Abstract
Background
Liquid biopsies offer less burdensome sensitive disease monitoring. Bone marrow (BM) metastases, common in various cancers including neuroblastoma, is associated with poor outcomes. In pediatric high-risk neuroblastoma most patients initially respond to treatment, but in the majority the disease recurs with only 40% long-term survivors, stressing the need for more sensitive detection of disseminated disease during therapy.
Methods
To validate sensitive neuroblastoma mRNA RT-qPCR BM testing, we prospectively assessed serial BM samples from 345 international high‐risk neuroblastoma patients, treated in trials NB2004 (GPOH) or NBL2009 (DCOG), using PHOX2B, TH, DDC, CHRNA3, and GAP43 RT-qPCR mRNA markers and BM GD2-immunocytology. Association between BM-infiltration levels and event-free survival (EFS) and overall survival (OS) was estimated by using Cox regression models and Kaplan-Meier’s methodology.
Results
BM infiltration >10% by RT-qPCR at diagnosis was prognostic for survival (adjusted hazard ratio (HR) 1.82 [95%CI 1.25‐2.63] and 2.04 [1.33‐3.14] for EFS and OS, respectively). Any post-induction RT-qPCR positivity correlated with poor EFS and OS, with a HR of 2.10 [1.27-3.49] and 1.76 [1.01-3.08] and 5-years EFS of 26.6% [standard error 5.2%] versus 60.4% [6.7] and OS of 43.8% [5.9] versus 65.7% [6.6] for RT-qPCR-positive patients versus RT-qPCR-negative patients. In contrast, post-induction immunocytology positivity was not associated with EFS or OS (HR 1.22 [0.68-2.19] and 1.26 [0.54-2.42]).
Conclusion
This study validates the association of not clearing of BM metastases by sensitive RT-qPCR detection with very poor outcome. We therefore propose implementation of RT-qPCR for minimal residual disease testing in neuroblastoma to guide therapy.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13046-024-03261-y.
Background
Liquid biopsies have shown promise in assisting diagnosis and monitoring therapy response in adult oncology [1] and have been implemented into clinical practice over the past years. The most widely adopted and investigated source for liquid biopsies is blood, although the term also comprises other body fluids such as urine, cerebrospinal fluid and bone marrow (BM). BM is a preferred site of metastatic disease in various adult and pediatric cancers, such as breast cancer, prostate cancer, and neuroblastoma [2]. Consequently, the monitoring of BM disease is pivotal to assess therapy response and disease recurrence. Neuroblastoma, the most common pediatric extracranial solid tumor has a broad spectrum of clinical behavior, ranging from spontaneous regression to incurable aggressive disease [3]. One of the most powerful predictors of outcome in patients with neuroblastoma is metastatic disease [4, 5]. BM is the most common site of metastatic disease at diagnosis [4, 6] and a frequent site of relapse [7]. In current clinical practice, assessment of treatment response is based on the International Neuroblastoma Response Criteria (INRC): Meta-iodobenzylguanidine (MIBG) scintigraphy, MRI, CT scans, PET scans, and BM examinations by histology or (immuno)cytology are combined to assess the extent of disease [8]. However, with current assessments, about 50% of all patients in presumed complete remission will relapse, leading to only 40% long term survivors [9, 10]. The prognosis of patients with recurrent or refractory neuroblastoma is dismal with 4-year progression free survival and overall survival (OS) of 6% and 20%, respectively [11]. This underlines the urgent need for more sensitive techniques to establish which patient will or will not be cured by the current therapies, and thus to stratify patients in function of response. As metastatic response to induction therapy is associated with outcome, at designated time points, bone marrow is tested for metastases by histology, cytology and immunocytology. Histology and cytology have a restricted sensitivity, can be more difficult to standardize and could therefore underestimate the BM infiltration during treatment [12]. Immunocytology is a more sensitive, standardized technique, and is implemented in several clinical protocols, but is time-consuming and requires experienced investigators [13, 14]. Reverse transcriptase quantitative polymerase chain reaction (RT-qPCR) provides highly sensitive means of detecting minimal residual disease (MRD) in BM and peripheral blood (PB) [5, 15]. In leukemia, MRD detection by (RT-)qPCR is a highly validated, inexpensive, easily standardized technique that has been implemented two decades ago and is performed worldwide to guide therapeutic decisions [16]. In neuroblastoma, detection of mRNA in BM by RT-qPCR during treatment has also been shown to be of prognostic value and a predictor of poor outcome [15, 17–19]. We were the first to describe paired-like homeobox 2b (PHOX2B) as a highly sensitive and neuroblastoma specific mRNA marker for MRD detection [20]. Yet, as PHOX2B expression levels vary between tumors, we further showed that adding other markers, tyrosine hydroxylase (TH), dopamine decarboxylase (DDC), cholinergic receptor nicotinic alpha 3 (CHRNA3), and growth associated protein 43 (GAP43), contributes to more sensitive MRD detection [21, 22] and the clinical utility for patients with neuroblastoma of all risk groups [15, 23, 24]. In a large prospective study of the European Society of Pediatric Oncology Neuroblastoma Group (SIOPEN), Viprey et al. showed in BM of high-risk patients, transcripts of TH, PHOX2B, or doublecortin (DCX) above a certain threshold, defined in a training cohort, to correlate with a poorer prognosis than patients showing no or lower levels of BM infiltration [17]. These thresholds were based on therapy responses, potentially requiring validation in different treatment regimens. In a retrospective study in patients with high-risk neuroblastoma, we demonstrated the prognostic value of our mRNA panel at diagnosis, and the significance of fast BM clearance [15]. In the study presented here, we prospectively validate these findings in an international cohort of children with high-risk neuroblastoma treated according to high-risk protocols NB2004 (GPOH) or NBL2009 (DCOG). The clinical significance of BM infiltration levels at diagnosis and clearing of the BM after induction therapy is measured by sensitive RT-qPCR for a neuroblastoma-specific mRNA panel and anti-GD2 immunocytology.
Methods
Patients and samples
Patients were included in this prospective study if they were (a) diagnosed with high-risk neuroblastoma between 2009 and 2017, (b) treated according to the German GPOH NB2004-HR trial [10] or the Dutch DCOG NBL2009 trial [25] (Supplemental Figure 1), and (c) if written informed consent from parents or guardians was obtained according to the declaration of Helsinki. Patients within the German GPOH NB2004-HR trial were randomized to receive prolonged induction therapy, but Berthold and colleagues reported equal outcomes for EFS and OS [10]. Within the high-risk protocol, standard induction therapy consisted of six alternating courses of N5 (vindesine, cisplatin, etoposide) and N6 (vincristine, dacarbacine, ifosfamide, doxorubicin), (identical in both GPOH and DCOG trials), which – depending on randomization results- were eventually preceded by, two additional courses of topotecan, cyclophosphamide and etoposide (N8) in the GPOH trial [10], or if clinically achievable, upfront 2 courses of MIBG therapy in the DCOG trial [25]. After induction therapy, patients received high dose chemotherapy with autologous hematopoietic stem cell rescue and isotretinoin for consolidation. Dutch patients diagnosed after 2012 (if eligible; patients in complete- or very good partial remission) received GD2 immunotherapy in the Children’s Hospital Philadelphia, USA [26]. For German patients, immunotherapy was not scheduled per protocol, but given to single patients after 2010.
Diagnostics and staging procedures were performed according to the International Neuroblastoma Staging System (INSS) [27, 28]. High-risk patients were defined as stage 4 over 1 year of age or all stages with MYCN amplification. The study was approved by the Medical Research Ethics Committees of the Academic Medical Center (Amsterdam, the Netherlands; MEC07/219#08.17.0836) and the University of Cologne (Cologne, Germany).
BM aspirates from two to four sites were collected in EDTA tubes at diagnosis and at dedicated time points during induction chemotherapy: after first 2 therapy courses (after 2x N8/ 2x MIBG therapy/ or the first N5/N6), and at the intended end of induction (Supplemental Figure 1). In Germany, bilateral BM samples were pooled prior to processing, the Dutch bilateral BM samples were analyzed individually by RT-qPCR. BM samples were transferred to PAXgene blood RNA tubes (QIAGEN, Venlo, the Netherlands) within 24 hours and then stored at -20°C. RNA isolation and RT-qPCR were performed in Amsterdam, the Netherlands, as described below.
RNA extraction and RT-qPCR
RNA was isolated from PAXgene blood RNA tubes (QIAGEN, Venlo, Netherlands) with the PAXgene Blood RNA Kit (QIAGEN). cDNA was synthesized from 2-3 µg of RNA, using 25 µmol/L random hexamers (Invitrogen, Carlsbad, CA, USA), 1 mmol/L dNTPs (Promega, Madison, WI, USA) and 100U of MMLV transcriptase (Invitrogen, ThermoFisher, Waltham, USA), in a total reaction volume of 40 µl and incubated at 42°C for 45 minutes. Finally, the reverse transcriptase was inactivated by heating and the volume was diluted to 100 µl. RT-qPCR for PHOX2B, TH, DDC, CHRNA3 and GAP43 was performed using beta-glucoronidase (GUSB) for normalization [21] on Step-One-Plus or Viia7 (Applied Biosystems, Carlsbad, CA, USA). Primers and probes sequences have been published previously [21, 29] and were synthesized by Eurogentec (Liege, Belgium). Reactions were carried out in 20 µL (10 µL TaqManTM Fast Universal PCR Master Mix (Applied Biosystems), 300 nM forward and reverse primer and 200 nM probe and 5 µL cDNA). In all RT-qPCR reactions, initial heating was done for 20 s at 95 °C, followed by 50 cycles of 1 s at 95 °C and 20 s at 60 °C. All RT-qPCR experiments were carried out in triplicate (except GUSB, which was performed in duplicate) and mean values were used for analysis. All samples with a Ct for GUSB >25 were excluded. Marker expression was normalized to GUSB expression using the following equation [(ΔCt) = Ctmarker – CtGUSB]. In short, PHOX2B was considered positive if there was any amplification, as the PHOX2B used by our lab has no background expression [20]; the other markers were considered positive if the Ct was <40 and ΔCt of the sample was <3 Ct than the ΔCt of the normal control BM samples, as described previously [21]. A sample was scored positive if the Ct of at least one out of five markers, was above the threshold for positivity, as has been described previously, determined in 51 pediatric BM samples [21]. To estimate the level of infiltration, the expression level of the mRNA RT-qPCR targets were related to the expression level of an external standard (neuroblastoma cell line IMR-32) according to the following formula: 2^-ΔΔCt (ΔCt sample - ΔCt IMR-32) * 100%. IMR-32 is one of the most frequently investigated cell lines in neuroblastoma research, elaborately tested for stability of our RT-qPCR markers [22] and used across SIOPEN laboratories for quality control and relative quantification [17, 30]. The median relative expression of the markers was used to calculate the level of infiltration of each individual patient/time point. Because Dutch BM samples were not pooled, results were averaged, and in case one site was negative for a marker(s) the positive quantity for that/those marker(s) was halved. If the adjusted marker scored above the threshold, the sample was regarded positive for this marker.
