levels of key mitochondrial biogenesis genes, including Mitochondrial transcription factor A (TFAM), POLG, and RNaseH1, are significantly reduced [105]. TFAM plays a critical role in regulating mtDNA replication and transcription, thereby influencing mitochondrial gene expression and functionality. Additionally, TFAM is instrumental in protecting mtDNA from oxidative stress-induced damage [106]. Peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α) is a master regulator of mitochondrial biogenesis and function. Cellular energy states, such as increased SIRT1 or NAD levels, promote the nuclear accumulation of PGC-1α, initiating the transcription of genes required for mitochondrial biogenesis and function [107]. SIRT1 regulates PGC-1α deacetylation through the AMPK signaling pathway, which is crucial for maintaining mitochondrial function in ovarian GCs [108]. A downregulated SIRT1/p-AMPK-PGC-1α pathway leads to decreased expression of mitochondrial transcription factors in GCs, impairing mitochondrial function and ultimately compromising oocyte quality [109]. Furthermore, reduced expression of mitochondrial biogenesis genes, such as PGC-1α and NRF1, in GCs of atretic follicles suppresses the mitogen-activated pathway kinase (MAPK)/extracellular signal-regulated kinases (ERK) signaling pathway, which is necessary for initiating mitochondrial biogenesis. This suppression contributes to an increased number of atretic follicles in PCOS mouse models [110]. Vitamin D3 enhances mitochondrial biogenesis and membrane integrity, improving mtDNA copy number in GCs of PCOS mice, and potentially enhancing follicle development and oocyte quality [111].
Mitochondria are highly dynamic organelles that undergo continuous processes of fusion and fission, altering their morphology, size, and position, collectively known as mitochondrial dynamics, to maintain mitochondrial homeostasis [112]. Mitochondrial fission and fusion play key roles in follicular development. Fission breaks down tubular mitochondrial networks into smaller organelles, facilitating the removal of depolarized mitochondria through mitophagy. In GCs from patients with PCOS, mitochondrial dynamics are disrupted, leading to increased fission and decreased fusion. In PCOS rat models, a reduction in rod-shaped mitochondria is observed, along with an increased proportion of rounded and contracted mitochondria. This is accompanied by the upregulation of the mitochondrial fission marker dynamin-related protein 1 (Drp1). Excessive Drp1 expression promotes mitochondrial fission, which further accelerates mitophagy and apoptosis, ultimately resulting in growth arrest of early antral follicles [113]. Mitochondrial fusion is typically viewed as a protective response, where two mitochondria merge their inner and outer membranes to form a tubular, interconnected network, thereby enhancing mitochondrial resilience. The fusion process is primarily regulated by the interactions of mitofusin 1 and 2 (Mfn1/2), located on the outer mitochondrial membrane, and optic atrophy 1 (OPA1), located on the inner mitochondrial membrane. However, in ovarian tissues of PCOS rats, the expression of Mfn1/2 is significantly downregulated [114]. Thus, disturbances in mitochondrial dynamics may lead to the accumulation of damaged mitochondria in the ovaries of PCOS models, ultimately impairing ovarian function.
Mitophagy refers to the selective degradation of damaged mitochondria by cells through autophagic mechanisms, thereby maintaining mitochondrial and cellular homeostasis. Among the key pathways involved in this process is the ubiquitin-dependent PINK1/Parkin-mediated pathway. Specifically, PINK1 acts as a sensor of mitochondrial damage and recruits Parkin (an E3 ubiquitin ligase) to damaged mitochondria, facilitating their clearance through Parkin-mediated ubiquitination and subsequent mitophagy [115]. However, excessive activation of mitophagy can lead to the degradation of essential cellular components, ultimately resulting in cellular dysfunction. In GCs of PCOS patients and androgen-treated KGN cells, PINK1/Parkin-mediated mitophagy is enhanced, as indicated by increased levels of autophagy-related proteins Beclin1 and LC3B-II, alongside elevated protein levels of PINK1 and Parkin, and decreased SIRT1 expression. Melatonin has been shown to ameliorate this excessive PINK1/Parkin-mediated mitophagy by enhancing SIRT1 expression in GCs [116]. However, the mechanisms of mitophagy in PCOS remain controversial. B-cell lymphoma-2 adenovirus E1B 19 kDa interacting protein 3 (BNIP3) induces mitophagy following autophagy stimulation, whereas BNIP3L promotes autophagosome accumulation. Excessive ROS levels may impair the hypoxia-inducible factor (HIF)-1α/BNIP3-mediated mitophagy pathway, thereby weakening mitophagy and exacerbating mitochondrial dysfunction [114].
Mitochondrial Ca2 + overload
OS disrupts intracellular calcium homeostasis, causing a significant release of Ca²⁺ from the endoplasmic reticulum (ER) and its subsequent influx into mitochondria. This process increases mitochondrial membrane permeability, leads to the loss of membrane potential, and triggers the release of pro-apoptotic factors [117]. The mitochondrial calcium uniporter (MCU), a highly selective Ca²⁺ channel located on the inner mitochondrial membrane, regulates the influx of cytoplasmic Ca²⁺ into mitochondria. Abnormal activation of the MCU can result in mitochondrial calcium overload, leading to mitochondrial dysfunction, impaired oxidative phosphorylation, loss of membrane potential, and defective opening of the mPTP [118]. In the ovaries of PCOS mouse models, increased MCU expression disrupts mitochondrial calcium homeostasis. Puerarin, a natural isoflavone, ameliorates this imbalance by inhibiting ERK1/2 and JNK phosphorylation, reducing cytoplasmic Ca²⁺ accumulation, and inactivating the calcineurin/Nfatc pathway targeting the MCU. These actions prevent excessive Ca²⁺ influx into mitochondria, thereby preserving mitochondrial function, improving the secretory function of GCs, and reducing apoptosis [119].
The effect of OS and energy metabolism disorder on PCOS reproduction
Ovarian GCs generate pyruvate through the glycolytic pathway, serving as the main energy source for oocytes. Additionally, GCs regulate the ovarian redox balance, playing a crucial role in the development, maturation, and ovulation process of follicles [14]. Mitochondrial dysfunction and restricted glycolysis in GCs may lead to decreased energy production and increased OS, potentially damaging oocytes in patients with PCOS [11, 12, 120].
The pathological role of OS in PCOS reproductive dysfunction
Studies have shown that OS levels in patients with PCOS are significantly higher than those in non-PCOS anovulatory patients [16]. OS is closely linked to decreased oocyte quality, reduced oocyte number, lower fertilization rates, diminished embryo quality, and reduced pregnancy rates in PCOS patients [14, 34, 62, 121]. OS activates the JNK pathway, enhancing the activity of forkhead box protein O1 (FoxO1), which subsequently induces apoptosis in GCs [122]. Since GCs tightly surround the oocyte, a high rate of GC apoptosis negatively impacts oocyte quality, thereby reducing the success rate of in vitro fertilization-embryo transfer (IVF-ET) in PCOS patients [34]. Moreover, OS induces the expression of pro-apoptotic gene Bax in oocytes, further exacerbating ovarian dysfunction in PCOS [123]. These mechanisms collectively impair the health of GCs and oocytes, contributing to ovarian dysfunction.These findings suggest that reducing OS with antioxidants may improve reproductive outcomes in PCOS patients. For example, melatonin alleviates GC autophagic death by inhibiting FoxO1 through the PI3K-Akt axis, thereby improving ovarian function in PCOS [124, 125]. Growth hormone (GH) also activates the PI3K/Akt signaling pathway, mitigating oxidative stress, enhancing mitochondrial function in GCs, and improving oocyte quality in PCOS patients [126, 127].
