Abstract
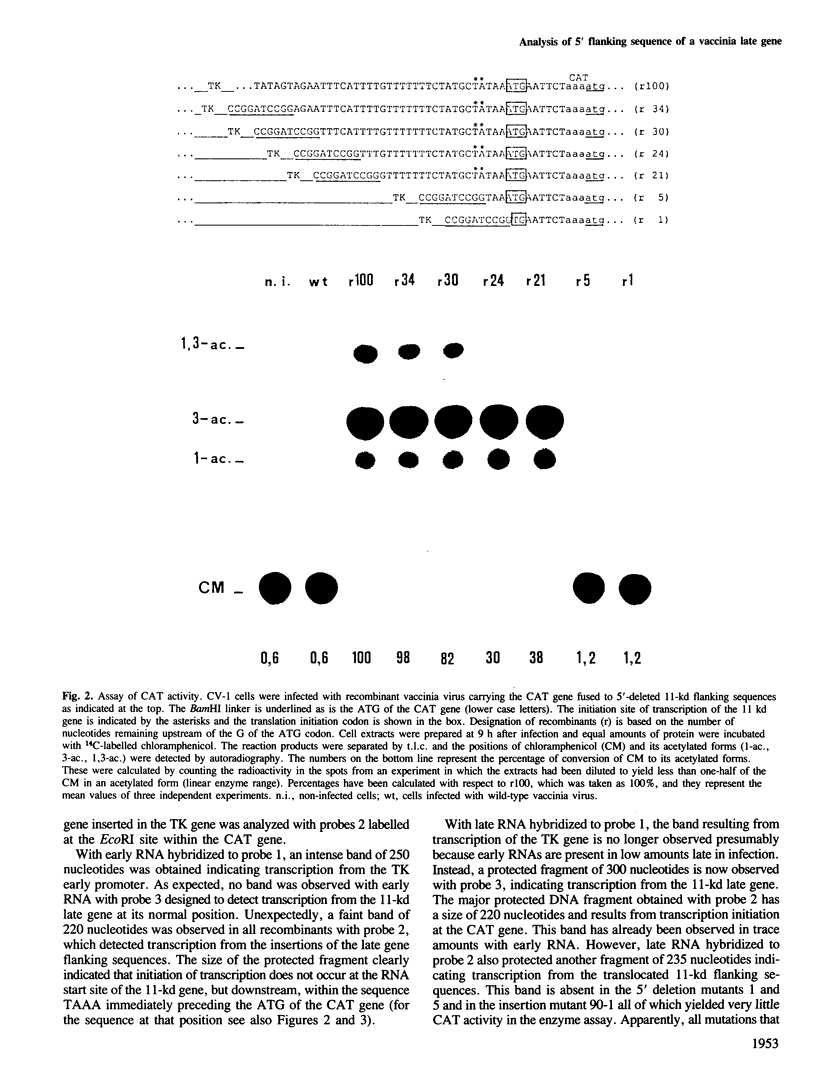
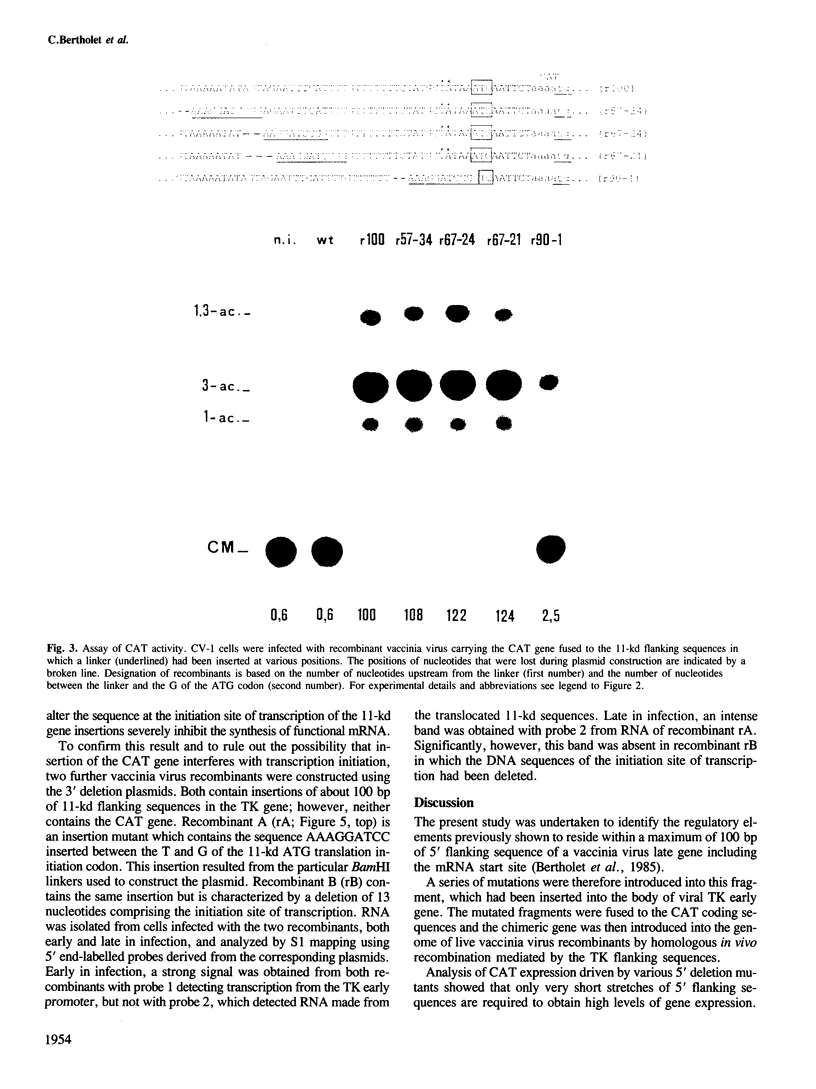
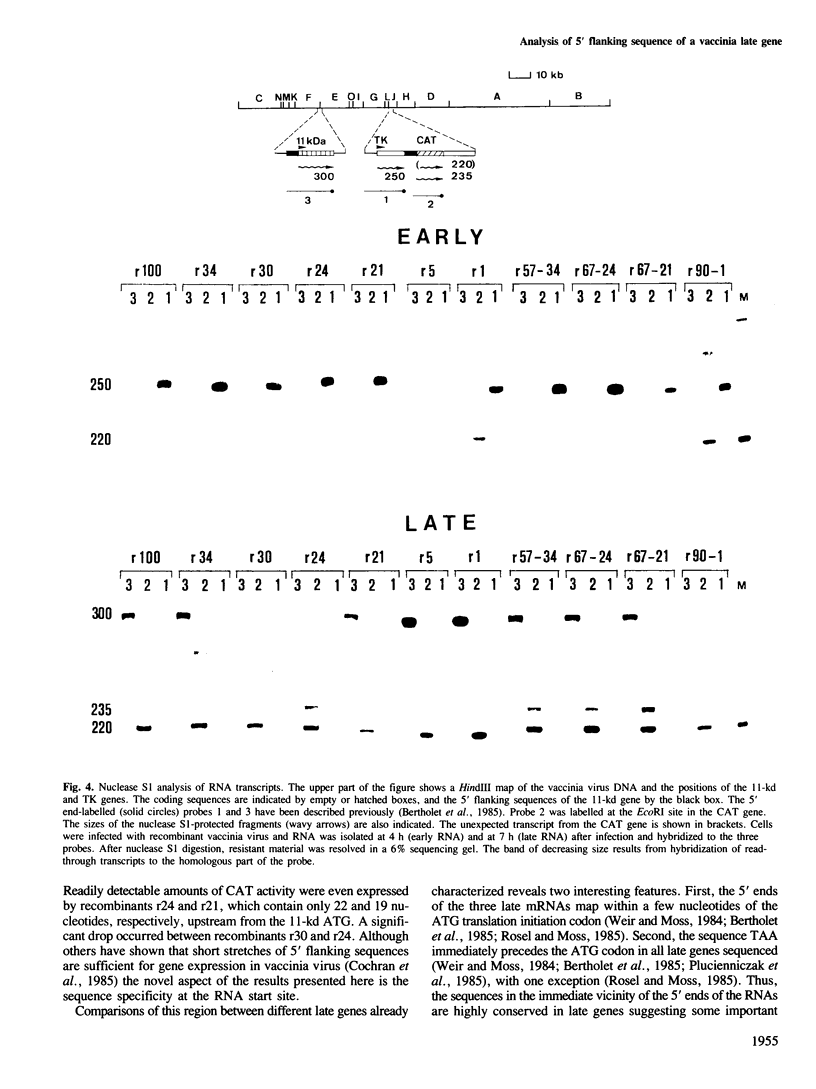
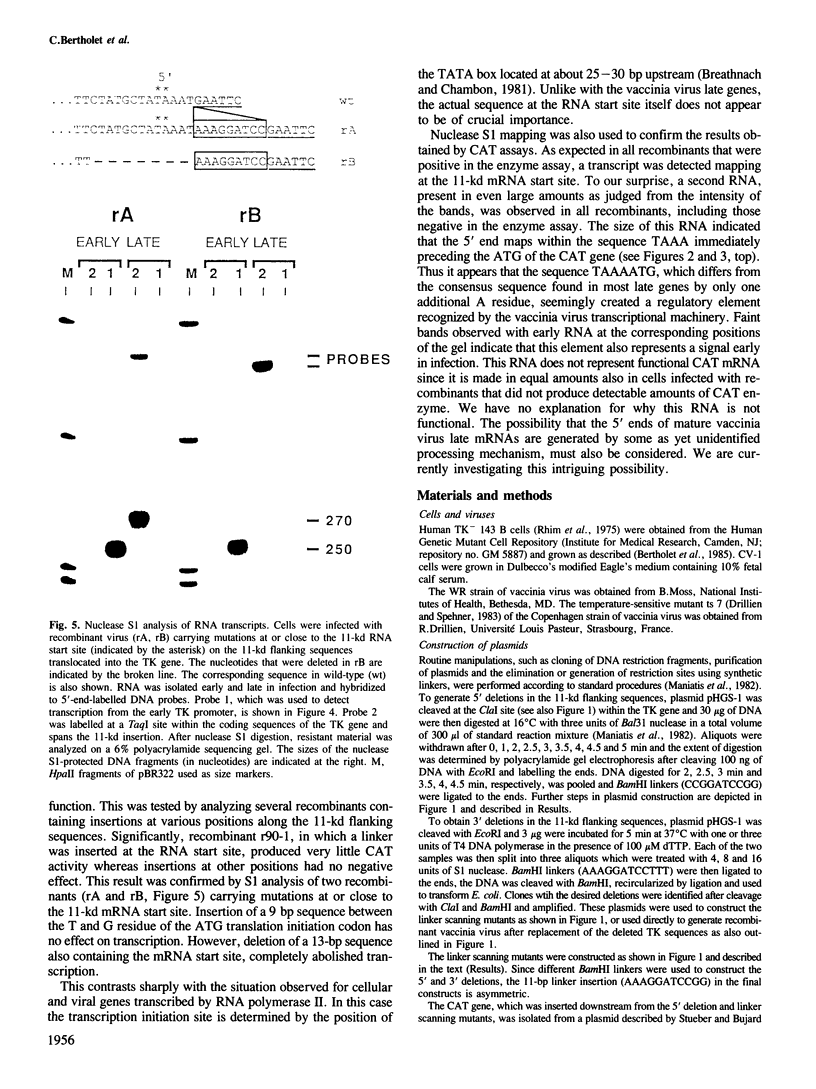
A series of mutations, including 5' and 3' deletions, as well as insertions were introduced into the 5' flanking nucleotide sequence of a vaccinia virus late gene. This DNA has been shown previously to contain all the necessary elements for correct regulation of the gene most probably transcribed by the viral RNA polymerase. To facilitate the assays, the mutated DNA was fused to the chloramphenicol acetyltransferase gene and inserted into the genome of live vaccinia virus. The effects of the mutations on expression of the chimeric gene were studied by both enzyme assays and nuclease S1 analysis. The results showed that 5' deletions up to about 15 bp from the putative initiation site of transcription still yielded high levels of gene expression. All mutations, however, that deleted the authentic late mRNA start site, abolished promoter activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajszár G., Wittek R., Weir J. P., Moss B. Vaccinia virus thymidine kinase and neighboring genes: mRNAs and polypeptides of wild-type virus and putative nonsense mutants. J Virol. 1983 Jan;45(1):62–72. doi: 10.1128/jvi.45.1.62-72.