GD2-immunocytology
For both the German and Dutch patients, immunocytology was carried out in Cologne, Germany, according to the internationally standardized protocols [5, 13, 31, 32]. BM samples, collected in EDTA tubes, from two to four sites were pooled and mononuclear cells were isolated by density gradient centrifugation. Cytospins were stained using the alkaline phosphatase anti-alkaline phosphatase -method. A minimum of one million cells were investigated. Results were given in the categories negative / <1% / 1-10% / 10-30% / 30-100% as estimated by the investigator (R.S.-K.).
Statistics
Kaplan-Meier’s methodology was used to estimate overall survival (OS) and event-free-survival (EFS, where event was defined as progressive disease, relapse and death) from time since sample acquisition; patients alive were censored at the last follow-up time. To assess the difference between survival outcomes the log-rank test was used. Reverse Kaplan Meier was employed to estimate the median follow-up [33]. A Cox univariable proportional hazards regression model was employed to quantify the effect of prognostics factors on survival outcomes. In addition, multivariable Cox models were estimated including MYCN amplification and age at diagnosis and information about relapse or progressive disease known at the landmark time in addition to RT-qPCR or immunocytology results [34]. A landmark analysis [34] with 2 landmarks points: at diagnosis and after 2 induction courses was employed to estimate the effect of RT-qPCR stratifications and relapse/progressive disease occurred within 2 years from diagnosis on overall survival (OS). In the landmark methodology, a fixed time after diagnosis and after 2 cycles of therapy are selected as landmark point for performing the analysis. Only patients alive at the landmark time are included in each analysis. In the Cox regression model for overall survival, from the landmark point 2 years after diagnosis and after 2 cycles of therapy, information about relapse or progressive disease known at the landmark time were included as risk factor in the model. An interaction term between RT-qPCR and occurrence of relapse/progressive disease known at the starting time of the analysis was also included in the Cox model. All statistical analyses were performed by using SPSS version 26.
Results
Patient characteristics
Three hundred forty-five children with high-risk neuroblastoma were included in this study, with a median age at diagnosis of 33.6 months (range 0.3-224 months), see Table 1 for patient characteristics. Patients were treated according to the German GPOH NB2004-HR trial [10] or the Dutch DCOG NBL2009 trial [25] (Supplemental Figure 1). The median follow-up time was 81.5 months [95% CI 76.1-86.9]. In 98% of patients, BM aspirates were available at diagnosis, in 74% after 2 therapy cycles and in 37% at end of induction, respectively (Supplemental Figure 2). We investigated whether previous GD2-immunocytology results (the clinically used standard) influenced sample acquisition. Of the 88 missing bone marrow samples after 2 cycles of therapy, 23 (26%) samples were from patients with a previous sample negative for immunocytology. Of the 257 sampled patients after 2 cycles of therapy, only 30 patients did have a negative previous sample or were not sampled before (12%). Of the 216 patients with missing samples at the end of induction, 102 patients (47%) had a previously negative sample for immunocytology, similarly to the 129 patient that were sampled at the end of induction (61 patients with a previously negative sample; 47%). Numbers at risk and 5-year survival rates for each estimated survival outcomes are presented in Supplemental Table 1.
Table 1.
Patient characteristics
| Patient characteristics | |
|---|---|
| Country | |
| Netherlands | 84 |
| Germany | 261 |
| INSS stage | |
| 2 | 6 |
| 3 | 19 |
| 4 | 312 |
| 4s | 7 |
| unknown | 1 |
| Age at diagnosis (months) | |
| median | 33,6 |
| range | 0.3-224.4 |
| Age at diagnosis | |
| <18 months | 62 |
| >18 months | 283 |
| Gender | |
| Male | 214 |
| Female | 131 |
| MYCN | |
| Amplification | 153 |
| No amplification | 188 |
| Unknown | 4 |
| Metastatic disease ánd age >18 months | |
| 266 | |
| Allocated treatment | |
| Standard arm | 222 |
| 2x N8 + standard arm | 100 |
| MIBG + standard arm | 23 |
| Follow up time (months) | |
| Median | 81,5 |
| 95% confidence interval | 76,1-86,9 |
Comparison between RT-qPCR and immunocytology
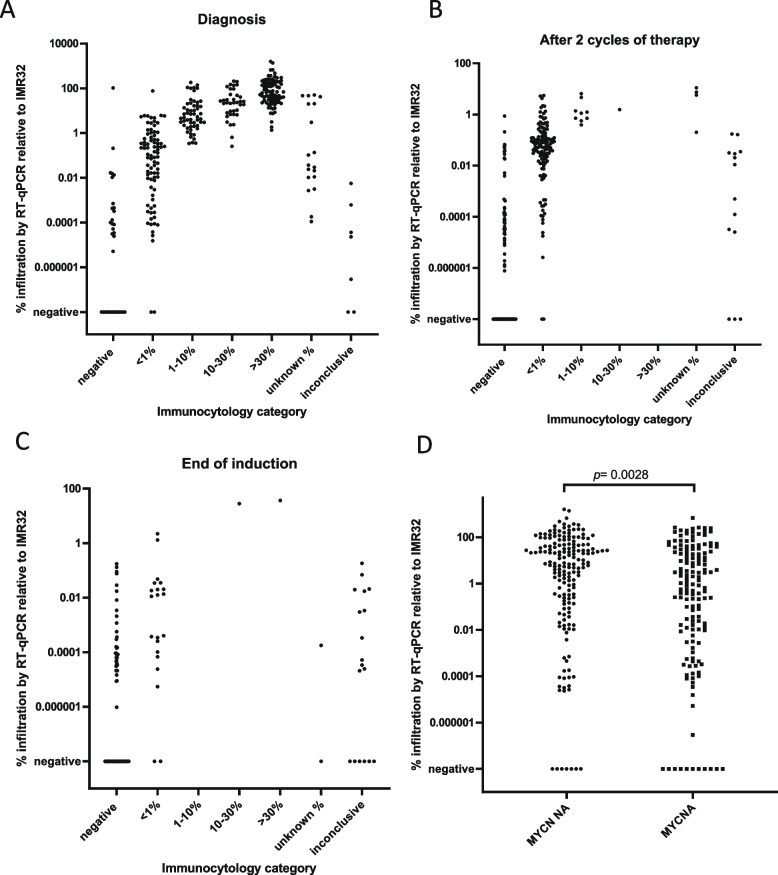
We observed concordance between the percentage BM infiltration estimated by the RT-qPCR mRNA panel and immunocytology in the paired BM samples. For both techniques, the number of positive BM samples and the levels of infiltration were lower after two therapy cycles and at end of induction, compared to the diagnostic samples (Figure 1, Supplemental Figure 3). At all three time-points, RT-qPCR was more sensitive compared to immunocytology, with 14 out 33 (42%), 42 out 86 (49%) and 43 out 80 (54%) RT-qPCR positive/immunocytology negative samples, at diagnosis, after 2 therapy cycles and at end of induction, respectively. At each time-point, only 2 RT-qPCR negative samples were immunocytology positive. At diagnosis, BM mRNA infiltration was significantly lower in BM of patients with MYCN amplified tumors compared to those with MYCN non-amplified tumors, but ranges overlap (median 2.1% [interquartile range 0.02-31.2] versus 10.4% [0.34-51.12], p=0.0028 (Figure 1D).
Fig 1.