The effect of energy metabolism imbalance on the reproductive function of PCOS
Ovarian energy metabolism significantly influences oocyte maturation and ovulation. AMP-activated protein kinase (AMPK) is a key sensor of cellular energy status, maintaining energy balance by regulating the synthesis and degradation of cellular metabolites [128]. he hypoxia-inducible factor 1 (HIF1) pathway promotes follicular development and prevents follicular atresia; however, energy deficiency activates AMPK, which in turn inhibits the mTOR and HIF1 signaling pathways, leading to follicular atresia [129]. GCs enhance glycolysis and reduce AMPK activity to activate the mTOR signaling pathway, subsequently initiating the PI3K-Akt-FOXO3a signaling pathway within oocytes, thereby promoting the activation of primordial follicles [130]. In PCOS, impaired glycolysis in GCs is hypothesized to activate AMPK, inhibiting mTOR signaling, which may contribute to the restricted activation of primordial follicles and lead to follicular atresia. Additionally, impaired glycolytic metabolism results in insufficient energy substrates for both oocytes and GCs, leading to mitochondrial OXPHOS dysfunction in GCs. This dysfunction impairs ATP synthesis and generates excessive ROS. These ROS further damage mitochondria, induce OS and ultimately cause insufficient energy supply to oocytes and apoptosis in GCs [81].
Treatment strategies for energy metabolism disorder and OS in PCOS
Analysis of oxidative markers and antioxidant parameters in PCOS patients, along with their associations with hormonal, glucose, lipid metabolism disturbances, and inflammatory factors, suggests that elevated OS levels may be a key contributor to adverse pregnancy outcomes. This indicates that antioxidant therapy may hold clinical significance in the management of PCOS. Several antioxidant agents, such as resveratrol, coenzyme Q10, and astaxanthin, have already been reported for use in PCOS. In addition, supplementation with trace elements has been shown to effectively reduce OS and improve endocrine dysfunction associated with PCOS [75]. Besides the antioxidants mentioned in the text, various agents used in PCOS to reduce oxidative stress, enhance mitochondrial function, and regulate energy metabolism are summarized in Table 3. This refined assessment provides a comprehensive overview of the therapeutic strategies involving antioxidants, mitochondrial regulators, and energy metabolism modulators tailored for patients with PCOS. Such interventions hold promise in ameliorating the pathophysiological aspects of PCOS and warrant further investigation for better patient outcomes.
Table 3.
Testing of antioxidants and drugs to improve energy metabolism in animals and humans
| Drugs | Subjects | Mechanism | Effect | References |
|---|---|---|---|---|
| Soy Isoflavones | PCOS rats | Inhibits NF-κB p65signaling pathway |
a. Alleviates OS, inflammation; b. Improves ovarian morphology, hormonal disturbances |
[131] |
| Soy Isoflavones | PCOS patients | - |
a. Alleviates OS; b. Improves hormonal disturbances and IR |
[132] |
| Sulforaphane | PCOS patients’ GLCs | Activates AMPK/AKT/Nrf2 signaling pathway |
a. Alleviates OS; b. Reduces cell apoptosis |
[133] |
| MitoQ10 | PCOS-IR rats | Improves mitochondrial function; Regulates cell death-related proteins |
a. Alleviates OS; b. Reverses endocrine and reproductive conditions |
[134] |
| Acetyl L-carnitine | PCOS mice |
a. Antioxidant properties; b. Enhances mitochondrial function |
a. Alleviates OS; b. Improves ovarian dysfunction |
[135] |
| Humanin | PCOS rats | Activates Keap1/Nrf2 signaling pathway | Alleviates OS | [136] |
| Genistein | PCOS mice | Enhances antioxidant capacity via ER-Nrf2-Foxo1 pathway |
a. Alleviates OS; b. Protects ovarian function |
[137] |
| Luteolin | PCOS rats | Activates Nrf2 pathway to enhance antioxidant capacity |
a. Alleviates OS; b. Improves IR |
[138] |
| Curcumin | PCOS patients | Increases gene expression of PGC1-α | Alleviates OS | [139] |
| Hydroxysafflor yellow A | PCOS mice | Antioxidant properties |
a. Alleviates OS; b. Restores hormone secretion |
[140] |
| Astaxanthin | PCOS patients | Activates Nrf2/HO-1 signaling pathway |
a. Alleviates OS; b. Enhances oocyte and embryo quality |
[8] |
| Oligosaccharide | KGN | Inhibits HIF-1α and VEGFA gene expression |
a. Alleviates OS, inflammation; b. Inhibits GCs apoptosis; c. Improves follicle development |
[141] |
| Metformin | PCOS mice |
a. Alleviates OS; b. Enhances oocyte development |
[142] | |
| Metformin and Resveratrol | PCOS rats |
a. Activates SIRT1 antioxidant pathway; b. Activates AMPK anti-inflammatory system |
a. Alleviates OS, inflammation; b. Improves hormonal disturbances, follicle quality |
[143] |
| Selenium | PCOS rats |
a. Regulates mitochondrial dynamics; b. Anti-apoptotic effects |
a. Alleviates OS; b. Improves mitochondrial function; c. Improves endocrine and metabolic disturbances |
[144] |
| Vitamin C/E | PCOS mice | Upregulates antioxidant enzyme expression |
a. Alleviates OS; b. Restores ovarian function |
[145] |
| Vitamin D | PCOS mice | (a) Activates MAPK-ERK1/2 signaling pathway; (b) Improves GCs mitochondrial biosynthesis |
a. Alleviates OS; b. Improves ovarian mitochondrial and follicular damage |
[110] |
| Melatonin | PCOS mice | Enhances SIRT1 expression to inhibit PINK1/Parkin-mediated mitochondrial autophagy | Protects GCs mitochondrial function | [116] |
| N-acetyl cysteine | PCOS mice | (a) Improves mitochondrial function; (b) Alleviates OS | [65] | |
| Resveratrol | PCOS rats | Upregulates SIRT2 to promote expression of glycolytic enzyme | Restores glycolysis process, improving ovarian energy metabolism dysfunction | [82] |
| Diane-35 and Metformin | PCOS rats | increases the expression of PKM2 and LDHA | Restores glycolysis pathway to improve ovarian energy metabolism | [83] |
| Bu-Shen-Tian-Jing Formula (BSTJF) | PCOS GCs | Upregulates SIRT3, reducing the production of mitochondrial ROS and inhibiting the activation of p38 MAPK |
a. Alleviates OS; b. Improves glucose metabolism |
[146] |
| Mogroside V | PCOS rats | Upregulates LDHA, HK2, and PKM2 in GCs |
a. Enhances lactate and energy production; b. Improves follicle development and ovulation |
[147] |
Conclusions
This review emphasizes the importance of energy metabolism balance and antioxidation in PCOS to ensure efficient energy utilization and maintenance of mitochondrial function. Mitochondrial dysfunction primarily affects the energy metabolism of GCs, which may result from disruptions in the mitochondrial quality control system, genetic mutations, oxidative stress, and structural mitochondrial damage. Additionally, various factors—including obesity, inflammation, hyperlipidemia, hyperglycemia, androgen excess, insulin resistance, and heavy metal exposure—independently or synergistically contribute to increased OS in PCOS. Consequently, mitochondrial-targeted therapies have emerged as promising interventions to mitigate OS and improve energy metabolism. However, further studies are required to clarify the exact role of mitochondrial dysfunction in the pathogenesis of PCOS, specifically whether it serves as a primary or secondary factor.