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berk A. J., Sharp P. A. Sizing and mapping of early adenovirus mRNAs by gel electrophoresis of S1 endonuclease-digested hybrids. Cell. 1977 Nov;12(3):721–732. doi: 10.1016/0092-8674(77)90272-0. [DOI] [PubMed] [Google Scholar]
- Bertholet C., Drillien R., Wittek R. One hundred base pairs of 5' flanking sequence of a vaccinia virus late gene are sufficient to temporally regulate late transcription. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2096–2100. doi: 10.1073/pnas.82.7.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Chambon P. Organization and expression of eucaryotic split genes coding for proteins. Annu Rev Biochem. 1981;50:349–383. doi: 10.1146/annurev.bi.50.070181.002025. [DOI] [PubMed] [Google Scholar]
- Cochran M. A., Puckett C., Moss B. In vitro mutagenesis of the promoter region for a vaccinia virus gene: evidence for tandem early and late regulatory signals. J Virol. 1985 Apr;54(1):30–37. doi: 10.1128/jvi.54.1.30-37.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drillien R., Spehner D. Physical mapping of vaccinia virus temperature-sensitive mutations. Virology. 1983 Dec;131(2):385–393. doi: 10.1016/0042-6822(83)90506-8. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Ball L. A. Mapping and identification of the vaccinia virus thymidine kinase gene. J Virol. 