Level of infiltration by RT-qPCR relative to cell line IMR-32 versus GD2-immunocytology at diagnosis (A), after 2 cycles of therapy (B), end of induction therapy (C). D. Level of infiltration by RT-qPCR relative to cell line IMR-32 at diagnosis in patients with MYCN non-amplified tumors (MYCN NA) vs MYCN-amplified tumors (MYCNA)
Prognostic value of neuroblastoma mRNA level in BM at diagnosis
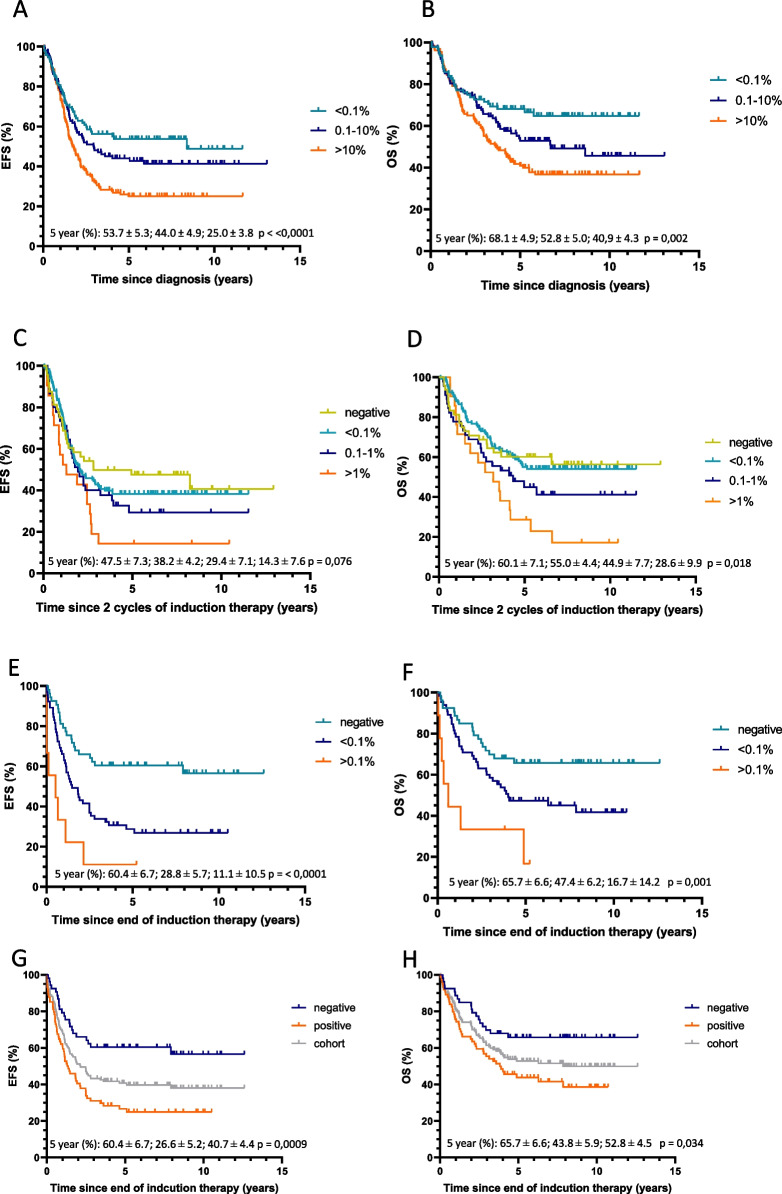
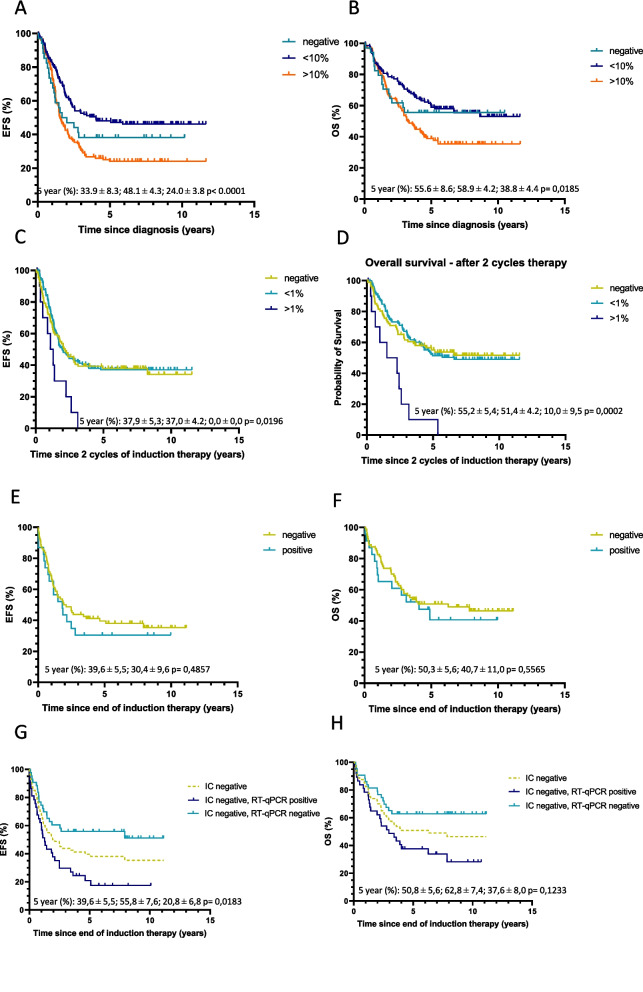
At diagnosis, 135 out 329 patients (41%) had high infiltration levels of >10% BM determined by RT-qPCR. These high neuroblastoma mRNA levels are strongly associated with outcome, both EFS and OS in univariable and multivariable analysis: the adjusted hazard ratio (HR) for RT-qPCR group >10% was 1.82 [95% CI 1.25‐2.63] and 2.04 [1.33‐3.14] for EFS and OS, respectively (Figure 2A-B, Table 2), with a 5-year EFS and OS of 25.0% (SE 3.8) and 40.9 (4.3) versus 53.7% (5.3) and 68.1 (4.9) for the <0.1% infiltration group (Supplemental Table 1). The adjusted HR for RT-qPCR infiltration 0.1-10% at diagnosis was 1.29 [0.87-1.93] and 1.52 [0.97-2.40] for EFS and OS, respectively. Also in patients older than 18 months with metastatic disease, HR for RT-qPCR group >10% was significant in multivariable analysis for OS only (Supplemental Figure 4A-B, Table 2). To study the effect of high infiltration levels in the BM at diagnosis after completing therapy, we performed a landmark analysis, with landmark point 2 years after diagnosis. This confirms the prognostic effect of infiltration on overall survival (HR for OS RT-qPCR 0.1-10% 2.42 [1.13-5.19) and RT-qPCR>10% 2.76 [1.32-5.77]), where occurrence of relapse/progressive disease within two years after diagnosis is included in the model as risk factor (Supplemental Figure 5A-C, Table 2).
Fig 2.
Kaplan-Meier event-free and overall survival curves (EFS on the left and OS on the right, respectively) according to the level of mRNA infiltration by RT-qPCR detected in bone marrow at diagnosis (A, B), after 2 cycles of therapy (C, D) and after induction therapy (E, F). Stratification by RT-qPCR at end of induction compared to the total cohort (G, H). 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement
Table 2.
Event-free survival (EFS) and overall survival (OS) in Cox proportional hazards regression models
| Univariable | Multivariable (adjusted for MYCN and age >18 months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| EFS | OS | EFS | OS | ||||||
| Variable | patients (No.) | HR | 95% CI | HR | 95% CI | HR | 95% CI | HR | 95% CI |
| Diagnosis, RT-qPCR group | |||||||||
| <0.1% | 92 | ||||||||
| 0.1-10% | 102 | 1.29 | 0.87-1.91 | 1.49 | 0.95-2.33 | 1.29 | 0.87-1.93 | 1.52 | 0.97-2.40 |
| 10-100% | 135 | 1.95 | 1.36-2.79 | 2.04 | 1.35-3.09 | 1.82 | 1.25-2.63 | 2.04 | 1.33-3.14 |
| After 2 cycles of therapy, RT-qPCR group | |||||||||
| negative | 48 | ||||||||
| <0.1% | 133 | 1.16 | 0.74-1.80 | 1.01 | 0.61-1.68 | 1.11 | 0.70-1.75 | 1.07 | 0.63-1.81 |
| 0.1-1% | 45 | 1.37 | 0.82-2.31 | 1.47 | 0.81-2.64 | 1.29 | 0.74-2.24 | 1.60 | 0.85-3.00 |
| 1-100% | 21 | 2.08 | 1.14-3.80 | 2.22 | 1.16-4.24 | 1.95 | 1.02-3.72 | 2.52 | 1.24-5.09 |
| End of induction, RT-qPCR group | |||||||||
| negative | 53 | ||||||||
| <0.1% | 65 | 2.30 | 1.38-3.82 | 1.85 | 1.05-3.26 | 1.94 | 1.16-3.26 | 1.60 | 0.90-2.84 |
| 0.1-100% | 9 | 5.38 | 2.38-12.17 | 4.55 | 1.89-10.95 | 4.40 | 1.92-10.08 | 4.00 | 1.63-9.78 |
| negative | 53 | ||||||||
| positive | 74 | 2.50 | 1.52-4.11 | 2.05 | 1.18-3.55 | 2.10 | 1.27-3.49 | 1.76 | 1.01-3.08 |
| Diagnosis, immunocytology group | |||||||||
| negative | 34 | ||||||||
| <10% | 140 | 0.70 | 0,43-1,14 | 0.83 | 0,47-1,46 | 0.65 | 0,40-1,07 | 0.80 | 0,45-1,42 |
| >10% | 127 | 1.28 | 0,80-2,05 | 1.43 | 0,83-2,49 | 1.12 | 0,69-1,82 | 1.35 | 0,77-2,39 |
| After 2 cycles of therapy, immunocytology group | |||||||||
| negative | 86 | ||||||||
| <1% | 125 | 0.95 | 0.67-1.34 | 0.97 | 0.65-1.45 | 0.95 | 0.66-1.37 | 0.99 | 0.65-1.51 |
| >1% | 10 | 2.39 | 1.21-4.71 | 3.68 | 1.83-7.40 | 2.19 | 1.11-4.33 | 3.48 | 1.72-7.06 |
| End of induction, immunocytology group | |||||||||
| negative | 80 | ||||||||
| positive | 23 | 1.22 | 0.70-2.15 | 1.21 | 0.65-2.25 | 1.22 | 0.68-2.19 | 1.26 | 0.54-2.42 |
| Diagnosis, immunocytology negative | |||||||||
| RT-qPCR negative | 14 | ||||||||
| RT-qPCR positive | 19 | 1.97 | 0.75-5.16 | 3.06 | 0.85-11.02 | 1.10 | 0.36-3.39 | 1.92 | 0.49-7.50 |
| End of induction, immunocytology negative | |||||||||
| RT-qPCR negative | 43 | ||||||||
| RT-qPCR positive | 37 | 2.45 | 1.38-4.34 | 2.16 | 1.15-4.06 | 2.05 | 1.14-367 | 1.83 | 0.97-3.45 |
| MYCN status | |||||||||
| not amplified | |||||||||
| amplified | 0.99 | 0.75-1.30 | 1.14 | 0.84-1.55 | |||||
| Age at diagnosis | |||||||||
| < 18 months | |||||||||
| > 18 months | 1.95 | 1.27-2.99 | 1.56 | 0.99-2.45 | |||||
| Patients > 18 months and metastatic disease only | |||||||||
| Diagnosis, RT-qPCR group | |||||||||
| <0.1% | 49 | ||||||||
| 0.1-10% | 75 | 0.94 | 0,59-1,48 | 1.09 | 0,64-1,87 | 0.94 | 0,59-1,48 | 1.08 | 0,63-1,86 |
| 10-100% | 128 | 1.39 | 0,92-2,09 | 1.61 | 0,99-2,60 | 1.39 | 0,92-2,10 | 1.63 | 1,01-2,64 |
| After 2 cycles of therapy, RT-qPCR group | |||||||||
| negative | 30 | ||||||||
| <0.1% | 116 | 0.9 | 0,56-1,46 | 0.89 | 0,50-1,58 | 0.87 | 0,53-1,42 | 0.93 | 0,52-1,67 |
| 0.1-1% | 44 | 1.02 | 0,59-1,78 | 1.24 | 0,65-2,35 | 1.02 | 0,57-1,80 | 1.39 | 0,72-2,72 |
| 1-100% | 21 | 1.5 | 0,80-2,81 | 1.83 | 0,91-3,67 | 1.51 | 0,78-2,90 | 2.16 | 1,04-4,51 |
| End of induction, RT-qPCR group | |||||||||
| negative | 39 | ||||||||
| <0.1% | 59 | 1.70 | 1,01-2,87 | 1.47 | 0,82-2,62 | 1.73 | 1,03-2,93 | 1.56 | 0,87-2,81 |
| 0.1-100% | 9 | 3.88 | 1,71-8,81 | 3.40 | 1,40-8,24 | 4.05 | 1,77-9,25 | 3.93 | 1,60-9,65 |
| negative | 39 | ||||||||
| positive | 68 | 1.86 | 1,12-3,10 | 1.62 | 0,92-2,85 | 1.89 | 1,14-3,16 | 1.73 | 0,98-3,06 |
| Diagnosis, immunocytology group | |||||||||
| negative | 17 | ||||||||
| <10% | 103 | 0.34 | 0,19-0,59 | 0.39 | 0,21-0,74 | 0.36 | 0,19-0,59 | 0.38 | 0,20-0,72 |
| >10% | 120 | 0.55 | 0,32-0,95 | 0.66 | 0,36-1,22 | 0.55 | 0,32-0,95 | 0.65 | 0,35-1,21 |
| After 2 cycles of therapy, immunocytology group | |||||||||
| negative | 64 | ||||||||
| <1% | 113 | 0.92 | 0,64-1,34 | 0.99 | 0,64-1,52 | 0.94 | 0,65-1,38 | 1.03 | 0,66-1,60 |
| >1% | 10 | 2.15 | 1,08-4,28 | 3.52 | 1,72-7,21 | 2.17 | 1,09-4,32 | 3.60 | 1,75-7,39 |
| End of induction, immunocytology group | |||||||||
| negative | 70 | ||||||||
| positive | 20 | 1.09 | 0,60-1,98 | 1.03 | 0,53-2,01 | 1.15 | 0,63-2,10 | 1.15 | 0,58-2,27 |
| Landmark analysis | |||||||||
| Relapse/progressive disease in first 2 years | |||||||||
| no | |||||||||
| yes | 7.79 | 4.81-12.61 | |||||||
| Landmark analysis, 2 years after sample taken, RT-qPCR group at diagnosisa | |||||||||