Acknowledgements
Not applicable.
Abbreviations
- 8-OHdG
8-hydroxy-2’-deoxyguanosine
- ATP
Adenosine Triphosphate
- AGEs
Advanced Glycation End Products
- ARE
Antioxidant Response Elements
- ADMA
Asymmetric Dimethylarginine
- BAX
BCL2-Associated X Protein
- BCAAs
Branched-Chain Amino Acids
- CAT
Catalase
- Cyt c
Cytochrome c
- Drp1
Dynamin-Related Protein 1
- ER
Endoplasmic Reticulum
- ERK1/2
Extracellular Signal-Regulated Kinases
- FoxO1
Forkhead Box O1
- GCL
Glutamate Cysteine Ligase
- GCs
Granulosa Cells
- GLUT1
Glucose Transporter Type 1
- GLUT4
Glucose Transporter Type 4
- GPx
Glutathione Peroxidase
- GR
Glutathione Reductase
- GSH
Glutathione
- GSSG
Oxidized Glutathione
- GST
Glutathione-S-Transferase
- H2O2
Hydrogen Peroxide
- HA
Hyperandrogenemia
- HCY
Homocysteine
- HIF-1α
Hypoxia-Inducible Factor-1α
- HK2
Hexokinase 2
- HO-1
Heme Oxygenase 1
- IRS-1
Insulin Receptor Substrate 1
- IVF-ET
In Vitro Fertilization-Embryo Transfer
- JNK
c-Jun N-Terminal Kinase
- Keap
Kelch-like ECH-associated protein 1
- LDHA
Lactate Dehydrogenase A
- MAPK
Mitogen-Activated Pathway Kinase
- Mcu
Mitochondrial calcium uniporter
- MDA
Malondialdehyde
- Mfn1/2
Membrane Fusion Proteins 1 and 2
- MMP
Mitochondrial Membrane Potential
- mPTP
mitochondrial Permeability Transition Pore
- NAD+
Nicotinamide Adenine Dinucleotide
- NDUFS1-AC
The Acetylation Status of NDUFS1
- NO
Nitric Oxide
- Nrf1/2
Nuclear Respiratory Factor 1 and 2
- Nrf2
Nuclear Factor Erythroid 2-related factor 2
- OXPHOS
Oxidative Phosphorylation
- PC
Protein Carbonyl
- PCG-1α
Peroxisome Proliferator-Activated Receptor γ Coactivator-1α
- PFPK
Phosphofructokinase
- PKM2
Pyruvate Kinase M2
- PON1
Paraoxonase 1
- PPM1K
Protein Phosphatase Mg2+/Mn2+-dependent 1 K
- RET
Retinol
- ROS
Reactive Oxygen Species
- SIRT3
Sirtuin 3
- SOA
Superoxide Anion
- SOD
Superoxide Dismutase
- TAC
Total Antioxidant Capacity
- TCA cycle
Tricarboxylic Acid Cycle
- TLR2
Toll-Like Receptor 2
- TOS
Total Oxidant Status
- TFAM
Mitochondrial Transcription Factor A
- VEGFA
Vascular Endothelial Growth Factor A
- XO
Xanthine Oxidase
- p38MAPK
p38 Mitogen-Activated Protein Kinase
Author contributions
HY and LW drafted the manuscript, conducted a systematic literature search, compiled and summarized the findings, and designed detailed illustrations to enhance the manuscript visually. GZ, NL, YZ, JL, MJ, XD, QZ, DX, LH, and ZZ edited and proofread specific sections of the manuscript, ensuring linguistic accuracy, logical flow, and strict adherence to formatting and journal guidelines. WL and ML conceptualized the study design, provided critical feedback, and supervised the revision process to ensure clarity, coherence, and scientific rigor.
Funding
This work was supported by the Key project of Sichuan Science and Technology Department (2023YFS0057), Youth Fund of Sichuan Natural Science Foundation (2024NSFSC1674), school science and technology fund of Chengdu Medical College (CYZYB23-03), the Key projects of Sichuan Medical and Health Care Promotion Institution (KY2023SJ0034).
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Heqiu Yan and Li Wang are joint first authors.
Contributor Information
Mengjun Luo, Email: luomengjun2023@163.com.
Weixin Liu, Email: liuweixind@163.com.