1982 Aug;43(2):403–409. doi: 10.1128/jvi.43.2.403-409.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E., Maki R. A., Miller D. B., Ball L. A. Fine structure analysis and nucleotide sequence of the vaccinia virus thymidine kinase gene. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3411–3415. doi: 10.1073/pnas.80.11.3411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight S. L., Kingsbury R. Transcriptional control signals of a eukaryotic protein-coding gene. Science. 1982 Jul 23;217(4557):316–324. doi: 10.1126/science.6283634. [DOI] [PubMed] [Google Scholar]
- Plucienniczak A., Schroeder E., Zettlmeissl G., Streeck R. E. Nucleotide sequence of a cluster of early and late genes in a conserved segment of the vaccinia virus genome. Nucleic Acids Res. 1985 Feb 11;13(3):985–998. doi: 10.1093/nar/13.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhim J. S., Cho H. Y., Huebner R. J. Non-producer human cells induced by murine sarcoma virus. Int J Cancer. 1975 Jan 15;15(1):23–29. doi: 10.1002/ijc.2910150104. [DOI] [PubMed] [Google Scholar]
- Rosel J., Moss B. Transcriptional and translational mapping and nucleotide sequence analysis of a vaccinia virus gene encoding the precursor of the major core polypeptide 4b. J Virol. 1985 Dec;56(3):830–838. doi: 10.1128/jvi.56.3.830-838.