| <0.1% | 69 | ||||||||
| 0.1-10% | 77 | 2.42 | 1.13-5.19 | ||||||
| 10-100% | 88 | 2.76 | 1.32-5.77 | ||||||
| Landmark analysis, 2 years after sample taken, RT-qPCR group after 2 cycles of therapya | |||||||||
| negative | 34 | ||||||||
| <0.1% | 102 | 1.08 | 0.44-2.64 | ||||||
| 0.1-1% | 31 | 1.75 | 0.64-4.76 | ||||||
| 1-100% | 13 | 3.32 | 1.17-9.42 | ||||||
| 8.51 | 4.79-15.12 | ||||||||
Hazard Ratio (HR) and 95% confidence intervals (CI) of univariate and multivariate Cox model analyses are indicated for levels of bone marrow infiltration by RT-qPCR or GD2-immunocytology. In multivariate analysis, MYCN status and age >18 months are included as variables
aIn the landmark analysis, relapse or progressive disease within 2 years after sample collection was included as a variable, MYCN status and age were no variables in this analysis
Prognostic value of GD2-immunocytology level in BM at diagnosis
For immunocytology, the level of infiltration was negatively associated with outcome (Fig 3A-B), however, it did not differ in terms of EFS and OS from immunocytology negative patients (Table 2, Supplemental Figure 6 A-B), possibly due to the relative poor outcome of those patients with immunocytology negative BM. Within the small cohort of 33 immunocytology negative samples (for 1 sample no material was available for RT-qPCR), a positive RT-qPCR appeared to predict a worse prognosis, however this difference did not reach significance (Table 2; Supplemental Figure 6C-D). In patients older than 18 months with metastatic disease, patients with GD-2 negative BM had a remarkably poor prognosis (Supplemental Figure 4G-H, Table 2).
Fig 3.
Kaplan-Meier event-free and overall survival curves (EFS on the left and OS on the right, respectively) according to the level of GD2- immunocytology (IC) detected in bone marrow at diagnosis (A, B), after 2 cycles of therapy (C, D) and after induction therapy (E, F). Patients with GD2-immunocytology negative bone marrow at the end of induction therapy, stratified for RT-qPCR results (G, H). 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement
Clinical significance of BM infiltration after two induction therapy courses
We previously showed in a retrospective cohort that fast-response correlated with better outcome. To study prospectively the clinical significance of early BM clearance, BM infiltration was analyzed by RT-qPCR after 2 induction therapy courses (n=247). Fast response after two therapy courses was associated with survival, a significantly poor prognosis was only seen for children with RT-qPCR BM infiltration >1% (adjusted HR 1.95 [95% CI 1.02-3.72] and 2.52 [1.24‐5.09] for EFS and OS, respectively) (Figure 2C-D, Table 2). This was also shown in the subgroup analysis of patients with metastatic disease, older than 18 months (Supplemental Figure 4C-D, Table 2) and in the landmark analysis starting 2 years after the sample was taken, (HR for OS RT-qPCR 0.1-1% 3.32 [1.17-9.42] and RT-qPCR>1% 8.51 [4.79-15.12]) (Table 2, Supplemental Figure 5D-F). Immunocytology after 2 cycles of therapy also confirmed the poor prognostic value of >1% BM infiltration, with a 5-year EFS of 0% versus 37.9 (5.3) and OS of 10% (9.5) versus 55.2 (5.4), also in the cohort of patients older than 18 months with metastatic disease (Table 2, Figure 3C-D, Supplemental Figure 4I-J).
Clinical significance of BM clearance at the end of induction therapy
To validate the correlation of BM response with outcome, we tested both RT-qPCR and immunocytology at end of induction therapy (median 184 days since diagnosis). In 74 out 127 patients neuroblastoma mRNA was still detected (poor-responders), which correlated with poor outcome: 5-years EFS was 26.6% (5.2) versus 60.4% (6.7) and OS was 43.8% (5.9) versus 65.7% (6.6) for RT-qPCR positive patients versus RT-qPCR negative patients (Figure 2E-H, Supplemental Table 1). Compared to the outcome of the whole cohort, any RT-qPCR-positivity correlated with poorer outcome (Figures 2G-H). In multivariate Cox regression model, end of induction RT-qPCR positivity was associated with poor EFS and OS, with a HR of 2.10 [1.27-3.49] and 1.76 [1.01-3.08] respectively (Table 2), also for the patients with metastatic disease, older than 18 months (Supplemental Figure 4E-F, Table 2). In contrast, end of induction immunocytology positivity was not associated with EFS or OS (HR 1.22 [0.68-2.19] and 1.26 [0.54-2.42]) (Table 2, Figure 3E-F), neither in the total high-risk cohort, nor in the subgroup of patients older than 18 months with metastatic disease only. Moreover, because we analyzed RT-qPCR and immunocytology in the same samples, we could classify the immunocytology-negative samples according to their RT-qPCR score. This clearly resulted in a significantly poorer outcome for the immunocytology-negative/RT-qPCR positive group, versus negative for both techniques (univariable HR 2.45 [1.38-4.35] and 2.16 [1.15-4.06] for EFS and OS respectively, and 5-year EFS of 20.8 (6.8) versus 55.8 (7.6) and 5-year OS of 37.6 (8.0) versus 62.8 (7.4)). Difference in survival in this immunocytology negative group remains significant in multivariate analysis for EFS (HR 2.05 [1.14-3.67]) (Table 2, Figure 3G-H). Immunocytology positive results correspond mainly to RT-qPCR positive BM, with only 2 immunocytology positive/RT-qPCR negative BM samples (Supplemental Figure 6E-F). We conclude that at the end of induction therapy, any RT-qPCR positivity, in contrast to immunocytology, identifies patients within the high-risk cohort with a very poor outcome.
Contribution of the different RT-qPCR markers
All samples collected at three different timepoints were included in this analysis. During an interim analysis in 2014 [35] DDC was the least informative marker and has therefore been excluded for further testing since then. At diagnosis, 311/329 samples (95%) showed positive results for one or more markers with 74% being positive for all markers (Figure 4A). PHOX2B was the most sensitive marker and was positive in 100% of positive diagnostic samples. In the follow-up samples, different markers/marker-combinations contributed to positivity (Figure 4B-C), again with PHOX2B positivity most frequently observed (80% after 2 therapy cycles and 56% at end of induction). Of the positive samples, only 1.0% after 2 cycles and 2.4% at end of induction were PHOX2B-negative. Supplemental Figures 7-9 and Supplemental Table 2 show the prognostic value of each individual marker. PHOX2B positivity at end of induction was predictive of EFS and OS in univariate analysis, similar to DDC, CHRNA3 and GAP43. Nevertheless, none of the individual markers, except for DDC, which was only performed in a small cohort, was more predictive of outcome compared to the combined RT-qPCR panel.
Fig 4.