References
- 1.Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22:687–708. [DOI] [PubMed] [Google Scholar]
- 2.Barber TM. Why are women with polycystic ovary syndrome obese? Br Med Bull. 2022;143:4–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liao B, Qi X, Yun C, Qiao J, Pang Y. Effects of Androgen excess-related metabolic disturbances on Granulosa cell function and Follicular Development. Front Endocrinol (Lausanne). 2022;13:815968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teede HJ, Hutchison SK, Zoungas S. The management of insulin resistance in polycystic ovary syndrome. Trends Endocrinol Metabolism. 2007;18:273–9. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C-H, Liu X-Y, Wang J. Essential role of Granulosa Cell glucose and lipid metabolism on oocytes and the potential metabolic imbalance in polycystic ovary syndrome. Int J Mol Sci. 2023;24:16247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang J, Bao Y, Zhou X, Zheng L. Polycystic ovary syndrome and mitochondrial dysfunction. Reprod Biol Endocrinol. 2019;17:67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Forman HJ, Zhang H. Targeting oxidative stress in disease: promise and limitations of antioxidant therapy. Nat Rev Drug Discov. 2021;20:689–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gharaei R, Alyasin A, Mahdavinezhad F, Samadian E, Ashrafnezhad Z, Amidi F. Randomized controlled trial of astaxanthin impacts on antioxidant status and assisted reproductive technology outcomes in women with polycystic ovarian syndrome. J Assist Reprod Genet. 2022;39:995–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang J, Wu X. The effects of mitochondrial dysfunction on energy metabolism switch by HIF-1α signalling in granulosa cells of polycystic ovary syndrome. Endokrynol Pol. 2020;71:134–45. [DOI] [PubMed] [Google Scholar]
- 10.Zhao H, Zhao Y, Li T, Li M, Li J, Li R, et al. Metabolism alteration in follicular niche: the nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic Biol Med. 2015;86:295–307. [DOI] [PubMed] [Google Scholar]
- 11.Mazloomi S, Farimani MS, Tavilani H, Karimi J, Amiri I, Abbasi E, et al. Granulosa cells from immature follicles exhibit restricted glycolysis and reduced energy production: a dominant problem in polycystic ovary syndrome. J Assist Reprod Genet. 2023;40:343–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhao Y-K, Gao Y-N, Wang L-C, Wang J, Wang G-J, Wu H-L. Correlation between abnormal energy metabolism of ovarian granulosa cells and in vitro fertilization-embryo transfer outcomes in patients with polycystic ovary syndrome and obesity. J Ovarian Res. 2023;16:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bannigida DM, Nayak BS, Vijayaraghavan R. Insulin resistance and oxidative marker in women with PCOS. Arch Physiol Biochem. 2020;126:183–6. [DOI] [PubMed] [Google Scholar]
- 14.Naigaonkar A, Dadachanji R, Hinduja I, Mukherjee S. Altered redox status may contribute to aberrant folliculogenesis and poor reproductive outcomes in women with polycystic ovary syndrome. J Assist Reprod Genet. 2021;38:2609–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garg D, Merhi Z. Relationship between Advanced Glycation End products and steroidogenesis in PCOS. Reprod Biol Endocrinol. 2016;14:71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Artimani T, Karimi J, Mehdizadeh M, Yavangi M, Khanlarzadeh E, Ghorbani M, et al. Evaluation of pro-oxidant-antioxidant balance (PAB) and its association with inflammatory cytokines in polycystic ovary syndrome (PCOS). Gynecol Endocrinol. 2018;34:148–52. [DOI] [PubMed] [Google Scholar]
- 17.Hilali N, Vural M, Camuzcuoglu H, Camuzcuoglu A, Aksoy N. Increased prolidase activity and oxidative stress in PCOS. Clin Endocrinol (Oxf). 2013;79:105–10. [DOI] [PubMed] [Google Scholar]
- 18.Abudawood M, Alnuaim L, Tabassum H, Ghneim HK, Alfhili MA, Alanazi ST, et al. An insight into the Impact of Serum Tellurium, Thallium, Osmium and Antimony on the Antioxidant/Redox status of PCOS patients: a Comprehensive Study. Int J Mol Sci. 2023;24:2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang H, Ruan X, Li Y, Cheng J, Mueck AO. Oxidative stress indicators in Chinese women with PCOS and correlation with features of metabolic syndrome and dependency on lipid patterns. Arch Gynecol Obstet. 2019;300:1413–21. [DOI] [PubMed] [Google Scholar]
- 20.Enechukwu CI, Onuegbu AJ, Olisekodiaka MJ, Eleje GU, Ikechebelu JI, Ugboaja JO, et al. Oxidative stress markers and lipid profiles of patients with polycystic ovary syndrome in a Nigerian tertiary hospital. Obstet Gynecol Sci. 2019;62:335–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeelani H, Ganie MA, Masood A, Amin S, Kawa IA, Fatima Q, et al. Assessment of PON1 activity and circulating TF levels in relation to BMI, testosterone, HOMA-IR, HDL-C, LDL-C, CHO, SOD activity and TAC in women with PCOS: an observational study. Diabetes Metab Syndr. 2019;13:2907–15. [DOI] [PubMed] [Google Scholar]
- 22.Murri M, Luque-Ramírez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF. Circulating markers of oxidative stress and polycystic ovary syndrome (PCOS): a systematic review and meta-analysis. Hum Reprod Update. 2013;19:268–88. [DOI] [PubMed] [Google Scholar]
- 23.Baskol G, Aygen E, Erdem F, Caniklioğlu A, Narin F, Sahin Y, et al. Assessment of paraoxonase 1, xanthine oxidase and glutathione peroxidase activities, nitric oxide and thiol levels in women with polycystic ovary syndrome. Acta Obstet Gynecol Scand. 2012;91:326–30. [DOI] [PubMed] [Google Scholar]
- 24.Nori W, Helmi ZR. Can follicular fluid 8-oxo-2’-deoxyguanosine predict the clinical outcomes in ICSI cycle among couples with normospermia male? Obstet Gynecol Sci. 2023;66:430–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Merhi Z, Kandaraki EA, Diamanti-Kandarakis E. Implications and future perspectives of AGEs in PCOS Pathophysiology. Trends Endocrinol Metab. 2019;30:150–62. [DOI] [PubMed] [Google Scholar]
- 26.Fatima Q, Amin S, Kawa IA, Jeelani H, Manzoor S, Rizvi SM, et al. Evaluation of antioxidant defense markers in relation to hormonal and insulin parameters in women with polycystic ovary syndrome (PCOS): a case-control study. Diabetes Metab Syndr. 2019;13:1957–61. [DOI] [PubMed] [Google Scholar]
- 27.Seyyed Anvari S, Dehgan GH, Razi M. Preliminary findings of platelet-rich plasma-Induced Ameliorative Effect on polycystic ovarian syndrome. Cell J. 2019;21:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uyanikoglu H, Sabuncu T, Dursun H, Sezen H, Aksoy N. Circulating levels of apoptotic markers and oxidative stress parameters in women with polycystic ovary syndrome: a case-controlled descriptive study. Biomarkers. 2017;22:643–7. [DOI] [PubMed] [Google Scholar]
- 29.Johnson LJ, Meacham SL, Kruskall LJ. The antioxidants–vitamin C,vitamin E, selenium, and carotenoids. J Agromedicine. 2003;9:65–82. [DOI] [PubMed] [Google Scholar]