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stueber D., Bujard H. Transcription from efficient promoters can interfere with plasmid replication and diminish expression of plasmid specified genes. EMBO J. 1982;1(11):1399–1404. doi: 10.1002/j.1460-2075.1982.tb01329.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver R. F., Weissmann C. Mapping of RNA by a modification of the Berk-Sharp procedure: the 5' termini of 15 S beta-globin mRNA precursor and mature 10 s beta-globin mRNA have identical map coordinates. Nucleic Acids Res. 1979 Nov 10;7(5):1175–1193. doi: 10.1093/nar/7.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir J. P., Moss B. Nucleotide sequence of the vaccinia virus thymidine kinase gene and the nature of spontaneous frameshift mutations. J Virol. 1983 May;46(2):530–537. doi: 10.1128/jvi.46.2.530-537.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
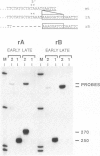
- Weir J. P., Moss B. Regulation of expression and nucleotide sequence of a late vaccinia virus gene. J Virol. 1984 Sep;51(3):662–669. doi: 10.1128/jvi.51.3.662-669.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittek R., Hänggi M., Hiller G. Mapping of a gene coding for a major late structural polypeptide on the vaccinia virus genome. J Virol. 1984 Feb;49(2):371–378. doi: 10.1128/jvi.49.2.371-378.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]