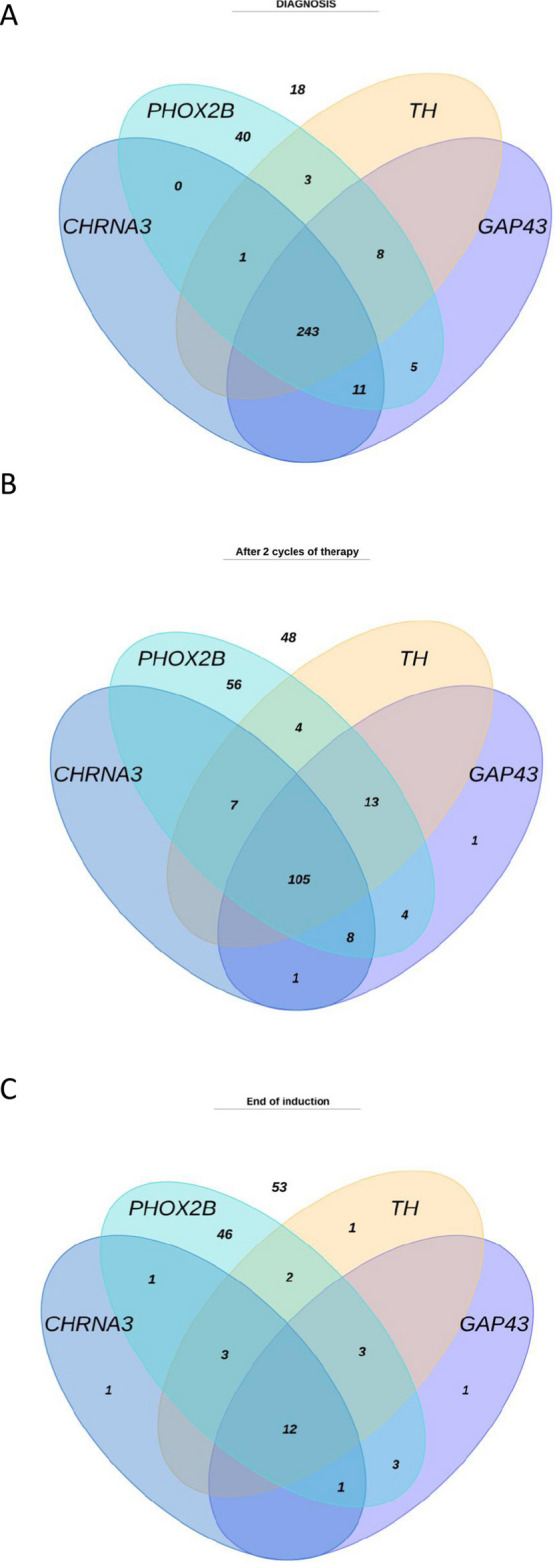
Contribution of the different mRNA markers to the positive samples at (A) diagnosis; (B) after 2 cycles of therapy and (C) end of induction therapy. Each ellipse represents positive results of one marker. The number of RT-qPCR negative samples are stated in white on top of the diagram
Discussion
While a great portion of high-risk neuroblastoma patients achieve remission at the end of first line treatment, unfortunately more than half of these patients still experience relapse. MRD in the BM is thought to be the leading cause of relapse, even after intensive treatment [6]. Early MRD detection is necessary for disease monitoring and predicting therapy response to achieve optimal outcome [36, 37]. GD2-immunocytology is recommended for BM evaluation by the INRC BM working group [5, 8], and is shown to be more sensitive than conventional cytology [13], as was RT-qPCR [21]. We show in one of the largest published prospective high-risk cohorts the prognostic value of neuroblastoma mRNA detection in BM at diagnosis, during, and at end of induction therapy, and compare these with the current diagnostic standard for BM infiltration, GD2-immunocytology. We show that RT-qPCR detecting our panel of neuroblastoma mRNA markers is an independent predictor of outcome. Very sensitive BM MRD detection by RT-qPCR for our mRNA panel is superior to GD2-immunocytology, not only in terms of prognostic value but also for sensitive and reliable detection of BM clearance. At diagnosis we already identify a subgroup (41%) with BM infiltration >10% that are at high-risk for suffering from relapse or progressive disease. At end of induction therapy, when patients proceed to consolidation therapy, neuroblastoma mRNA RT-qPCR identifies a large group (58%) which still has BM infiltration and poor prognosis. End of induction GD2-immunocytology results did not correlate with outcome. Strikingly, RT-qPCR stratified the immunocytology negative patients in those with truly negative BM and those with still detectable MRD. These findings show that RT-qPCR has superior sensitivity, and that low levels of BM disease, detected only by RT-qPCR, correlate with poor outcome. This is in line with other studies from Viprey, Träger and Yáñez [17, 38, 39]. For patients within this high-risk group novel treatment options should be discussed, e.g. tandem autologous stem cell transplant [40], promising chemo-immunotherapies [41, 42] or precision medicine programs such as the INFORM Registry [43], and evaluated in clinical trials. Studies should furthermore investigate if these are the patients with SIOPEN >3 skeletal scores who have a poorer outcome [44–46], and if alternative therapy approaches, evaluated in clinical trials, can rescue these patients. Our study furthermore demonstrates the robustness of RT-qPCR mRNA detection, as it validates the clinical significance of neuroblastoma-specific mRNA detection in BM in a different high-risk chemotherapy background, and using a different marker panel and cut points then Viprey et al [17].
Multiple studies made use of distinct marker panels and different methods for defining a threshold for positivity of MRD [15, 17, 18, 21, 47–49]. Viprey et al. defined a threshold for positivity by calculating a cut point for mRNA based on a test cohort that is associated with survival [17]. Efforts to validate both the SIOPEN and DCOG/GPOH approach are ongoing. To avoid false-positive results, we base our thresholds for positivity on expression of the markers in normal control BM samples [20]. Furthermore, these thresholds are not dependent on changes in treatment protocols. Although in our study we estimate BM infiltration levels by relating the expression levels of the mRNA panel to that in the neuroblastoma cell line IMR-32, the estimated infiltration percentage by RT-qPCR, which are mRNA transcripts, overall corresponds to the infiltration by GD2-immunocytology. Several MRD markers were tested in the search for optimal markers in neuroblastoma over the past years. Due to the heterogeneous expression of marker genes amongst patients, and within an individual tumor and its metastasis, the use of a marker panel is superior to using a single marker [21, 22, 47, 50, 51]. In our study, PHOX2B is the most specific and sensitive marker. It should be noted that this PHOX2B assay, developed by Stutterheim et al., is different from the one used by Viprey and colleagues [17, 20]. During an interim analysis in 2014 [35], DDC was the least informative marker, being solely positive in only one follow-up sample and was subsequently excluded. As none of the markers contributed individually more to the outcome than the whole panel, we propose to use the panel of four markers (PHOX2B, TH, CHRNA3, GAP43). Although this panel of markers is superior to current techniques to detect neuroblastoma presence in the BM, we unfortunately still see a group of patients, free from BM infiltration, who still suffer from recurrent disease. It might be that the residual tumor cells causing the relapse do not reside in the BM, or the tumor cells residing in the BM escape surveillance due to a low expression of the used MRD markers. The commonly used MRD markers are selected on expression levels in primary tumors and cell lines, but not on treated tumors. Epithelial-to-mesenchymal transition (EMT)—the process by which epithelial cells transform to a mesenchymal phenotype—is associated with tumor progression, metastasis, and therapy resistance in several cancer types [52]. The process of EMT, or in case of neuroblastoma, adrenergic to mesenchymal transition, has also been demonstrated to generate cellular heterogeneity in neuroblastoma [53, 54]. We confirmed that the commonly used neuroblastoma MRD markers (including PHOX2B, TH, CHRNA3, GAP43) are rarely expressed in mesenchymal neuroblastoma cell lines. We therefore identified a panel of markers, specific for the detection of the mesenchymal neuroblastoma cells [55]. In a follow-up study, we are currently investigating the clinical significance of these markers in the same cohort used in this study. As the number of MRD mRNA markers is expanding with the addition of these markers, we developed a reliable and sensitive multiplex panel for (adrenergic and mesenchymal) MRD markers, after completing this prospective study, to save time, make optimal use of these precious samples, and to facilitate the use of a panel of mRNA markers in the clinic [51]. This study has two main limitations, which also are present in studies of Viprey and Yáñez [17, 39]. Similar to other studies, the first limitation is the number of missing samples during and at end of induction therapy. This was mostly the result of logistical or clinical failures, previous GD2-immunocytology results, which were reported back to the treating physician, did not influence subsequent sampling. The second limitation is the fact that in the rare disease neuroblastoma, international prospective studies take many years to complete, so these often lack prospectively reported data on other disease evaluation modalities such as MIBG/imaging scans or urine catecholamines. Marachelian et al. already showed the additional value of neuroblastoma mRNA for disease evaluation compared to MIBG and BM morphology in cohort with relapsed/refractory patients [47]. These data will be prospectively collected in the SIOPEN High-Risk Neuroblastoma 2 trial (ClinicalTrials.gov Identifier: CT04221035). In this trial, we will also analyze the value of bilateral bone marrow sampling versus single site sampling only by comparing highest, lowest and median infiltration. In this cohort, this was not possible as most samples (all German samples) were pooled before analysis [7, 41].
MRD analysis by RT-qPCR has several advantages: quantification can be reliably performed [22], it is relatively inexpensive, less dependent on quality of smears and not dependent on interobserver variability or experience of the investigator, compared to cytology & immunocytology [32]. While the emerging technique droplet digital PCR (ddPCR) can robustly quantify low levels of tumor derived nucleic acids with high precision, it is less suited for materials with high RNA or DNA content, such as the bone marrow. (RT-)qPCR is very well suited for MRD detection in a broad dynamic range, such as the expression levels of neuroblastoma mRNA in BM [56]. The use of cell-free DNA (cfDNA) in liquid biopsies is successfully being studied in adult cancers, and starting to emerge in pediatric cancers. The question can arise if mRNA or cfDNA is more optimal for MRD detection. Our studies in neuroblastoma have shown not only the concordance but also the discordance when testing both our mRNA-panel as well as circulating tumor-derived DNA in paired samples, demonstrating the need to implement both techniques as future clinical test [57, 58].