- 30.Vašková J, Kočan L, Vaško L, Perjési P. Glutathione-related enzymes and proteins: a review. Molecules. 2023;28:1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Di Meo S, Reed TT, Venditti P, Victor VM. Role of ROS and RNS sources in physiological and pathological conditions. Oxid Med Cell Longev. 2016;2016:1245049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vermot A, Petit-Härtlein I, Smith SME, Fieschi F. NADPH oxidases (NOX): an overview from Discovery, Molecular mechanisms to Physiology and Pathology. Antioxid (Basel). 2021;10:890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lai Q, Xiang W, Li Q, Zhang H, Li Y, Zhu G, et al. Oxidative stress in granulosa cells contributes to poor oocyte quality and IVF-ET outcomes in women with polycystic ovary syndrome. Front Med. 2018;12:518–24. [DOI] [PubMed] [Google Scholar]
- 35.Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–33. [DOI] [PubMed] [Google Scholar]
- 36.Lu J, Wang Z, Cao J, Chen Y, Dong Y. A novel and compact review on the role of oxidative stress in female reproduction. Reprod Biol Endocrinol. 2018;16:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabel AM, Al-Shehri AH, Al-Talhi RA, Abd Elmaaboud MA. The promising effect of linagliptin and/or indole-3-carbinol on experimentally-induced polycystic ovarian syndrome. Chem Biol Interact. 2017;273:190–9. [DOI] [PubMed] [Google Scholar]
- 38.Hui M, Hu S, Ye L, Zhang M, Jing X, Hong Y. PAK2/beta-catenin/c-Myc/PKM2 signal transduction suppresses ovarian granulosa cell apoptosis in polycystic ovary syndrome. Biochem Biophys Res Commun. 2023;677:54–62. [DOI] [PubMed] [Google Scholar]
- 39.Ji R, Jia F, Chen X, Gao Y, Yang J. Carnosol inhibits KGN cells oxidative stress and apoptosis and attenuates polycystic ovary syndrome phenotypes in mice through Keap1-mediated Nrf2/HO-1 activation. Phytother Res. 2023;37:1405–21. [DOI] [PubMed] [Google Scholar]
- 40.Morgan MJ, Liu Z. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011;21:103–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zeber-Lubecka N, Ciebiera M, Hennig EE. Polycystic ovary syndrome and oxidative stress—from bench to Bedside. Int J Mol Sci. 2023;24:14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yifu P. Evidence for causal effects of polycystic ovary syndrome on oxidative stress: a two-sample mendelian randomisation study. BMC Med Genomics. 2023;16:141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kurek Eken M, Sahin Ersoy G, Yayla Abide C, Sanverdi İ, Devranoglu B, Kutlu T, et al. Association between circulating neuregulin 4 levels and metabolic, aterogenic, and AMH profile of polycystic ovary syndrome. J Obstet Gynaecol. 2019;39:975–80. [DOI] [PubMed] [Google Scholar]
- 44.Luc K, Schramm-Luc A, Guzik TJ, Mikolajczyk TP. Oxidative stress and inflammatory markers in prediabetes and diabetes. J Physiol Pharmacol. 2019;70. [DOI] [PubMed]
- 45.Malin SK, Kirwan JP, Sia CL, González F. Pancreatic β-cell dysfunction in polycystic ovary syndrome: role of hyperglycemia-induced nuclear factor-κB activation and systemic inflammation. Am J Physiol Endocrinol Metab. 2015;308:E770–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Malin SK, Kirwan JP, Sia CL, González F. Glucose-stimulated oxidative stress in mononuclear cells is related to pancreatic β-cell dysfunction in polycystic ovary syndrome. J Clin Endocrinol Metab. 2014;99:322–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zuo T, Zhu M, Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancers. Oxid Med Cell Longev. 2016;2016:8589318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sztretye M, Dienes B, Gönczi M, Czirják T, Csernoch L, Dux L, et al. Astaxanthin: a potential mitochondrial-targeted antioxidant treatment in diseases and with aging. Oxid Med Cell Longev. 2019;2019:3849692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang R, Liu H, Bai H, Zhang Y, Liu Q, Guan L, et al. Oxidative stress status in Chinese women with different clinical phenotypes of polycystic ovary syndrome. Clin Endocrinol (Oxf). 2017;86:88–96. [DOI] [PubMed] [Google Scholar]
- 50.Zhang J, Zhang Y, Liu H, Bai H, Wang Y, Jiang C, et al. Antioxidant properties of high-density lipoproteins are impaired in women with polycystic ovary syndrome. Fertil Steril. 2015;103:1346–54. [DOI] [PubMed] [Google Scholar]
- 51.Perovic Blagojevic IM, Vekic JZ, Macut DP, Ignjatovic SD, Miljkovic-Trailovic MM, Zeljkovic AR, et al. Overweight and obesity in polycystic ovary syndrome: association with inflammation, oxidative stress and dyslipidaemia. Br J Nutr. 2022;128:604–12. [DOI] [PubMed] [Google Scholar]
- 52.Macut D, Damjanovic S, Panidis D, Spanos N, Glisic B, Petakov M, et al. Oxidised low-density lipoprotein concentration - early marker of an altered lipid metabolism in young women with PCOS. Eur J Endocrinol. 2006;155:131–6. [DOI] [PubMed] [Google Scholar]
- 53.Kumariya S, Ubba V, Jha RK, Gayen JR. Autophagy in ovary and polycystic ovary syndrome: role, dispute and future perspective. Autophagy 17:2706–33. [DOI] [PMC free article] [PubMed]
- 54.Ding Y, Jiang Y, Zhu M, Zhu Q, He Y, Lu Y, et al. Follicular fluid lipidomic profiling reveals potential biomarkers of polycystic ovary syndrome: a pilot study. Front Endocrinol (Lausanne). 2022;13:960274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Si C, Wang N, Wang M, Liu Y, Niu Z, Ding Z. TMT-based proteomic and bioinformatic analyses of human granulosa cells from obese and normal-weight female subjects. Reprod Biol Endocrinol. 2021;19:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ma Y, Zheng L, Wang Y, Gao Y, Xu Y. Arachidonic acid in Follicular Fluid of PCOS induces oxidative stress in a human ovarian granulosa Tumor Cell Line (KGN) and upregulates GDF15 expression as a response. Front Endocrinol (Lausanne). 2022;13:865748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Niu Z, Lin N, Gu R, Sun Y, Feng Y. Associations between insulin resistance, free fatty acids, and Oocyte Quality in Polycystic Ovary Syndrome during in Vitro Fertilization. J Clin Endocrinol Metab. 2014;99:E2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kobayashi M, Yoshino O, Nakashima A, Ito M, Nishio K, Ono Y, et al. Inhibition of autophagy in theca cells induces CYP17A1 and PAI-1 expression via ROS/p38 and JNK signalling during the development of polycystic ovary syndrome. Mol Cell Endocrinol. 2020;508:110792. [DOI] [PubMed] [Google Scholar]
- 59.Chełchowska M, Jurczewska J, Gajewska J, Mazur J, Szostak-Węgierek D, Rudnicka E, et al. Antioxidant defense expressed as glutathione status and Keap1-Nrf2 system action in relation to Anthropometric Parameters and body composition in Young women with polycystic ovary syndrome. Antioxid (Basel). 2023;12:730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Andreas E, Reid M, Zhang W, Moley KH. The effect of maternal high-fat/high-sugar diet on offspring oocytes and early embryo development. Mol Hum Reprod. 2019;25:717–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Dandona P, Mohanty P, Ghanim H, Aljada A, Browne R, Hamouda W, et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab. 2001;86:355–62. [DOI] [PubMed] [Google Scholar]
- 62.Liu Y, Yu Z, Zhao S, Cheng L, Man Y, Gao X, et al. Oxidative stress markers in the follicular fluid of patients with polycystic ovary syndrome correlate with a decrease in embryo quality. J Assist Reprod Genet. 2021;38:471–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.González F, Nair KS, Daniels JK, Basal E, Schimke JM, Blair HE. Hyperandrogenism sensitizes leukocytes to hyperglycemia to promote oxidative stress in lean reproductive-age women. J Clin Endocrinol Metab. 2012;97:2836–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Weng Y, Zhang Y, Wang D, Wang R, Xiang Z, Shen S, et al. Exercise-induced irisin improves follicular dysfunction by inhibiting IRE1α-TXNIP/ROS-NLRP3 pathway in PCOS. J Ovarian Res. 2023;16:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao Q, Zou X, Liu S, Wu H, Shen Q, Kang J. Oxidative stress as a contributor to Insulin Resistance in the skeletal muscles of mice with polycystic ovary syndrome. Int J Mol Sci. 2022;23:11384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Forghani N, Karimi Z, Mokhtari M, Shariati M, Masjedi F. Association of Oxidative Stress with Kidney Injury in a hyperandrogenemic female rat model. Iran J Med Sci. 2023;48:187–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang D, Wang T, Wang R, Zhang X, Wang L, Xiang Z, et al. Suppression of p66Shc prevents hyperandrogenism-induced ovarian oxidative stress and fibrosis. J Transl Med. 2020;18:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhao H, Zhang J, Cheng X, Nie X, He B. Insulin resistance in polycystic ovary syndrome across various tissues: an updated review of pathogenesis, evaluation, and treatment. J Ovarian Res. 2023;16:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sun Y, Li S, Liu H, Bai H, Hu K, Zhang R, et al. Oxidative stress promotes hyperandrogenism by reducing sex hormone-binding globulin in polycystic ovary syndrome. Fertil Steril. 2021;116:1641–50. [DOI] [PubMed] [Google Scholar]
- 70.Dabravolski SA, Nikiforov NG, Eid AH, Nedosugova LV, Starodubova AV, Popkova TV, et al. Mitochondrial Dysfunction and Chronic Inflammation in Polycystic Ovary Syndrome. Int J Mol Sci. 2021;22:3923. [DOI] [PMC free article] [PubMed]
- 71.Yuan B, Luo S, Feng L, Wang J, Mao J, Luo B. Resveratrol regulates the inflammation and oxidative stress of granulosa cells in PCOS via targeting TLR2. J Bioenerg Biomembr. 2022;54:191–201. [DOI] [PubMed] [Google Scholar]
- 72.Swanson KV, Deng M, Ting JP-Y. The NLRP3 inflammasome: molecular activation and regulation to therapeutics. Nat Rev Immunol. 2019;19:477–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Liu Y, Liu H, Li Z, Fan H, Yan X, Liu X, et al. The release of Peripheral Immune Inflammatory cytokines promote an inflammatory Cascade in PCOS patients via altering the Follicular Microenvironment. Front Immunol. 2021;12:685724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang Y, Yang Q, Wang H, Zhu J, Cong L, Li H, et al. NAD + deficiency and mitochondrial dysfunction in granulosa cells of women with polycystic ovary syndrome‡. Biol Reprod. 2021;105:371–80. [DOI] [PubMed] [Google Scholar]
- 75.Srnovršnik T, Virant-Klun I, Pinter B. Heavy Metals and Essential Elements in Association with oxidative stress in women with polycystic ovary Syndrome-A systematic review. Antioxid (Basel). 2023;12:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.da Costa CS, Oliveira TF, Freitas-Lima LC, Padilha AS, Krause M, Carneiro MTWD, et al. Subacute cadmium exposure disrupts the hypothalamic-pituitary-gonadal axis, leading to polycystic ovarian syndrome and premature ovarian failure features in female rats. Environ Pollut. 2021;269:116154. [DOI] [PubMed] [Google Scholar]
- 77.Kurdoglu Z, Kurdoglu M, Demir H, Sahin HG. Serum trace elements and heavy metals in polycystic ovary syndrome. Hum Exp Toxicol. 2012;31:452–6. [DOI] [PubMed] [Google Scholar]
- 78.Jiang X, Xing X, Zhang Y, Zhang C, Wu Y, Chen Y, et al. Lead exposure activates the Nrf2/Keap1 pathway, aggravates oxidative stress, and induces reproductive damage in female mice. Ecotoxicol Environ Saf. 2021;207:111231. [DOI] [PubMed] [Google Scholar]
- 79.Yin J, Hong X, Ma J, Bu Y, Liu R. Serum Trace Elements in patients with polycystic ovary syndrome: a systematic review and Meta-analysis. Front Endocrinol (Lausanne). 2020;11:572384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sutton-McDowall ML, Gilchrist RB, Thompson JG. The pivotal role of glucose metabolism in determining oocyte developmental competence. Reproduction. 2010;139:685–95. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Q, Ren J, Wang F, Pan M, Cui L, Li M, et al. Mitochondrial and glucose metabolic dysfunctions in granulosa cells induce impaired oocytes of polycystic ovary syndrome through Sirtuin 3. Life (Basel). 2022;187:1–16. [DOI] [PubMed] [Google Scholar]
- 82.Liang A, Huang L, Liu H, He W, Lei X, Li M, et al. Resveratrol improves Follicular Development of PCOS rats by regulating the glycolytic pathway. Mol Nutr Food Res. 2021;65:e2100457. [DOI] [PubMed] [Google Scholar]
- 83.Zhang S, Tu H, Yao J, Le J, Jiang Z, Tang Q, et al. Combined use of Diane-35 and metformin improves the ovulation in the PCOS rat model possibly via regulating glycolysis pathway. Reprod Biol Endocrinol. 2020;18:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li T, Zhang Z, Kolwicz SC, Abell L, Roe ND, Kim M, et al. Defective branched-chain amino acid catabolism disrupts glucose metabolism and sensitizes the heart to Ischemia-Reperfusion Injury. Cell Metabol. 2017;25:374–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chang AY, Lalia AZ, Jenkins GD, Dutta T, Carter RE, Singh RJ, et al. Combining a nontargeted and targeted metabolomics approach to identify metabolic pathways significantly altered in polycystic ovary syndrome. Metabolism. 2017;71:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mu L, Ye Z, Hu J, Zhang Y, Chen K, Sun H, et al. PPM1K-regulated impaired catabolism of branched-chain amino acids orchestrates polycystic ovary syndrome. EBioMedicine. 2023;89:104492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Yu Y, Tan P, Zhuang Z, Wang Z, Zhu L, Qiu R, et al. Untargeted metabolomic approach to study the serum metabolites in women with polycystic ovary syndrome. BMC Med Genomics. 2021;14:206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril. 2015;103:303–16. [DOI] [PubMed] [Google Scholar]
- 89.Lai Y, Ye Z, Mu L, Zhang Y, Long X, Zhang C, et al. Elevated levels of follicular fatty acids induce ovarian inflammation via ERK1/2 and Inflammasome activation in PCOS. J Clin Endocrinol Metabolism. 2022;107:2307–17. [DOI] [PubMed] [Google Scholar]