Conclusion
In this prospective study, we show the clinical relevance of MRD detection by RT-qPCR in neuroblastoma. The mRNA panel currently studied shows a strong association between BM infiltration levels and EFS and OS at different time-points. Any end of induction BM positivity by RT-qPCR is significantly associated with poor survival and identifies patients at risk for relapse. Molecular detection of MRD by RT-qPCR was more sensitive and of higher prognostic value than immunocytology for neuroblastoma BM infiltration. Thus, we suggest the implementation of MRD detection by RT-qPCR in clinical practice.
Supplementary Information
Supplementary Material 1: Supplemental Figure 1. Schematic overview of Dutch (DCOG NBL2009 trial) and German (GPOH NB2004-HR) trials. Dutch patients received 2 upfront courses of MIBG therapy, if clinically achievable. German patients were randomized to standard induction therapy, or two additional courses of N8 upfront. N5 = vindesine, cisplatin, etoposide; N6= vincristine, dacarbacine, ifosfamide, doxorubicin; N8 = topotecan, cyclophosphamide and etoposide; HD chemo = melphalan, carboplatin, etoposide, followed by autologous stem cell transplantation; RTx= radiotherapy, IT= immune therapy. Blue arrows indicate time points of sample acquisition. Supplemental Figure 2. Sample consort diagram. We investigated whether previous negative immunocytology results influenced sample acquisition. Of the 88 missing bone marrow samples after 2 cycles of therapy, 23 (26%) samples were from patients with a previous sample negative for immunocytology, while of the 257 sampled patients after 2 cycles of therapy, only 30 patients did have a negative previous sample or were not sampled before (12%). Of the 216 patients with missing samples at the end of induction, 102 patients (47%) had a previously negative sample for immunocytology, similarly to the 129 patient that were sampled at the end of induction (61 patients with a previously negative sample; 47%). Supplemental Figure 3 (A) Number of samples grouped by infiltration by RT-qPCR at diagnosis, after 2 cycles of therapy (2 CT) and at the end of induction therapy. (B) Number of samples grouped by infiltration based on GD2-immunocytology at diagnosis, after 2 cycles of therapy (2 CT) and at the end of induction therapy. Supplemental Figure 4. Kaplan-Meier event-free and overall survival curves (EFS on the left and OS on the right, respectively) of the cohort with patients stage M and age >18 months, according to the level of mRNA infiltration by RT-qPCR detected in bone marrow at diagnosis (A, B), after 2 cycles of therapy (C, D), after induction therapy (E, F) and according to the level of GD2- immunocytology (IC) detected in bone marrow at diagnosis (G,H), after 2 cycles of therapy (I,J) and after induction therapy (K,L). 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement. Supplemental Figure 5. Landmark Kaplan-Meier overall surival (OS) analysis, starting 2 years after sample collection for A. diagnosis, stratified for relapse or progressive disease in the first 2 years after sample collection; B. level of bone marrow infiltration by RT-qPCR at diagnosis; C. diagnosis, stratified for RT-qPCR group and relapse/progressive disease; D. after 2 cycles of therapy, stratified for relapse or progressive disease in the first 2 years after sample collection; E. level of bone marrow infiltration by RT-qPCR after 2 cycles of therapy; F. after 2 cycles of therapy, stratified for RT-qPCR group and relapse/progressive disease. 5-year survival rates are given in order according to the legend.The number of children at risk with time for each group is provided in the Data Supplement. Supplemental Figure 6. Kaplan-Meier event-free and overall survival curves (EFS on the left and OS on the right, respectively) according to the level of GD2- immunocytology (IC) detected in bone marrow at diagnosis (A, B). Kaplan-Meier curves for patients with GD-2 IC negative bone marrow at diagnosis, stratified for RT-qPCR results (C, D) and patients with GD2- immunocytology positive bone marrow at the end of induction, stratified for RT-qPCR results (E, F). 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement. Supplemental Figure 7. Kaplan-Meier event-free and overall survival curves (EFS on the left and OS on the right, respectively) according to individual marker positivity in bone marrow samples at diagnosis. If a marker had a Ct value<40 but ΔCt of the sample was within 3 Ct of the ΔCt of the normal control BM samples, it was regarded as negative for analysis, but is depicted here as‘below threshold’. 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement. Supplemental Figure 8. Kaplan-Meier event-free and overall curves (EFS on the left and OS on the right, respectively) according to individual marker positivity in bone marrow samples after 2 cycles of therapy. If a marker had a Ct value<40 but ΔCt of the sample was within 3 Ct of the ΔCt of the normal control BM samples, it was regarded as negative for analysis, but is depicted here as ‘below threshold’. 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement. Supplemental Figure 9. Kaplan-Meier event-free and overall curves (EFS on the left and OS on the right, respectively) according to individual marker positivity in bone marrow samples at the end of induction therapy. If a marker had a Ct value<40 but ΔCt of the sample was within 3 Ct of the ΔCt of the normal control BM samples, it was regarded as negative for analysis, but is depicted here as ‘below threshold’. 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement.
Supplementary Material 2: Supplemental Table 1. Number of children at risk with time in Kaplan Meier plots shown in Figure 2, 3, Supplemental Figures 4, 5, 6, 7-9.
Supplementary Material 3: Supplemental Table 2. Event-free survival (EFS) and overall survival (OS) in Cox proportional hazards regression models per individual RT-qPCR marker. Hazard Ratio (HR) and 95% confidence intervals (CI) of univariable and multivariable Cox model analyses are indicated for each RT-qPCR marker. In multivariate analysis, RT-qPCR group,MYCN status and age >18 months are included as variables.
Acknowledgements
Not applicable.
Abbreviations
- BM
Bone marrow
- cfDNA
Cell-free DNA
- CHRNA3
Cholinergic receptor nicotinic alpha 3
- DCOG
Dutch Childhood Oncology Group
- DCX
Doublecortin
- DDC
Dopamine decarboxylase
- EFS
Event free survival
- GAP43
Growth associated protein 43
- GD2
Disialoganglioside GD2
- GPOH
Gesellschaft für Pädiatrische Onkologie und Hämatologie
- GUSB
Beta-glucoronidase
- HR
Hazard Ratio
- INRC
International Neuroblastoma Response Criteria
- INSS
International Neuroblastoma Staging System
- MIBG
Metaiodobenzylguanidine
- MRD
Minimal residual disease
- MRI
Magnetic resonance imaging
- MYCN
N-Myc proto-oncogen
- N5
Chemotherapy regimen consisting of vindesine, cisplatin, etoposide
- N6
Chemotherapy regimen consisting of vincristine, dacarbacine, ifosfamide, doxorubicin
- N8
Chemotherapy regimen consisting of topotecan, cyclophosphamide and etoposide
- OS
Overall survival
- PB
Peripheral blood
- PET
Positron emission tomography
- PHOX2B
Paired-like homeobox 2b
- RT-qPCR
Reverse transcriptase quantitative polymerase chain reaction
- SIOPEN
European Society of Pediatric Oncology Neuroblastoma Group
- TH
Tyrosine hydroxylase
Authors’ contributions
Conceptualization: LMJVZ, CEVDS, BH, JS, GAMT; Methodology/design: LMJVZ, EMVW, CEVDS, BH, JS, GAMT; Validation: LMJVZ, EMVW; Formal analysis: LMJVZ, MF; Investigation: LMJVZ, BD, EMVW, LZK, NUG, RSK; Resources: LMJVZ, TS, FB, MMN, BH, JS, GAMT; Data curation: LMJVZ, BD, LZK, FB, BH, CEVDS, JS, GAMT; Writing original draft: LMJVZ, GAMT; Writing-review: LMJVZ, BD, EMVW, LZK, NUG, RSK, TS, FB, MMN, MF, CEVDS, JS, GAMT; Visualization: LMJVZ; Project administration: BH, GAMT; Funding acquisition: GAMT; All authors read and approved the final manuscript.
Funding
This study was funded by KWF Kankerbestrijding, grants UVA 2010-4738 and TRANSCAN 8352/TRS-2018-00000715.
Data availability
The data during and/or analysed during the current study available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the Medical Research Ethics Committees of the Academic Medical Center (Amsterdam, the Netherlands; MEC07/219#08.17.0836) and the University of Cologne (Cologne, Germany). Written informed consent from parents or guardians was obtained according to the declaration of Helsinki.
Consent for publication
Not applicable.
Competing interests
The authors report no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Barbara Hero, Janine Stutterheim and Godelieve A. M. Tytgat shared last authorship.