- 90.Placidi M, Di Emidio G, Virmani A, D’Alfonso A, Artini PG, D’Alessandro AM, et al. Carnitines as mitochondrial modulators of oocyte and Embryo Bioenergetics. Antioxidants. 2022;11:745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fenkci SM, Fenkci V, Oztekin O, Rota S, Karagenc N. Serum total L-carnitine levels in non-obese women with polycystic ovary syndrome. Hum Reprod. 2008;23:1602–6. [DOI] [PubMed] [Google Scholar]
- 92.Song L, Yu J, Zhang D, Li X, Chen L, Cai Z, et al. Androgen excess Induced mitochondrial abnormality in ovarian granulosa cells in a rat model of polycystic ovary syndrome. Front Endocrinol (Lausanne). 2022;13:789008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Gao Y, Zou Y, Wu G, Zheng L. Oxidative stress and mitochondrial dysfunction of granulosa cells in polycystic ovarian syndrome. Front Med (Lausanne). 2023;10:1193749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kroemer G, Galluzzi L, Brenner C. Mitochondrial membrane permeabilization in cell death. Physiol Rev. 2007;87:99–163. [DOI] [PubMed] [Google Scholar]
- 95.Zheng B, Meng J, Zhu Y, Ding M, Zhang Y, Zhou J. Melatonin enhances SIRT1 to ameliorate mitochondrial membrane damage by activating PDK1/Akt in granulosa cells of PCOS. J Ovarian Res. 2021;14:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Navarro A, Boveris A. The mitochondrial energy transduction system and the aging process. Am J Physiol Cell Physiol. 2007;292:C670–686. [DOI] [PubMed] [Google Scholar]
- 97.Hahn A, Zuryn S. Mitochondrial genome (mtDNA) mutations that generate reactive oxygen species. Antioxid (Basel). 2019;8:392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moosa A, Ghani M, O’Neill HC. Genetic associations with polycystic ovary syndrome: the role of the mitochondrial genome; a systematic review and meta-analysis. J Clin Pathol. 2022;75:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ding Y, Xia B-H, Zhang C-J, Zhuo G-C. Mitochondrial tRNALeu(UUR) C3275T, tRNAGln T4363C and tRNALys A8343G mutations may be associated with PCOS and metabolic syndrome. Gene. 2018;642:299–306. [DOI] [PubMed] [Google Scholar]
- 100.Zhuo G, Ding Y, Feng G, Yu L, Jiang Y. Analysis of mitochondrial DNA sequence variants in patients with polycystic ovary syndrome. Arch Gynecol Obstet. 2012;286:653–9. [DOI] [PubMed] [Google Scholar]
- 101.Zhu J, Vinothkumar KR, Hirst J. Structure of mammalian respiratory complex I. Nature. 2016;536:354–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ding Y, Xia B-H, Zhang C-J, Zhuo G-C. Mutations in mitochondrial tRNA genes may be related to insulin resistance in women with polycystic ovary syndrome. Am J Transl Res. 2017;9:2984–96. [PMC free article] [PubMed] [Google Scholar]
- 103.Dong X-C, Liu C, Zhuo G-C, Ding Y. Potential roles of mtDNA mutations in PCOS-IR: a review. Diabetes Metab Syndr Obes. 2023;16:139–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ogino M, Tsubamoto H, Sakata K, Oohama N, Hayakawa H, Kojima T, et al. Mitochondrial DNA copy number in cumulus cells is a strong predictor of obtaining good-quality embryos after IVF. J Assist Reprod Genet. 2016;33:367–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Qasemi M, Aleyasin A, Mahdian R, Ghanami Gashti N, Shabani Nashtaei M, Ashrafnezhad Z, et al. Cell-free mtDNA level and its biomarker potency for ART outcome are different in follicular fluid of PCOS and non-PCOS women. Mitochondrion. 2021;59:30–6. [DOI] [PubMed] [Google Scholar]
- 106.Piantadosi CA. Mitochondrial DNA, oxidants, and innate immunity. Free Radic Biol Med. 2020;152:455–61. [DOI] [PubMed] [Google Scholar]
- 107.Tang BL. Sirt1 and the Mitochondria. Mol Cells. 2016;39:87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Di Emidio G, Placidi M, Rea F, Rossi G, Falone S, Cristiano L, et al. Methylglyoxal-dependent glycative stress and deregulation of SIRT1 Functional Network in the Ovary of PCOS mice. Cells. 2020;9:209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Xie C, Lu H, Zhang X, An Z, Chen T, Yu W, et al. Mitochondrial abnormality in ovarian granulosa cells of patients with polycystic ovary syndrome. Mol Med Rep. 2023;29:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Safaei Z, Bakhshalizadeh S, Nasr-Esfahani MH, Akbari Sene A, Najafzadeh V, Soleimani M, et al. Vitamin D3 affects mitochondrial biogenesis through mitogen-activated protein kinase in polycystic ovary syndrome mouse model. J Cell Physiol. 2020;235:6113–26. [DOI] [PubMed] [Google Scholar]
- 111.Safaei Z, Bakhshalizadeh S, Nasr Esfahani MH, Akbari Sene A, Najafzadeh V, Soleimani M, et al. Effect of vitamin D3 on mitochondrial Biogenesis in Granulosa cells derived from polycystic ovary syndrome. Int J Fertil Steril. 2020;14:143–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Zhou H, Ren J, Toan S, Mui D. Role of mitochondrial quality surveillance in myocardial infarction: from bench to bedside. Ageing Res Rev. 2021;66:101250. [DOI] [PubMed] [Google Scholar]
- 113.Salehi R, Mazier HL, Nivet A-L, Reunov AA, Lima P, Wang Q, et al. Ovarian mitochondrial dynamics and cell fate regulation in an androgen-induced rat model of polycystic ovarian syndrome. Sci Rep. 2020;10:1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wang F, Han J, Wang X, Liu Y, Zhang Z. Roles of HIF-1α/BNIP3 mediated mitophagy in mitochondrial dysfunction of letrozole-induced PCOS rats. J Mol Histol. 2022;53:833–42. [DOI] [PubMed] [Google Scholar]
- 115.Jiang Y, Shen M, Chen Y, Wei Y, Tao J, Liu H. Melatonin represses Mitophagy to protect mouse granulosa cells from oxidative damage. Biomolecules. 2021;11:968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yi S, Zheng B, Zhu Y, Cai Y, Sun H, Zhou J. Melatonin ameliorates excessive PINK1/Parkin-mediated mitophagy by enhancing SIRT1 expression in granulosa cells of PCOS. Am J Physiol Endocrinol Metab. 2020;319:E91–101. [DOI] [PubMed] [Google Scholar]
- 117.Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2 + transfer in the control of apoptosis. Oncogene. 2008;27:6407–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Garbincius JF, Elrod JW. Mitochondrial calcium exchange in physiology and disease. Physiol Rev. 2022;102:893–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang Y-S, Li B-Y, Xing Y-F, Huang J-C, Chen Z-S, Yue L, et al. Puerarin ameliorated PCOS through preventing mitochondrial dysfunction dependent on the maintenance of intracellular calcium homeostasis. J Agric Food Chem. 2024;72:2963–76. [DOI] [PubMed] [Google Scholar]
- 120.Peng Y, Yang X, Luo X, Liu C, Cao X, Wang H, et al. Novel mechanisms underlying anti-polycystic ovary like syndrome effects of electroacupuncture in rats: suppressing SREBP1 to mitigate insulin resistance, mitochondrial dysfunction and oxidative stress. Biol Res. 2020;53:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Huang B, Li, Ren X, Ai J, Zhu L, Jin L. Free radical scavenging window of infertile patients with polycystic ovary syndrome: correlation with embryo quality. Front Med. 2017;11:247–52. [DOI] [PubMed] [Google Scholar]