References
- 1.Wan JCM, Massie C, Garcia-Corbacho J, et al. Liquid biopsies come of age: towards implementation of circulating tumour DNA. Nat Rev Cancer. 2017;17(4):223–38. 10.1038/nrc.2017.7. [DOI] [PubMed] [Google Scholar]
- 2.Sai B, Xiang J. Disseminated tumour cells in bone marrow are the source of cancer relapse after therapy. J Cell Mol Med. 2018;22(12):5776–86. 10.1111/jcmm.13867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speleman F, Park JR, Henderson TO. Neuroblastoma: A Tough Nut to Crack. Am Soc Clin Oncol Educ Book. 2016;36:e548–57. 10.1200/EDBK_159169. [DOI] [PubMed] [Google Scholar]
- 4.Cohn SL, Pearson AD, London WB, et al. The International Neuroblastoma Risk Group (INRG) classification system: an INRG Task Force report. J Clin Oncol. 2009;27(2):289–97. 10.1200/JCO.2008.16.6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burchill SA, Beiske K, Shimada H, et al. Recommendations for the standardization of bone marrow disease assessment and reporting in children with neuroblastoma on behalf of the International Neuroblastoma Response Criteria Bone Marrow Working Group. Cancer. 2017;123(7):1095–105. [DOI] [PubMed] [Google Scholar]
- 6.Maris JM. Recent advances in neuroblastoma. N Engl J Med. 2010;362(23):2202–11. 10.1056/NEJMra0804577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kushner BH, Kramer K, Modak S, Cheung NK. Sensitivity of surveillance studies for detecting asymptomatic and unsuspected relapse of high-risk neuroblastoma. J Clin Oncol. 2009;27(7):1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park JR, Bagatell R, Cohn SL, et al. Revisions to the international neuroblastoma response criteria: a consensus statement from the National Cancer Institute clinical trials planning meeting. J Clin Oncol. 2017;35(22):2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ladenstein R, Pötschger U, Pearson ADJ, et al. Busulfan and melphalan versus carboplatin, etoposide, and melphalan as high-dose chemotherapy for high-risk neuroblastoma (HR-NBL1/SIOPEN): an international, randomised, multi-arm, open-label, phase 3 trial. Lancet Oncol. 2017;18(4):500–14. 10.1016/S1470-2045(17)30070-0. [DOI] [PubMed] [Google Scholar]
- 10.Berthold F, Faldum A, Ernst A, et al. Extended induction chemotherapy does not improve the outcome for high-risk neuroblastoma patients: results of the randomized open-label GPOH trial NB2004-HR. Ann Oncol. 2020;31(3):422–9. 10.1016/j.annonc.2019.11.011. [DOI] [PubMed] [Google Scholar]
- 11.London WB, Bagatell R, Weigel BJ, et al. Historical time to disease progression and progression-free survival in patients with recurrent/refractory neuroblastoma treated in the modern era on Children’s Oncology Group early-phase trials. Cancer. 2017;123(24):4914–23. 10.1002/cncr.30934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Méhes G, Luegmayr A, Kornmüller R, et al. Detection of disseminated tumor cells in neuroblastoma: 3 log improvement in sensitivity by automatic immunofluorescence plus FISH (AIPF) analysis compared with classical bone marrow cytology. Am J Pathol. 2003;163(2):393–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Swerts K, Ambros PF, Brouzes C, et al. Standardization of the immunocytochemical detection of neuroblastoma cells in bone marrow. J Histochem Cytochem. 2005;53(12):1433–40. 10.1369/jhc.5C6661.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Beiske K, Burchill SA, Cheung IY, et al. Consensus criteria for sensitive detection of minimal neuroblastoma cells in bone marrow, blood and stem cell preparations by immunocytology and QRT-PCR: recommendations by the International Neuroblastoma Risk Group Task Force. Br J Cancer. 2009;100(10):1627–37. 10.1038/sj.bjc.6605029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stutterheim J, Zappeij-Kannegieter L, Versteeg R, Caron HN, van der Schoot CE, Tytgat GA. The prognostic value of fast molecular response of marrow disease in patients aged over 1 year with stage 4 neuroblastoma. Eur J Cancer. 2011;47(8):1193–202. 10.1016/j.ejca.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 16.van der Velden VHJ, Dombrink I, Alten J, et al. Analysis of measurable residual disease by IG/TR gene rearrangements: quality assurance and updated EuroMRD guidelines. Leukemia. 2024;38(6):1315–22. 10.1038/s41375-024-02272-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Viprey VF, Gregory WM, Corrias MV, et al. Neuroblastoma mRNAs predict outcome in children with stage 4 neuroblastoma: a European HR-NBL1/SIOPEN study. J Clin Oncol. 2014;32(10):1074–83. 10.1200/JCO.2013.53.3604. [DOI] [PubMed] [Google Scholar]
- 18.Cheung IY, Feng Y, Cheung NK. Early negative minimal residual disease in bone marrow after immunotherapy is less predictive of late or non-marrow relapse among patients with high-risk stage 4 neuroblastoma. Pediatr Blood Cancer. 2013;60(7):E32-4. 10.1002/pbc.24469. [DOI] [PubMed] [Google Scholar]
- 19.Tchirkov A, Paillard C, Halle P, et al. Significance of molecular quantification of minimal residual disease in metastatic neuroblastoma. J Hematother Stem Cell Res. 2003;12(4):435–42. [DOI] [PubMed] [Google Scholar]
- 20.Stutterheim J, Gerritsen A, Zappeij-Kannegieter L, et al. PHOX2B is a novel and specific marker for minimal residual disease testing in neuroblastoma. J Clin Oncol. 2008;26(33):5443–9. 10.1200/JCO.2007.13.6531. [DOI] [PubMed] [Google Scholar]
- 21.Stutterheim J, Gerritsen A, Zappeij-Kannegieter L, et al. Detecting minimal residual disease in neuroblastoma: the superiority of a panel of real-time quantitative PCR markers. Clin Chem. 2009;55(7):1316–26. 10.1373/clinchem.2008.117945. [DOI] [PubMed] [Google Scholar]
- 22.Stutterheim J, Zappeij-Kannegieter L, Ora I, et al. Stability of PCR targets for monitoring minimal residual disease in neuroblastoma. J Mol Diagn. 2012;14(2):168–75. 10.1016/j.jmoldx.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 23.van Wezel EM, Decarolis B, Stutterheim J, et al. Neuroblastoma messenger RNA is frequently detected in bone marrow at diagnosis of localised neuroblastoma patients. Eur J Cancer. 2016;54:149–58. 10.1016/j.ejca.2015.11.007. [DOI] [PubMed] [Google Scholar]
- 24.van Wezel EM, Stutterheim J, Vree F, et al. Minimal residual disease detection in autologous stem cell grafts from patients with high risk neuroblastoma. Pediatr Blood Cancer. 2015;62(8):1368–73. 10.1002/pbc.25507. [DOI] [PubMed] [Google Scholar]
- 25.Kraal KCJM, Bleeker GM, van Eck-Smit BLF, et al. Feasibility, toxicity and response of upfront metaiodobenzylguanidine therapy therapy followed by German Pediatric Oncology Group Neuroblastoma 2004 protocol in newly diagnosed stage 4 neuroblastoma patients. Eur J Cancer. 2017;76:188–96. 10.1016/j.ejca.2016.12.013. [DOI] [PubMed] [Google Scholar]
- 26.Yu AL, Gilman AL, Ozkaynak MF, et al. Anti-GD2 antibody with GM-CSF, interleukin-2, and isotretinoin for neuroblastoma. N Engl J Med. 2010;363(14):1324–34. 10.1056/NEJMoa0911123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brodeur GM, Pritchard J, Berthold F, et al. Revisions of the international criteria for neuroblastoma diagnosis, staging, and response to treatment. J Clin Oncol. 1993;11(8):1466–77 (http://www.ncbi.nlm.nih.gov/pubmed/8336186). [DOI] [PubMed] [Google Scholar]
- 28.Monclair T, Brodeur GM, Ambros PF, et al. The International Neuroblastoma Risk Group (INRG) staging system: an INRG Task Force report. J Clin Oncol. 2009;27(2):298–303. 10.1200/JCO.2008.16.6876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beillard E, Pallisgaard N, van der Velden VHJ, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) – a Europe against cancer program. Leukemia. 2003;17(12):2474–86. 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- 30.Viprey VF, Corrias MV, Kagedal B, et al. Standardisation of operating procedures for the detection of minimal disease by QRT-PCR in children with neuroblastoma: Quality assurance on behalf of SIOPEN-R-NET. Eur J Cancer. 2007;43(2):341–50. 10.1016/j.ejca.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Schumacher-Kuckelkorn R, Volland R, Gradehandt A, Hero B, Simon T, Berthold F. Lack of immunocytological GD2 expression on neuroblastoma cells in bone marrow at diagnosis, during treatment, and at recurrence*. Pediatr Blood Cancer. 2017;64(1):46–56. 10.1002/pbc.26184. [DOI] [PubMed] [Google Scholar]
- 32.Schumacher-Kuckelkorn R, Atra A, Belli ML, et al. The reliability of bone marrow cytology as response criterion in metastatic neuroblastoma. Pediatr Blood Cancer. 2021;68(3):e28819. 10.1002/pbc.28819. [DOI] [PubMed] [Google Scholar]
- 33.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Control Clin Trials. 1996;17(4):343–6. 10.1016/0197-2456(96)00075-X. [DOI] [PubMed] [Google Scholar]
- 34.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1(11):710–9. 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 35.van Wezel EM. Minimal Residual Disease Monitoring in Neuroblastoma. [Phd-Thesis - Research and graduation internal, University of Amsterdam]. 2016. https://pure.amsterdamumc.nl/en/publications/minimal-residual-disease-monitoring-in-neuroblastoma.
- 36.Seeger RC, Reynolds CP, Gallego R, Stram DO, Gerbing RB, Matthay KK. Quantitative tumor cell content of bone marrow and blood as a predictor of outcome in stage IV neuroblastoma: a Children’s Cancer Group Study. J Clin Oncol. 2000;18(24):4067–76 (http://www.ncbi.nlm.nih.gov/pubmed/11118468). [DOI] [PubMed] [Google Scholar]
- 37.Cai JY, Pan C, Tang YJ, et al. Minimal Residual Disease is a Prognostic Marker for Neuroblastoma With Bone Marrow Infiltration. Am J Clin Oncol. 2012;35(3):275–8. 10.1097/COC.0b013e318210f51b. [DOI] [PubMed] [Google Scholar]
- 38.Trager C, Vernby A, Kullman A, Ora I, Kogner P, Kagedal B. mRNAs of tyrosine hydroxylase and dopa decarboxylase but not of GD2 synthase are specific for neuroblastoma minimal disease and predicts outcome for children with high-risk disease when measured at diagnosis. Int J Cancer. 2008;123(12):2849–55. 10.1002/ijc.23846. [DOI] [PubMed] [Google Scholar]
- 39.Yáñez Y, Hervás D, Grau E, et al. TH and DCX mRNAs in peripheral blood and bone marrow predict outcome in metastatic neuroblastoma patients. J Cancer Res Clin Oncol. 2016;142(3):573–80. 10.1007/s00432-015-2054-7. [DOI] [PubMed] [Google Scholar]
- 40.Park JR, Kreissman SG, London WB, et al. Effect of Tandem Autologous Stem Cell Transplant vs Single Transplant on Event-Free Survival in Patients With High-Risk Neuroblastoma. JAMA. 2019;322(8):746. 10.1001/jama.2019.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anderson J, Majzner RG, Sondel PM. Immunotherapy of Neuroblastoma: Facts and Hopes. Clin Cancer Res. 2022;28(15):3196–206. 10.1158/1078-0432.CCR-21-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mody R, Yu AL, Naranjo A, et al. Irinotecan, Temozolomide, and Dinutuximab With GM-CSF in Children With Refractory or Relapsed Neuroblastoma: A Report From the Children’s Oncology Group. J Clin Oncol. 2020;38(19):2160–9. 10.1200/JCO.20.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Tilburg CM, Pfaff E, Pajtler KW, et al. The Pediatric Precision Oncology INFORM Registry: Clinical Outcome and Benefit for Patients with Very High-Evidence Targets. Cancer Discov. 2021;11(11):2764–79. 10.1158/2159-8290.CD-21-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yanik GA, Parisi MT, Naranjo A, et al. Validation of Postinduction Curie Scores in High-Risk Neuroblastoma: A Children’s Oncology Group and SIOPEN Group Report on SIOPEN/HR-NBL1. J Nuclear Med. 2018;59(3):502–8. 10.2967/jnumed.117.195883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Decarolis B, Schneider C, Hero B, et al. Iodine-123 Metaiodobenzylguanidine Scintigraphy Scoring Allows Prediction of Outcome in Patients With Stage 4 Neuroblastoma: Results of the Cologne Interscore Comparison Study. J Clin Oncol. 2013;31(7):944–51. 10.1200/JCO.2012.45.8794. [DOI] [PubMed] [Google Scholar]
- 46.Ladenstein R, Lambert B, Pötschger U, et al. Validation of the mIBG skeletal SIOPEN scoring method in two independent high-risk neuroblastoma populations: the SIOPEN/HR-NBL1 and COG-A3973 trials. Eur J Nucl Med Mol Imaging. 2018;45(2):292–305. 10.1007/s00259-017-3829-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Marachelian A, Villablanca JG, Liu CW, et al. Expression of five neuroblastoma genes in bone marrow or blood of patients with relapsed/refractory neuroblastoma provides a new biomarker for disease and prognosis. Clin Cancer Res. 2017;23(18):5374–83. 10.1158/1078-0432.CCR-16-2647. [DOI] [PubMed] [Google Scholar]
- 48.Hartomo TB, Kozaki A, Hasegawa D, et al. Minimal residual disease monitoring in neuroblastoma patients based on the expression of a set of real-time RT-PCR markers in tumor-initiating cells. Oncol Rep. 2013;29(4):1629–36. 10.3892/or.2013.2286. [DOI] [PubMed] [Google Scholar]
- 49.Thwin KKM, Ishida T, Uemura S, et al. Level of Seven Neuroblastoma-Associated mRNAs Detected by Droplet Digital PCR Is Associated with Tumor Relapse/Regrowth of High-Risk Neuroblastoma Patients. J Mol Diagn. 2020;22(2):236–46. 10.1016/j.jmoldx.2019.10.012. [DOI] [PubMed] [Google Scholar]
- 50.Cheung IY, Feng Y, Gerald W, Cheung NK. Exploiting gene expression profiling to identify novel minimal residual disease markers of neuroblastoma. Clin Cancer Res. 2008;14(21):7020–7. 10.1158/1078-0432.CCR-08-0541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.van Zogchel LMJ, Zappeij-Kannegieter L, Javadi A, et al. Specific and Sensitive Detection of Neuroblastoma mRNA Markers by Multiplex RT-qPCR. Cancers (Basel). 2021;13(1):150. 10.3390/cancers13010150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dave B, Mittal V, Tan NM, Chang JC. Epithelial-mesenchymal transition, cancer stem cells and treatment resistance. Breast Cancer Res. 2012;14(1):202. 10.1186/bcr2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.van Groningen T, Koster J, Valentijn LJ, et al. Neuroblastoma is composed of two super-enhancer-associated differentiation states. Nat Genet. 2017;49(8):1261–6. 10.1038/ng.3899. [DOI] [PubMed] [Google Scholar]
- 54.Thirant C, Peltier A, Durand S, et al. Reversible transitions between noradrenergic and mesenchymal tumor identities define cell plasticity in neuroblastoma. Nat Commun. 2023;14(1):2575. 10.1038/s41467-023-38239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.van Wezel EM, van Zogchel LMJ, van Wijk J, et al. Mesenchymal Neuroblastoma Cells Are Undetected by Current mRNA Marker Panels: The Development of a Specific Neuroblastoma Mesenchymal Minimal Residual Disease Panel. JCO Precis Oncol. 2019;3:1–11. 10.1200/PO.18.00413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Taylor SC, Laperriere G, Germain H. Droplet Digital PCR versus qPCR for gene expression analysis with low abundant targets: from variable nonsense to publication quality data. Sci Rep. 2017;7(1):2409. 10.1038/s41598-017-02217-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Zogchel LMJ, van Wezel EM, van Wijk J, et al. Hypermethylated RASSF1A as Circulating Tumor DNA Marker for Disease Monitoring in Neuroblastoma. JCO Precis Oncol. 2020;4:291–306. 10.1200/PO.19.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van Zogchel LMJ, Lak NSM, Verhagen OJHM, et al. Novel Circulating Hypermethylated RASSF1A ddPCR for Liquid Biopsies in Patients With Pediatric Solid Tumors. JCO Precis Oncol. 2021;5:1738–48. 10.1200/PO.21.00130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material 1: Supplemental Figure 1. Schematic overview of Dutch (DCOG NBL2009 trial) and German (GPOH NB2004-HR) trials. Dutch patients received 2 upfront courses of MIBG therapy, if clinically achievable. German patients were randomized to standard induction therapy, or two additional courses of N8 upfront. N5 = vindesine, cisplatin, etoposide; N6= vincristine, dacarbacine, ifosfamide, doxorubicin; N8 = topotecan, cyclophosphamide and etoposide; HD chemo = melphalan, carboplatin, etoposide, followed by autologous stem cell transplantation; RTx= radiotherapy, IT= immune therapy. Blue arrows indicate time points of sample acquisition. Supplemental Figure 2. Sample consort diagram. We investigated whether previous negative immunocytology results influenced sample acquisition. Of the 88 missing bone marrow samples after 2 cycles of therapy, 23 (26%) samples were from patients with a previous sample negative for immunocytology, while of the 257 sampled patients after 2 cycles of therapy, only 30 patients did have a negative previous sample or were not sampled before (12%). Of the 216 patients with missing samples at the end of induction, 102 patients (47%) had a previously negative sample for immunocytology, similarly to the 129 patient that were sampled at the end of induction (61 patients with a previously negative sample; 47%). Supplemental Figure 3 (A) Number of samples grouped by infiltration by RT-qPCR at diagnosis, after 2 cycles of therapy (2 CT) and at the end of induction therapy. (B) Number of samples grouped by infiltration based on GD2-immunocytology at diagnosis, after 2 cycles of therapy (2 CT) and at the end of induction therapy. Supplemental Figure 4. Kaplan-Meier event-free and overall survival curves (EFS on the left and OS on the right, respectively) of the cohort with patients stage M and age >18 months, according to the level of mRNA infiltration by RT-qPCR detected in bone marrow at diagnosis (A, B), after 2 cycles of therapy (C, D), after induction therapy (E, F) and according to the level of GD2- immunocytology (IC) detected in bone marrow at diagnosis (G,H), after 2 cycles of therapy (I,J) and after induction therapy (K,L). 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement. Supplemental Figure 5. Landmark Kaplan-Meier overall surival (OS) analysis, starting 2 years after sample collection for A. diagnosis, stratified for relapse or progressive disease in the first 2 years after sample collection; B. level of bone marrow infiltration by RT-qPCR at diagnosis; C. diagnosis, stratified for RT-qPCR group and relapse/progressive disease; D. after 2 cycles of therapy, stratified for relapse or progressive disease in the first 2 years after sample collection; E. level of bone marrow infiltration by RT-qPCR after 2 cycles of therapy; F. after 2 cycles of therapy, stratified for RT-qPCR group and relapse/progressive disease. 5-year survival rates are given in order according to the legend.The number of children at risk with time for each group is provided in the Data Supplement. Supplemental Figure 6. Kaplan-Meier event-free and overall survival curves (EFS on the left and OS on the right, respectively) according to the level of GD2- immunocytology (IC) detected in bone marrow at diagnosis (A, B). Kaplan-Meier curves for patients with GD-2 IC negative bone marrow at diagnosis, stratified for RT-qPCR results (C, D) and patients with GD2- immunocytology positive bone marrow at the end of induction, stratified for RT-qPCR results (E, F). 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement. Supplemental Figure 7. Kaplan-Meier event-free and overall survival curves (EFS on the left and OS on the right, respectively) according to individual marker positivity in bone marrow samples at diagnosis. If a marker had a Ct value<40 but ΔCt of the sample was within 3 Ct of the ΔCt of the normal control BM samples, it was regarded as negative for analysis, but is depicted here as‘below threshold’. 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement. Supplemental Figure 8. Kaplan-Meier event-free and overall curves (EFS on the left and OS on the right, respectively) according to individual marker positivity in bone marrow samples after 2 cycles of therapy. If a marker had a Ct value<40 but ΔCt of the sample was within 3 Ct of the ΔCt of the normal control BM samples, it was regarded as negative for analysis, but is depicted here as ‘below threshold’. 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement. Supplemental Figure 9. Kaplan-Meier event-free and overall curves (EFS on the left and OS on the right, respectively) according to individual marker positivity in bone marrow samples at the end of induction therapy. If a marker had a Ct value<40 but ΔCt of the sample was within 3 Ct of the ΔCt of the normal control BM samples, it was regarded as negative for analysis, but is depicted here as ‘below threshold’. 5-year survival rates are given in order according to the legend. The number of children at risk with time for each group is provided in the Data Supplement.
Supplementary Material 2: Supplemental Table 1. Number of children at risk with time in Kaplan Meier plots shown in Figure 2, 3, Supplemental Figures 4, 5, 6, 7-9.
Supplementary Material 3: Supplemental Table 2. Event-free survival (EFS) and overall survival (OS) in Cox proportional hazards regression models per individual RT-qPCR marker. Hazard Ratio (HR) and 95% confidence intervals (CI) of univariable and multivariable Cox model analyses are indicated for each RT-qPCR marker. In multivariate analysis, RT-qPCR group,MYCN status and age >18 months are included as variables.
Data Availability Statement
The data during and/or analysed during the current study available from the corresponding author on reasonable request.