- 122.Weng Q, Liu Z, Li B, Liu K, Wu W, Liu H. Oxidative stress induces mouse follicular granulosa cells apoptosis via JNK/FoxO1 pathway. PLoS ONE. 2016;11:e0167869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nikmard F, Hosseini E, Bakhtiyari M, Ashrafi M, Amidi F, Aflatoonian R. The boosting effects of melatonin on the expression of related genes to oocyte maturation and antioxidant pathways: a polycystic ovary syndrome- mouse model. J Ovarian Res. 2022;15:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shen M, Cao Y, Jiang Y, Wei Y, Liu H. Melatonin protects mouse granulosa cells against oxidative damage by inhibiting FOXO1-mediated autophagy: implication of an antioxidation-independent mechanism. Redox Biol. 2018;18:138–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Xie F, Zhang J, Zhai M, Liu Y, Hu H, Yu Z, et al. Melatonin ameliorates ovarian dysfunction by regulating autophagy in PCOS via the PI3K-Akt pathway. Reproduction. 2021;162:73–82. [DOI] [PubMed] [Google Scholar]
- 126.Gong Y, Luo S, Fan P, Jin S, Zhu H, Deng T, et al. Growth hormone alleviates oxidative stress and improves oocyte quality in Chinese women with polycystic ovary syndrome: a randomized controlled trial. Sci Rep. 2020;10:18769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gong Y, Luo S, Fan P, Zhu H, Li Y, Huang W. Growth hormone activates PI3K/Akt signaling and inhibits ROS accumulation and apoptosis in granulosa cells of patients with polycystic ovary syndrome. Reprod Biol Endocrinol. 2020;18:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Garcia D, Shaw RJ. AMPK: mechanisms of Cellular Energy Sensing and Restoration of metabolic balance. Mol Cell. 2017;66:789–800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Liu L, Hao M, Zhang J, Chen Z, Zhou J, Wang C, et al. FSHR-mTOR-HIF1 signaling alleviates mouse follicles from AMPK-induced atresia. Cell Rep. 2023;42:113158. [DOI] [PubMed]
- 130.Zhang X, Zhang W, Wang Z, Zheng N, Yuan F, Li B, et al. Enhanced glycolysis in granulosa cells promotes the activation of primordial follicles through mTOR signaling. Cell Death Dis. 2022;13:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ma X, Li X, Ma L, Chen Y, He S. Soy isoflavones alleviate polycystic ovary syndrome in rats by regulating NF- κB signaling pathway. Bioengineered. 2021;12:7215–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jamilian M, Asemi Z. The effects of Soy isoflavones on metabolic status of patients with polycystic ovary syndrome. J Clin Endocrinol Metab. 2016;101:3386–94. [DOI] [PubMed] [Google Scholar]
- 133.The protective effect. Of sulforaphane against oxidative stress in granulosa cells of patients with polycystic ovary syndrome (PCOS) through activation of AMPK/AKT/NRF2 signaling pathway. Reprod Biol. 2021;21:100563. [DOI] [PubMed] [Google Scholar]
- 134.Ding Y, Jiang Z, Xia B, Zhang L, Zhang C, Leng J. Mitochondria-targeted antioxidant therapy for an animal model of PCOS-IR. Int J Mol Med. 2019;43:316–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Di Emidio G, Rea F, Placidi M, Rossi G, Cocciolone D, Virmani A, et al. Regulatory functions of L-Carnitine, Acetyl, and Propionyl L-Carnitine in a PCOS Mouse Model: focus on Antioxidant/Antiglycative Molecular Pathways in the ovarian microenvironment. Antioxid (Basel). 2020;9:867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang Y, Li N, Zeng Z, Tang L, Zhao S, Zhou F, et al. Humanin regulates oxidative stress in the ovaries of polycystic ovary syndrome patients via the Keap1/Nrf2 pathway. Mol Hum Reprod. 2021;27:gaaa081. [DOI] [PubMed] [Google Scholar]
- 137.Luo M, Zheng L-W, Wang Y-S, Huang J-C, Yang Z-Q, Yue Z-P, et al. Genistein exhibits therapeutic potential for PCOS mice via the ER-Nrf2-Foxo1-ROS pathway. Food Funct. 2021;12:8800–11. [DOI] [PubMed] [Google Scholar]
- 138.Huang Y, Zhang X. Luteolin alleviates polycystic ovary syndrome in rats by resolving insulin resistance and oxidative stress. Am J Physiol Endocrinol Metab. 2021;320:E1085–92. [DOI] [PubMed] [Google Scholar]
- 139.Heshmati J, Golab F, Morvaridzadeh M, Potter E, Akbari-Fakhrabadi M, Farsi F, et al. The effects of curcumin supplementation on oxidative stress, Sirtuin-1 and peroxisome proliferator activated receptor γ coactivator 1α gene expression in polycystic ovarian syndrome (PCOS) patients: a randomized placebo-controlled clinical trial. Diabetes Metab Syndr. 2020;14:77–82. [DOI] [PubMed] [Google Scholar]
- 140.Luo M, Huang J-C, Yang Z-Q, Wang Y-S, Guo B, Yue Z-P. Hydroxysafflor yellow A exerts beneficial effects by restoring hormone secretion and alleviating oxidative stress in polycystic ovary syndrome mice. Exp Physiol. 2020;105:282–92. [DOI] [PubMed] [Google Scholar]
- 141.Xie Q, Hong W, Li Y, Ling S, Zhou Z, Dai Y, et al. Chitosan oligosaccharide improves ovarian granulosa cells inflammation and oxidative stress in patients with polycystic ovary syndrome. Front Immunol. 2023;14:1086232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Amani Abkenari S, Safdarian L, Amidi F, Hosseini A, Aryanpour R, Salahi E, et al. Metformin improves epigenetic modification involved in oocyte growth and embryo development in polycystic ovary syndrome mice model. Mol Reprod Dev. 2021;88:817–29. [DOI] [PubMed] [Google Scholar]
- 143.Furat Rencber S, Kurnaz Ozbek S, Eraldemır C, Sezer Z, Kum T, Ceylan S, et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res. 2018;11:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Atef MM, Abd-Ellatif RN, Emam MN, Abo El Gheit RE, Amer AI, Hafez YM. Therapeutic potential of sodium selenite in letrozole induced polycystic ovary syndrome rat model: targeting mitochondrial approach (selenium in PCOS). Arch Biochem Biophys. 2019;671:245–54. [DOI] [PubMed] [Google Scholar]
- 145.Bafor EE, Uchendu AP, Osayande OE, Omoruyi O, Omogiade UG, Panama EE, et al. Ascorbic acid and alpha-tocopherol contribute to the therapy of polycystic ovarian syndrome in mouse models. Reprod Sci. 2021;28:102–20. [DOI] [PubMed] [Google Scholar]
- 146.Zhang Q, Ren J, Wang F, Li M, Pan M, Zhang H, et al. Chinese herbal medicine alleviates the pathogenesis of polycystic ovary syndrome by improving oxidative stress and glucose metabolism via mitochondrial sirtuin 3 signaling. Phytomedicine. 2023;109:154556. [DOI] [PubMed] [Google Scholar]
- 147.Huang L, Liang A, Li T, Lei X, Chen X, Liao B, et al. Mogroside V improves Follicular Development and Ovulation in Young-Adult PCOS rats Induced by Letrozole and High-Fat Diet through promoting Glycolysis. Front Endocrinol (Lausanne). 2022;13:838204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.